Abstract
Objectives
Epidural analgesia is recommended for the provision of analgesia following major abdominal surgery. Continuous local anaesthetic wound infiltration may be an effective alternative. A prospective randomized trial was undertaken to compare these two methods following open liver resection. The primary outcome was length of time required to fulfil criteria for discharge from hospital.
Methods
Patients undergoing open liver resection were randomized to receive either epidural (EP group) or local anaesthetic wound infiltration plus patient-controlled opiate analgesia (WI group) for the first 2 days postoperatively. All other care followed a standardized enhanced recovery protocol. Time to fulfil discharge criteria, pain scores, physical activity measurements and complications were recorded.
Results
Between August 2009 and July 2010, 65 patients were randomized to EP (n= 32) or WI (n= 33). The mean time required to fulfil discharge criteria was 4.5 days (range: 2.5–63.5 days) in the WI group and 6.0 days (range: 3.0–42.5 days) in the EP group (P= 0.044). During the first 48 h following surgery, pain scores were significantly lower in the EP group both at rest and on movement. Resting pain scores within both groups were rated as mild (range: 0–3). There was no significant difference between the groups in time to first mobilization or overall complication rate (48.5% in the WI group vs. 58.1% in the EP group; P= 0.443).
Conclusions
Local anaesthetic wound infiltration combined with patient-controlled opiate analgesia reduces the length of time required to fulfil criteria for discharge from hospital compared with epidural analgesia following open liver resection. Epidural analgesia provides superior analgesia, but does not confer benefits in terms of faster mobilization or recovery.
Keywords: analgesia, epidural, liver resection, bupivacaine, wound catheter, enhanced recovery
Introduction
The provision of adequate pain relief following open liver resection is vital to minimize patient distress, allow effective mobilization and prevent complications. Ideally, analgesia should be safe, easy to administer and have few side-effects. Unfortunately, this can be difficult to achieve and some degree of compromise is usually required.
Intravenous opiate patient-controlled analgesia (PCA) is associated with good patient satisfaction and is straightforward to administer and monitor.1 However, the side-effects of opiate analgesia may prove troublesome in attempts to achieve adequate analgesia following major surgery.
An early meta-analysis reported the widespread benefits of epidural analgesia over opioid analgesia in terms of mortality and major morbidity.2 Two subsequent large randomized trials failed to demonstrate many of these reported benefits, but did show improved analgesia and a reduced incidence of postoperative respiratory failure, specifically in high-risk patients undergoing high-risk procedures.3,4 Improved pain relief and avoidance of opiates are likely to contribute to the beneficial effects on postoperative ileus seen with epidural analgesia.5 Epidural analgesia remains popular, is considered by many to represent the reference standard in major abdominal surgery, and is advocated in enhanced recovery programmes.
However, it is widely recognized that the epidural technique is not universally successful and that inadequate analgesia is experienced by 20–30% of patients.6,7 The management of poorly functioning epidurals is time-consuming and may be particularly difficult to achieve out of hours when the time of appropriately trained staff is subject to increased demands. Epidural-associated hypotension is a frequently encountered problem. Appropriate management includes i.v. fluid administration and the use of vasoactive drugs. However, i.v. fluid administration may be significant and may result in a patient receiving an excessive volume of fluid.8
Infrequent but potentially devastating complications such as epidural haematoma or abscess formation must always be considered.9 In major hepatic resection, in which some degree of postoperative coagulopathy is frequently encountered, this point merits special consideration.10 There is little evidence to support or refute the theory of an increased risk for epidural haematoma following liver resection as this complication remains extremely rare. However, this issue is an area of ongoing concern for some11 and although epidural analgesia remains popular in the UK following liver resection, it is not universally employed worldwide.
Continuous infiltration of local anaesthetic using wound catheters placed in the abdominal wall has recently emerged as an alternative means of providing postoperative analgesia. An initial meta-analysis of randomized trials involving local anaesthetic wound catheters reported mixed results.12 It has become clear that catheter placement is key to the success of the technique. Recent studies in open nephrectomy and colorectal resection have demonstrated that the placement of catheters in the appropriate muscle layers can achieve a significant reduction in requirements for additional opiates.13,14 Use of this technique in liver resection was first described by Basu et al.15 Chan et al. subsequently confirmed its efficacy in this context in a randomized controlled trial (RCT).16 What remains to be investigated is how this analgesic technique compares with the current reference standard, epidural analgesia.
