Abstract
Background
Orthotopic liver transplantation (LT) in non-alcoholic steatohepatitis (NASH) is increasing in parallel with the obesity epidemic.
Methods
This study retrospectively reviewed the clinical outcomes of LTs in NASH (n= 129) and non-NASH (n= 775) aetiologies carried out at a single centre between 1999 and 2009.
Results
Rates of 1-, 3- and 5-year overall survival in NASH (90%, 88% and 85%, respectively) were comparable with those in non-NASH (92%, 86% and 80%, respectively) patients. Mortality within 4 months of LT was twice as high in NASH as in non-NASH patients (8.5% vs. 4.2%; P= 0.04). Compared with non-NASH patients, post-LT mortality in NASH patients was more commonly caused by infectious (38% vs. 26%; P < 0.05) or cardiac (19% vs. 7%; P < 0.05) aetiologies. Five-year survival was lower in NASH patients with a high-risk phenotype (age >60 years, body mass index >30 kg/m2, with hypertension and diabetes) than in NASH patients without these characteristics (72% vs. 87%; P= 0.02). Subgroup analyses revealed that 5-year overall survival in NASH was equivalent to that in Laennec's cirrhosis (85% vs. 80%; P= 0.87), but lower than that in cirrhosis of cryptogenic aetiology (85% vs. 96%; P= 0.04).
Conclusions
Orthotopic LT in NASH was associated with increased early postoperative mortality, but 1-, 3- and 5-year overall survival rates were equivalent to those in non-NASH patients.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease in the USA,1–3 affecting 3–24% of the general population.4–6 The presence of NAFLD parallels the obesity epidemic and up to 84–96% of morbidly obese patients show histological evidence of NAFLD.7–9 Although most patients with NAFLD have only bland steatosis, a portion of patients will develop progressive disease characterized by steatosis with an associated necroinflammatory component known as non-alcoholic steatohepatitis (NASH).10 The latter has been estimated to affect 5–7% of the population2,11 and is strongly associated with the development of hepatic fibrosis and cirrhosis.10,12,13 A significant portion of patients with NASH-related cirrhosis decompensate and develop end-stage liver disease.14–16 Non-alcoholic steatohepatitis is also clearly associated with the development of hepatocellular carcinoma.16–18
Several studies have defined risk factors associated with the development of advanced fibrosis in the setting of NASH.7,19–22 Important predictive demographics include age >50 years, obesity, type 2 diabetes mellitus, hypertension and non-African American race.7,19–22 Because the US population is ageing and rates of obesity, type 2 diabetes mellitus and hypertension are escalating, the frequency of NASH-related cirrhosis is expected to increase in the future. A recent United Network for Organ Sharing (UNOS)-wide study confirmed that liver transplantation (LT) in NASH has increased from 1.2% prior to 2003 to 7.4% in 2010 and that NASH now represents the fourth most common indication for LT in the USA.23 This large study reported 1-, 3- and 5-year post-transplant overall survival rates in the NASH population equivalent to those in the non-NASH population.23 Although limited by missing data in 25% of NASH patients, this UNOS-wide study suggests that the cause of death following transplantation for NASH was more likely to reflect a cardiovascular aetiology and less likely to show graft failure.23
To date, three large, single-centre studies have provided detailed outcome data on causes of death following LT in NASH.24–26 The Baylor group published a clinico–pathological correlation study of recurrent hepatic steatosis and the development of NASH in scheduled liver biopsies following LT in patients with NASH or cryptogenic cirrhosis (many of whom were presumed to have undiagnosed NASH).26 Steatosis developed in 31% and NASH in 4% of this study's patient population and NASH was found to be a strong risk factor for the development of bridging fibrosis or cirrhosis.26 However, recurrent NASH-induced liver failure was a very rare cause of death.26 The Pittsburgh group reported on a cohort of 98 NASH patients with a 1-year mortality rate of 21.4% following LT.25 This group further defined a high-risk NASH phenotype [age >60 years, body mass index (BMI) >30 kg/m2, with diabetes and hypertension] in which 1-year mortality reached 50%.25 The final study, by the Miami group, reported on a cohort of 71 NASH patients and documented no statistical difference in post-transplant survival between patients with NASH and those with alcoholic liver disease.24 The detailed survival data published by the Pittsburgh and Miami groups highlighted the significant increase in the cardiac and sepsis aetiologies of death following LT for NASH.24,25
Together, these four important studies23–26 document that: (i) survival following transplantation in NASH is at least comparable with that in non-NASH aetiologies; (ii) recurrent NASH-induced liver failure is uncommon; (iii) the cause of death following transplantation for NASH commonly refers to cardiac and sepsis-related aetiologies, and (iv) certain patients with high-risk NASH phenotype characteristics may have very poor short-term survival. The purpose of this study is to measure survival outcomes after LT in NASH patients at the University of Alabama at Birmingham (UAB), a programme with a referral population that is among the most obese in the country and has a high prevalence of NASH-related cirrhosis.27 These data may inform future NASH transplant candidate evaluation and selection processes.
Materials and methods
Ethical approval for this study was obtained from the University of Alabama Institutional Review Board (Protocol #X100310006).
A retrospective chart review was performed for all patients aged >18 years who received a deceased donor LT at UAB between 1999 and 2009. Patients were identified from an internal transplant database that is used for clinical purposes. Information is entered into this database prospectively and this is the primary source of data for required UNOS reporting.
Defining NASH patients
Patients were diagnosed with NASH if they did not have other forms of liver disease and had a pre-transplant biopsy consistent with NASH or had pre-cirrhotic imaging demonstrating hepatic steatosis or met criteria for the NASH phenotype. Patients with a diagnosis of NASH or cryptogenic cirrhosis were included only if they fulfilled the criteria published in recent NASH transplant outcomes studies by Malik et al.25 and Bhagat et al.24 Briefly, NASH patients had: (i) no history of alcohol ingestion; (ii) negative serological studies for hepatitis B and hepatitis C; (iii) no laboratory or histological evidence of autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, haemochromatosis, alpha-1-antitripsin disease or Wilson's disease, and (iv) had a pre-transplant or pre-cirrhotic biopsy that demonstrated NASH or met criteria for the NASH phenotype defined as a BMI of >30 kg/m2 and concurrent treatment for type 2 diabetes mellitus. Waist circumference was not routinely measured and was not included in the definition of the NASH phenotype.
Candidate evaluation process
There were no maximum BMI criteria utilized during this study. Patients were required to have been ambulatory within the past month. All candidates were seen by a transplant surgeon, transplant hepatologist, social worker and dietician. Neuropsychiatric evaluations were performed selectively in candidates with a history of substance abuse or cognitive dysfunction. Non-intubated patients completed pulmonary function testing. Pre-transplant cardiac screening was identical in NASH and non-NASH candidates. All patients were given an electrocardiogram, trans-thoracic echocardiogram and a technetium sestamibi cardiac stress test (MIBI). A right heart catheterization was obtained for an estimated pulmonary artery pressure of >25 mmHg on the echocardiogram. A left heart catheterization was obtained in all patients with active angina, a positive MIBI test or a documented history of coronary artery disease and no angiographic interrogation in >2 years. Cardiology consultation was requested selectively for arrhythmias or for abnormalities appreciated on the echocardiogram or heart catheterizations. All patients were reviewed at a multidisciplinary liver disease conference to determine candidacy for transplantation.
Immunosuppression protocol
A post-transplant immunosuppression protocol was applied uniformly in NASH and non-NASH patients (except in recipients with hepatitis C; see below). Transplant recipients were universally treated with steroids, antimetabolites and calcineurin inhibitors, and selectively treated with interleukin-2 (IL-2) receptor inhibitors and mTOR inhibitors. Steroids were administered in a 1-g solumedrol bolus after recipient hepatectomy and a rapid i.v. solumedrol taper over 5 days, after which oral prednisone 20 mg was initiated and tapered off over 3 months. Antimetabolites were administered as mycophenylate mofetil 1 g b.i.d. initiated 12 h postoperatively, initially i.v. and subsequently orally. The dose was decreased in the event of side-effects and substituted with azathioprine if appropriate. Calcineurin inhibitors consisted of tacrolimus (titrated to a trough of 5–10 ng/ml) used preferentially and substituted with cyclosporine in the event of side-effects. Perioperative IL-2 receptor inhibitors were used selectively by the transplanting surgeon, preferentially for renal insufficiency and for steroid avoidance in recipients with hepatitis C; mTOR inhibitors were utilized infrequently, primarily for neurologic complications or for stage IV chronic renal insufficiency. No specific renal sparing protocols were employed peri-transplant. Acute rejection was treated with i.v. steroids or rarely with anti-lymphocyte regimens for steroid-resistant episodes. Rejection in recipients with hepatitis C was preferentially treated initially by escalating doses of calcineurin inhibitors and antimetabolites.
Data analysed
Recipient demographics, perioperative details and post-transplant data were collected. Recipient variables included: age; gender; ethnicity; BMI; specific comorbidities (diabetes mellitus, hypertension, hypertriglyceridaemia, hypercholesterolaemia, hypertension, renal insufficiency); Model for End-stage Liver Disease (MELD) score, and specific laboratory variables [bilirubin, international normalized ratio (INR), creatinine, albumin]. Perioperative variables included: recipient medical condition [home, hospitalized, intensive care unit (ICU)]; immediate pre-transplant dialysis; donor allograft cold ischaemia time and warm ischaemia time; blood products administered (red blood cells, fresh frozen plasma and platelets), and length of operation. Post-transplant variables included length of ICU stay and total post-transplant hospital length of stay.