This study aimed to evaluate the use of continuous infiltration of local anaesthetic as part of a multimodal analgesic regimen, as an alternative to epidural analgesia, within an enhanced recovery programme following open liver resection.
Materials and methods
Ethical approval for the study was obtained from the Lothian Research Ethics Committee. Potential participants were identified through the hepatopancreaticobiliary (HPB) multidisciplinary team meeting at the Royal Infirmary of Edinburgh and were approached in the outpatient department. All patients undergoing open liver resection for benign or malignant conditions were considered for inclusion. Exclusion criteria are listed in Fig. 1. All participants gave written consent prior to inclusion. Participants were counselled preoperatively on the daily targets that would be set postoperatively and the anticipated timeline for their recovery. Baseline characteristics recorded included age, gender, comorbidities and indications for surgery.
Figure 1.

Exclusion criteria
A sample size calculation was performed using local historic data on length of hospital stay following liver resection and pilot data on the use of local anaesthetic wound infiltration in this setting. The median length of hospital stay was 6 days. Using log-transformed data, the mean length of stay was calculated as 0.819 (standard deviation 0.204). To demonstrate a reduction in length of stay of 0.153 (approximately 1.5 days), with a level of statistical significance of 0.05 and a power of 0.85, it was calculated that 32 patients would be required in each arm of the study. The Royal Infirmary of Edinburgh provides a hepatobiliary service to patients who live a significant distance from the hospital and length of stay can be influenced by factors such as transport availability. Time required to fulfil criteria for discharge from hospital was therefore used as the primary endpoint of the study in order to reflect functional recovery more accurately than actual length of hospital stay.
Block randomization, stratified by age (<60 years or ≥60 years), was performed using sealed envelopes and was undertaken by anaesthesia staff prior to the arrival of the patient in the anaesthesia room. Patients were randomized to receive either local anaesthetic infiltration via wound catheters plus PCA (WI group), or epidural analgesia (EP group). A single-blind design was employed. Patients in both arms of the study received 2 mg midazolam on arrival in the anaesthesia room. Patients in the EP group underwent mid-thoracic epidural catheter insertion at approximately T7–8. A test dose of 4 ml 2% lignocaine was given, followed by infusion of 0.1% bupivacaine and 2 mcg/ml fentanyl commenced at 7–10 ml/h. Patients in the WI group had a sham epidural fixed to the back with standardized dressings, connected to a non-active epidural pump.
Antibiotic and thromboprophylaxis were given pre-procedure. Induction of anaesthesia in both groups followed a standardized protocol, using 1–2 mcg/kg fentanyl, 0.1–0.15 mg/kg vecuronium, and propofol. Patients in the WI group also received a loading dose of 5–10 mg i.v. morphine and further morphine as deemed appropriate by anaesthesia staff throughout the surgical procedure. Isoflurane or desflurane was administered for maintenance of anaesthesia. Intravenous fluids were administered at 100 ml/h maintenance (0.18% sodium chloride/4% dextrose) along with colloid boluses to replace blood loss. All patients received antiemetic ondansetron 4 mg i.v. before the reversal of the neuromuscular blockade and termination of anaesthesia.
Liver resection was performed through a right subcostal incision extended upwards to the midline or across the midline. Extent of resection, requirement for hepaticojejunostomy, operating time, estimated blood loss, wound length and intraoperative complications were all noted. Nasogastric tubes were not placed routinely; when nasogastric tubes were placed intraoperatively, they were removed before the end of the surgical procedure. Surgical and anaesthetic details were recorded on a standardized data collection form.
At the end of the surgical procedure, dual wound catheters (On-Q® SilverSoaker™; B. Braun Medical Ltd, Sheffield, UK), with an active length of 12.5 cm, were placed in patients in the WI group under direct vision in the deep muscle layers, between the transversus abdominus and internal oblique laterally, and in the posterior rectus sheath medially. These were flushed with a total of 20 ml 0.25% levobupivacaine and connected to an elastomeric pump (On-Q® PainBuster®; B. Braun Medical Ltd). The pump delivered 0.375% levobupivacaine at 4 ml/h over a 48-h period. Patients in the WI group also received an opiate PCA device, but were unaware of which randomization group this related to. In patients in the EP group, sham wound catheters were attached to the anterior abdominal wall in standardized positions using opaque dressings and were connected to a non-active elastomeric pump. All patients received paracetamol (acetaminophen) 1 g four times per day throughout the postoperative period, except those in whom there was concern regarding the size or quality of the liver remnant; this decision was left to the discretion of the operating surgeon.
In the postoperative period, patients in both arms of the study followed a standardized enhanced recovery protocol, modified from Hendry et al.17 (Fig. 2). This set targets for the resumption of normal dietary intake, the removal of catheters and lines, and mobilization. In the study institution, pain scores are measured hourly in the high dependency unit (HDU), using a 3-point scale (mild, moderate, severe). For the purposes of this study, a more sensitive numerical rating scale of 0–10 was employed, and for practical reasons pain scores were measured at 2 h, 6 h and 12 h postoperatively, plus once daily at 09.00 hours for the following 6 days. Nausea and sedation scores were also recorded at these time-points. In the initial postoperative period, patients were reviewed by the acute pain service (APS) and their randomized analgesic method optimized. Changes to epidural infusion rate and boluses were made as directed by the APS and were not patient-controlled. A standardized protocol for the management of poorly functioning epidurals was followed. Persistently high pain scores after a defined number of epidural boluses within a specified time period were considered to indicate the failure of the epidural technique and to indicate that a dislodged epidural catheter should be replaced (if it was safe and practical to do so) or that the patient should be converted to i.v. opiate PCA. Epidural-associated hypotension was managed prophylactically with 30 mg oral ephedrine, administered prior to mobilization, and persistent epidural-associated hypotension was managed with a continuous i.v. infusion of vasoactive drugs as required.
Figure 2.

Enhanced recovery care plan followed by both groups following open liver resection
A standardized oral analgesic regimen was commenced on the morning of the second postoperative day in both study arms. This comprised paracetamol 1 g four times per day, ibuprofen 400 mg three times per day (unless contraindicated, in which case tramadol 100 mg four times per day was used), plus oxycodone 10 mg as required for breakthrough pain relief.
Patient mobility was recorded using an activPAL™ physical activity logger (PAL Technologies Ltd, Glasgow, UK).18 This monitor was worn on the right thigh from entry to the recovery room until discharge from hospital, or for the first 7 days, whichever was sooner. This device recorded continuous information on the patient's position such as whether the patient was lying, standing or stepping.
Twice daily, patients were assessed against a set of criteria for discharge initially from the HDU and latterly from hospital (Fig. 3). The criteria for discharge from hospital assessed functional recovery and have been used previously in work published from this institution.17 In addition to five key discharge criteria, willingness to go home was included as a measure of a patient's satisfaction with his or her recovery. Both the length of time required to fulfil discharge criteria and actual length of stay were noted. Complications were recorded and graded using an established classification system.19
Figure 3.

Criteria for discharge from hospital following open liver resection
All data were entered into a database using Microsoft Excel 2004 (Microsoft Corp., Redmond, WA, USA). Data were analysed on an intention-to-treat basis, using spss Version 15.0 (SPSS, Inc., Chicago, IL, USA). Data on time required to fulfil criteria for discharge were normalized by taking the reciprocal, and an independent sample t-test was performed. Pearson's chi-squared and Fisher's exact tests were also used as appropriate. Pain scores were analysed using repeated-measures analysis of variance (anova), controlling for age, gender, body mass index (BMI) and wound length. Data are presented as medians and ranges, unless stated otherwise.
Results
Between August 2009 and July 2010, 116 patients were scheduled to undergo liver resection at the Royal Infirmary of Edinburgh. Of these, 26 patients were excluded and a further 25 declined to participate in the study for the reasons shown in Fig. 4. A total of 65 patients were randomized to wound infiltration plus PCA (WI group, n= 33) or to epidural analgesia (EP group, n= 32). One patient in the EP group experienced a cardiac event after the induction of anaesthesia and did not proceed to surgery; this patient was therefore removed from the analysis.
Figure 4.