Statistical analysis
Outcomes were compared between the NASH cohort and all patients who did not fulfil NASH criteria (referred to as the ‘non-NASH’ cohort). Because NASH-related cirrhosis/liver failure is clinically similar to Laennec's cirrhosis and cryptogenic cirrhosis, subgroup survival analyses also compared outcomes between patients with NASH and those with Laennec's cirrhosis, and between patients with NASH and those with cryptogenic cirrhosis.
The primary outcome measures were post-transplant survival and cause of death. Examination of the data began by examining measures of central tendency (sample mean and median), as well as measures of dispersion [variance, standard deviation (SD)]. The primary analytic approaches for dichotomous variables utilized Pearson chi-squared and Fisher exact test analyses. Data were expressed as the mean ± SD when normally distributed and as the median (including minimum to maximum values) when not normally distributed. The Student t-test was used to compare means between cohorts. The Wilcoxon rank-sum test was used to compare median values between cohorts. Kaplan–Meier curves were constructed to evaluate patient survival. Survival probabilities were analysed with the log-rank test. For all inferences, the probability of a type I error (α) was set to 0.05. All analyses were conducted using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline demographics
Patients with NASH represented 14% (129/904) of patients transplanted at UAB between 1999 and 2009 (Fig. 1). Recipients with NASH were older at the time of transplantation, were less likely to be male, were more likely to be White, and less likely to be African American compared with non-NASH LT recipients. The median laboratory MELD score at the time of transplant was higher in NASH patients than in non-NASH patients (23 vs. 21; P= 0.03) (Table 1).
Figure 1.
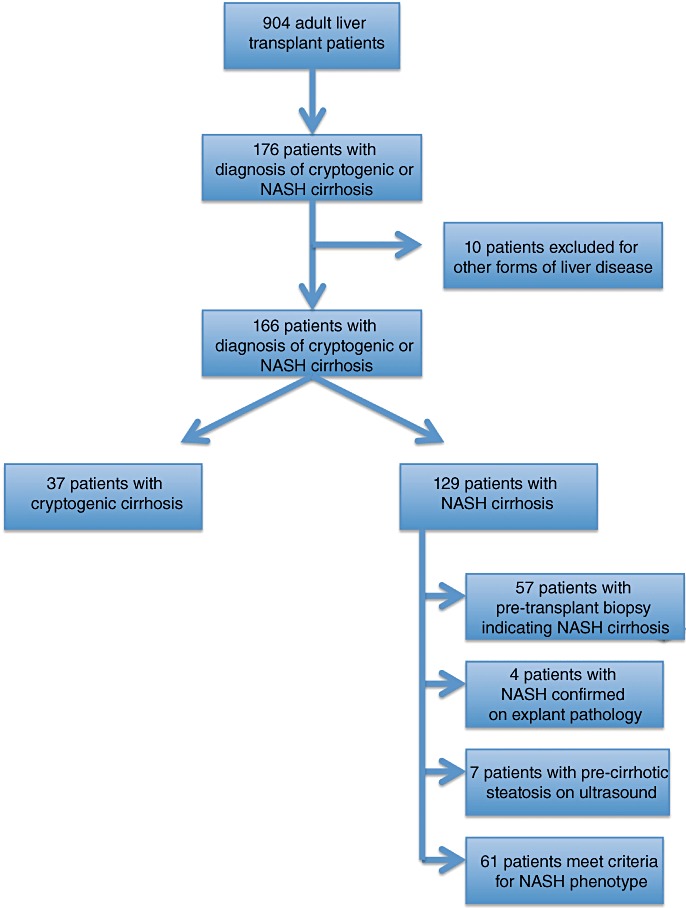
Flow diagram of 904 adult liver transplant patients demonstrating the criteria used to select patients with non-alcoholic steatohepatitis (NASH) cirrhosis
Table 1.
Baseline characteristics at transplant of non-alcoholic steatohepatitis (NASH) and non-NASH patients
| Variable | NASH patients (n= 129) | Non-NASH patients (n= 775) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 57 ± 9 | 48 ± 17 | <0.001 |
| Male gender, n (%) | 61 (47%) | 483 (62%) | 0.04 |
| White, n (%) | 119 (92%) | 635 (82%) | 0.05 |
| African American, n (%) | 8 (6%) | 108 (14%) | 0.01 |
| BMI, kg/m2, mean ± SD | 34 ± 7 | 28 ± 6 | <0.001 |
| MELD score, median (range) | 23 (6–40) | 21 (6–40) | 0.03 |
SD, standard deviation; BMI, body mass index; MELD, Model for End-stage Liver Disease.