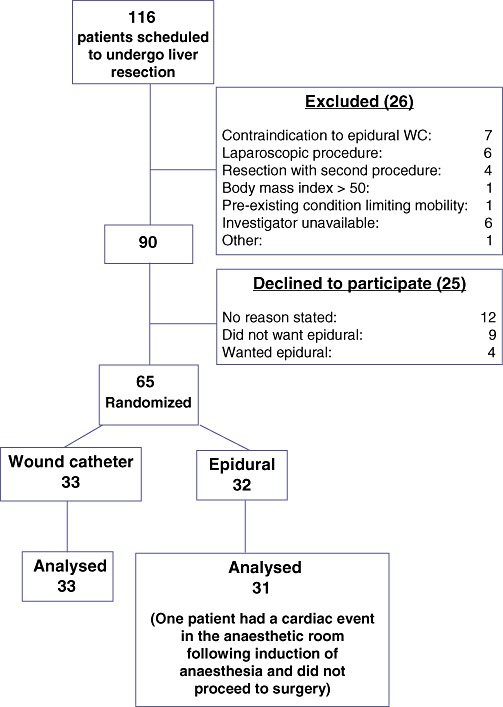
Flow diagram illustrating patient enrolment. WC, wound catheter
Analysis of baseline characteristics demonstrated that the two groups were well matched in most respects, although BMI was significantly greater in the WI group (Table 1). The predominant indication for liver resection was colorectal metastases and, accordingly, approximately 60% of patients in each group had undergone previous laparotomy. One patient in each group was taking regular opiate analgesia for pain prior to liver resection. Analysis of intraoperative details demonstrated that a significantly greater number of major resections (three or more segments) were performed in the WI group; this corresponded with a significantly greater operating time in this group (Table 1). Wound length was also significantly greater in the WI group.
Table 1.
Baseline characteristics and intraoperative details of patients undergoing open liver resection, randomized to either wound catheter plus patient-controlled i.v. opiate analgesia (WI group) or epidural analgesia (EP group)
| WI group | EP group | P-value | |
|---|---|---|---|
| (n= 33) | (n= 31) | ||
| Patient characteristics | |||
| Age, years, median (range) | 60 (39–84) | 60 (23–85) | 0.877 |
| Male gender, n (%) | 17 (51.5) | 19 (61.3) | 0.431 |
| BMI, median (range) | 25 (19–36) | 24 (18–33) | 0.044 |
| ASA physical status, n (%) | |||
| I | 2 (6.1) | 5 (16.1) | 0.107 |
| II | 20 (60.5) | 20 (64.5) | |
| III | 11 (33.3) | 6 (19.4) | |
| Indication for surgery, n (%) | |||
| Colorectal metastases | 19 (57.6) | 17 (54.8) | 0.816 |
| Cholangiocarcinoma | 4 (12.1) | 5 (16.1) | |
| Hepatocellular carcinoma | 2 (6.1) | 3 (9.7) | |
| Other malignancy | 2 (6.1) | 3 (9.7) | |
| Benign disease | 6 (18.2) | 3 (9.7) | |
| Previous laparotomy, n (%) | 19 (57.6) | 18 (58.1) | 0.968 |
| Intraoperative data | |||
| Operating time, min, median (range) | 265 (50–550) | 190 (90–540) | 0.006 |
| Extent of hepatic resection, n (%) | |||
| ≥ 3 segments | 24 (72.7) | 13 (41.9) | 0.042 |
| Section/segment/metastasectomy | 7 (21.2) | 15 (48.4) | |
| Unresectable | 2 (6.1) | 3 (9.7) | |
| Bile duct excision | 9 (27.3) | 8 (25.8) | 0.894 |
| Blood loss, ml, median (range) | 870 (0–5000) | 545 (150–2675) | 0.106 |
| Wound length, cm, median (range) | 32.0 (23.0–53.0) | 28.5 (16.0–35.0) | <0.001 |
BMI, body mass index; ASA, American Society of Anesthesiologists.
The primary outcome was length of time required to fulfil criteria for discharge from hospital. The median length of time was 4.5 days (range: 2.5–63.5 days) in the WI group and 6.0 days (range: 3.0–42.5 days) in the EP group (P= 0.044). Discharge was delayed in one patient in the WI group as a result of abnormal coagulation and one patient in the EP group as a result of thrombocytopenia. After meeting the five key criteria for discharge from hospital, three patients in the EP group and five patients in the WI group were unwilling to go home on that day. However, all these patients were willing to be discharged within the following 24 h. The median length of time required to fulfil criteria for discharge from the HDU was 1.5 days (range: 0–4.5 days) in the WI group and 1.5 days (range: 0.5–4.5 days) in the EP group (P= 0.002).
Pain scores were lower in the EP group both at rest and on movement (Fig. 5). The median pain scores at rest in both groups were scored as mild (0–3 on a numerical rating scale). As expected, total narcotic use was greater in the WI group as opiate PCA was routine in this group but not in the EP group.
Figure 5.
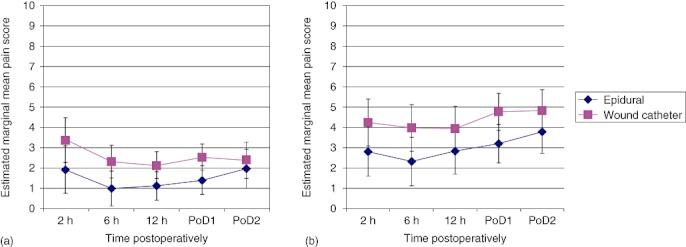
Mean pain scores (a) at rest (P= 0.015) and (b) on movement (P= 0.022) following open liver resection, in patients randomized to either wound catheter patient-controlled i.v. opiate analgesia or epidural analgesia. PoD, postoperative day
There was no significant difference between the groups in the timing of first mobilization. However, the number of steps taken in the first 48 h after surgery was significantly greater in the WI group [WI group: 6 steps (range: 0–560 steps); EP group: 2 steps (range: 0–570 steps); P= 0.040].
One death occurred in each group. There was no significant difference between the groups in overall complication rate or grade of complications (Table 2). There were no instances of epidural haematoma or abscess formation in either group.
Table 2.
Complication rates in patients undergoing open liver resection, randomized to either wound catheter plus patient-controlled i.v. opiate analgesia (WI group) or epidural analgesia (EP group)
| WI group | EP group | P-value | |
|---|---|---|---|
| (n= 33) | (n= 31) | ||
| Overall complication rate, n (%) | 16 (48.5) | 18 (58.1) | 0.443 |
| Highest grade of complication (Clavien–Dindo classification), n (%) | |||
| I | 4 (12.1) | 2 (6.5) | 0.601 |
| II | 8 (24.2) | 10 (32.3) | |
| IIIa | 2 (6.1) | 3 (9.7) | |
| IIIb | 0 | 2 (6.5) | |
| IVa | 1 (3.0) | 0 | |
| IVb | 0 | 0 | |
| V | 1 (3.0) | 1 (3.2) | |
Discussion
This study assessed the influence of postoperative analgesic method on length of time to fulfil criteria for discharge from hospital following open liver resection within an enhanced recovery protocol.
It was hypothesized that increased freedom to mobilize and a reduced requirement for intensive cardiovascular monitoring would allow the WI group to fulfil criteria for discharge from the HDU more quickly and to reach fitness for discharge from hospital in a shorter length of time than the EP group. The length of time required to fulfil criteria for discharge from hospital was indeed significantly shorter in the WI group. This occurred despite a significantly greater number of major resections in this group. A significant difference was also observed between the treatment groups in the time required to fulfil criteria for discharge from the HDU, in favour of the WI group.
In the present institution, patients with epidural analgesia are managed within a level 2 (HDU) environment as there is no facility for level 1 care. This has several implications. In the absence of other factors, invasive cardiovascular monitoring will prevent patients with epidural analgesia moving to a level 0 bed; patients in level 0 beds are more likely to be mobile (e.g. to walk to and from the toilet) than similar patients in level 2 care. Factors influencing mobility will be discussed further. This study confirmed that a proportion of patients are well enough to step down to the general ward environment within the first 24 h following open liver resection. The necessity to manage patients with epidural analgesia in a level 2 environment in the present institution is therefore very likely to account for the difference demonstrated between the groups in length of HDU stay. It may also account for the difference in overall recovery time. Although a formal cost analysis has not been performed, the practice of keeping patients in the HDU environment simply for the delivery of epidural analgesia also has resource and cost implications.
Analysis of pain scores demonstrated consistently better scores in the EP group. Although pain at rest was scored as mild (score range: 0–3) in both groups, it was rated as moderate (score range: 4–6) on movement in the WI group. Improved dynamic pain relief is a recognized advantage of epidural analgesia and intuitively should allow for better mobilization following major surgery. However, in this study, there was no difference between groups in time to first mobilization. The WI group took statistically more steps than the EP group in the first 48 h after surgery, but the clinical significance of this is negligible. These findings are consistent with those of Park et al., who found no difference in postoperative physical activity scores between patients managed with epidural and parenteral opioids, respectively, following major abdominal surgery.3
It is likely that mobility in the early postoperative phase is determined by factors other than adequate analgesia, including the presence of lines and tubes, haemodynamic instability, patient expectations, and cultural issues amongst medical and nursing staff regarding the management of epidural analgesia. Although a standardized care pathway outlined daily mobility targets, it is possible that the single-blind design of this study may have allowed the introduction of bias in terms of mobilization of patients. However, if this is so, it simply highlights the need to find a pragmatic approach to postoperative analgesia that works in the real world outside the environment of the RCT.
The data regarding postoperative mobility are remarkable in that the median number of steps taken within the first 48 h after surgery was less than 10 in both groups. This demonstrates the relative immobility of patients following major abdominal surgery, even those who are managed within an enhanced recovery programme that aims to pre-empt all of the issues limiting mobility discussed herein. Further accurate investigation of postoperative mobility using personal activity monitors is warranted in patients undergoing major abdominal surgery.
There was no significant difference in complications between the groups. The poorer dynamic pain relief associated with wound infiltration plus PCA did not, therefore, equate to delayed or complicated recovery in this study. This study, however, was not powered to look at postoperative complications and thus, although no major differences were seen, results must be interpreted with caution.
The study was of a single-blind design in an effort to reduce bias in the measurement of pain scores. It was neither practical nor safe for medical and nursing staff responsible for patient care to be blinded to randomization. Although every effort was made to maintain single blinding by avoiding discussion of analgesic techniques within earshot of patients, this proved extremely difficult and was not universally successful. Whether this had any influence on the pain scores recorded is unknown and it thus remains a potential source of bias. The present authors would counsel others who hope to conduct a study of this type against attempting this type of blinding.
Nine patients declined to participate in the study because they preferred not to receive epidural analgesia. Some had prior experience of epidural analgesia during previous surgery for primary colorectal malignancy. Others had taken the opportunity to find out more about epidurals in the weeks between being given information about the study and presenting for operation. Ease of access to information via the Internet means that patients are becoming increasingly informed about the risks and benefits of interventions such as epidural insertion. Conversely, four patients declined to participate in the study as they preferred to receive epidural analgesia postoperatively. This demonstrates that patients are increasingly aware of, and expect to have, choices with regard to analgesia.
As might be expected in a study of this size, there were no cases of epidural haematoma or abscess formation. However, these are recognized complications and whether or not patients are at higher risk following liver resection because of potential coagulopathy remains to be determined.
To date, no other studies have compared epidural analgesia with a multimodal analgesic regimen based around continuous local anaesthetic wound infiltration. It is clear that the success of enhanced recovery programmes depends on the combination of many different elements, including optimized analgesia. Each individual element must be investigated while the others are kept constant and it should be recognized that different types of surgical procedure may call for tailored protocols.
Continuous local anaesthetic wound infiltration, as part of a multimodal analgesic regime, offers a clinically acceptable alternative to epidural analgesia following open liver resection. This approach has been shown to reduce the length of time required to fulfil criteria for discharge from hospital. The cost of this reduction in time is poorer pain control within the early postoperative period, but, in the present study, this did not decrease mobility or overall outcome. When epidural is contraindicated, technically impossible or refused by the patient, an acceptable opiate-minimizing alternative has been demonstrated.
Acknowledgments
The authors are grateful for the support of Professor R. W. Parks, J. J. Powell, E. L. Hidalgo, R. Ravindran and M. Duxbury with the recruitment of patients, S. McNally for statistical advice, and the Acute Pain Service, at the Royal Infirmary of Edinburgh. This study was funded by the University of Edinburgh. Local anaesthetic wound catheters were donated by B. Braun Medical Ltd, Sheffield, UK, and physical activity loggers by PAL Technologies Ltd, Glasgow, UK. None of the supporting organizations had any input into the study design, data collection or analysis of results.
This study is registered under ClinicalTrials.gov: NCT01042054.
Conflicts of interest
None declared.
References
- 1.Hudcova J, McNicol ED, Quah CS, Lau J, Carr DB. Patient-controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2006;(4):CD003348. doi: 10.1002/14651858.CD003348.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Rogers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomized trials. BMJ. 2000;321:1493–1497. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park WY, Thompson JS, Lee KK. Effect of epidural anaesthesia and analgesia on perioperative outcome. A randomized, controlled Veterans Affairs Cooperative study. Ann Surg. 2001;234:560–571. doi: 10.1097/00000658-200110000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomized trial. Lancet. 2002;359:1276–1282. doi: 10.1016/S0140-6736(02)08266-1. [DOI] [PubMed] [Google Scholar]
- 5.Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systemic update of the evidence. Anesth Analg. 2007;104:689–702. doi: 10.1213/01.ane.0000255040.71600.41. [DOI] [PubMed] [Google Scholar]
- 6.Burstal R, Wegener F, Hayes C, Lantry G. Epidural analgesia: prospective audit of 1062 patients. Anaesth Intensive Care. 1998;26:165–172. doi: 10.1177/0310057X9802600206. [DOI] [PubMed] [Google Scholar]
- 7.McLeod G, Davies H, Munnoch N, Bannister J, MacRae W. Postoperative pain relief using thoracic epidural analgesia: outstanding success and disappointing failures. Anaesthesia. 2001;56:75–81. doi: 10.1046/j.1365-2044.2001.01763-7.x. [DOI] [PubMed] [Google Scholar]
- 8.Revie EJ, Massie LJ, McNally SJ, McKeown DW, Garden OJ, Wigmore SJ. Effectiveness of epidural analgesia following open liver resection. HPB. 2011;13:206–211. doi: 10.1111/j.1477-2574.2010.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook TM, Counsell D, Wildsmith JAW. Major complications of central neuraxial block: report on the third national audit project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179–190. doi: 10.1093/bja/aen360. [DOI] [PubMed] [Google Scholar]
- 10.Matot I, Scheinin O, Eid A, Jurim O. Epidural anaesthesia and analgesia in liver resection. Anesth Analg. 2002;95:1179–1181. doi: 10.1097/00000539-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg L, Scurrah N, Gunning K, McNicol L. Postoperative changes in prothrombin time following hepatic resection: implications for perioperative analgesia. Anaesth Intensive Care. 2006;34:438–443. doi: 10.1177/0310057X0603400405. [DOI] [PubMed] [Google Scholar]
- 12.Lui SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anaesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203:914–932. doi: 10.1016/j.jamcollsurg.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Forastiere E, Sofra M, Giannarelli D, Fabrizi L, Simone G. Effectiveness of continuous wound infusion of 0.5% ropivacaine by On-Q pain relief system for postoperative pain management after open nephrectomy. Br J Anaesth. 2008;101:841–847. doi: 10.1093/bja/aen309. [DOI] [PubMed] [Google Scholar]
- 14.Beaussier M, El'Ayoubi H, Schiffer E, Rolin M, Parc Y, Mazoit J-M, et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery. Anesthesiology. 2007;107:461–468. doi: 10.1097/01.anes.0000278903.91986.19. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Tamijmarane A, Bulters D, Wells JKG, John TG, Rees M. An alternative method of wound pain control following hepatic resection: a preliminary study. HPB. 2004;6:186–189. doi: 10.1080/13651820410030844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan SK, Lai PB, Li PT, Wong J, Karmakar MK, Lee KF, et al. The analgesic efficacy of continuous wound instillation with ropivacaine after open hepatic surgery. Anaesthesia. 2010;65:1180–1186. doi: 10.1111/j.1365-2044.2010.06530.x. [DOI] [PubMed] [Google Scholar]
- 17.Hendry PO, van Dam RM, Bukkems SFFW, McKeown DW, Parks RW, Preston T, et al. on behalf of the Enhanced Recovery After Surgery (ERAS) Group. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg. 2010;97:1198–1206. doi: 10.1002/bjs.7120. [DOI] [PubMed] [Google Scholar]
- 18.Ryan CG, Grant PM, Tigbe WW, Granat MH. The validity and reliability of a novel activity monitor as a measure of walking. Br J Sports Med. 2006;40:779–784. doi: 10.1136/bjsm.2006.027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]