All comorbidities associated with metabolic syndrome were significantly higher in the NASH cohort than in the non-NASH cohort (Table 2). Hypertension (75% vs. 41%; P < 0.001), diabetes mellitus (59% vs. 17%; P < 0.001) and hypercholesterolaemia (22% vs. 12%; P= 0.003) were substantially more prevalent in the NASH cohort. Correspondingly, obesity (defined as a BMI >30 kg/m2) was present in 68% of NASH patients compared with 28% of non-NASH patients (P < 0.001). Non-hepatic end-organ damage was more prevalent in NASH patients compared with non-NASH patients. Chronic renal insufficiency (16% vs. 5%; P < 0.001), coronary artery disease (6% vs. 3%; P= 0.04) and cerebrovascular disease (5% vs. 1%; P= 0.01) were each significantly more common in the NASH cohort (Table 2).
Table 2.
Comorbidities in non-alcoholic steatohepatitis (NASH) and non-NASH patients
| Variable | NASH patients (n= 129) | Non-NASH patients (n= 775) | P-value |
|---|---|---|---|
| Hypertensiona, n (%) | 97 (75%) | 315 (41%) | <0.001 |
| Diabetesa, n (%) | 76 (59%) | 135 (17%) | <0.001 |
| Obesity (BMI >30 kg/m2), n (%) | 88 (68%) | 211 (28%) | <0.001 |
| Hypercholesterolaemiaa, n (%) | 29 (22%) | 98 (12%) | 0.003 |
| Chronic renal insufficiencyb, n (%) | 21 (16%) | 41 (5%) | <0.001 |
| Coronary artery diseaseb, n (%) | 8 (6%) | 21 (3%) | 0.04 |
| Cerebrovascular diseaseb, n (%) | 6 (5%) | 11 (1%) | 0.01 |
Patients were included only if they were using a hypertensive medication (excluding Nadolol) or a diabetes medication including insulin or an oral hypoglycaemic, or a cholesterol-lowering agent at the time of listing for liver transplant.
Patients were included based upon medical history at the time of listing for liver transplant. Patients with acute or diuretic-induced renal dysfunction were not included.
BMI, body mass index.
Perioperative characteristics
The mean donor age was significantly increased in the NASH cohort compared with the non-NASH cohort (42 ± 18 years vs. 36 ± 18 years; P < 0.001). Cold ischaemia time, warm ischaemia time and operative length were similar in the NASH and non-NASH cohorts. Transfusions of packed red blood cells and fresh frozen plasma were nearly identical in quantity between cohorts, although significantly fewer units of platelets were given to NASH patients than to non-NASH patients (1.3 ± 2.0 units vs. 1.9 ± 3.9 units; P= 0.01) (Table 3).
Table 3.
Hospitalization and operative characteristics in non-alcoholic steatohepatitis (NASH) and non-NASH patients
| Variable | NASH patients (n= 129) | Non-NASH patients (n= 775) | P-value |
|---|---|---|---|
| Donor age, years, mean ± SD | 42 ± 18 | 36 ± 18 | 0.001 |
| Cold ischaemia time, min, mean ± SD | 401 ± 163 | 373 ± 158 | 0.07 |
| Warm ischaemia time, min, mean ± SD | 47 ± 13 | 45 ± 12 | 0.08 |
| Operative time, min, mean ± SD | 252 ± 121 | 251 ± 121 | 0.94 |
| Packed red blood cells, units, mean ± SD | 3.8 ± 2.9 | 3.6 ± 3.7 | 0.58 |
| Fresh frozen plasma, units, mean ± SD | 1.4 ± 1.9 | 1.4 ± 1.6 | 0.71 |
| Platelets, units, mean ± SD | 1.3 ± 2.0 | 1.9 ± 3.9 | 0.01 |
| Postoperative ICU stay, days, mean ± SD | 2.0 ± 2.0 | 2.0 ± 2.0 | 0.25 |
| Total length of stay, days, mean ± SD | 11 ± 10 | 11 ± 10 | 0.17 |
SD, standard deviation; ICU, intensive care unit.
There were no differences in median ICU stay (2.0 days vs. 2.0 days; P= 0.25) or median overall hospitalization (11 days vs. 11 days; P= 0.17) between the NASH and non-NASH cohorts (Table 3).
Retransplantation
Two of 129 (2%) patients in the NASH cohort underwent retransplantation (one for primary non-function at 3 days and one for hepatic artery thrombosis at 16 days), as did 16 of 775 (2%) patients in the non-NASH cohort.
Survival
Mortality amounted to 16% (n= 21/129) in the NASH cohort and 22% (n= 167/775) in the non-NASH cohort. The most common aetiology of death in each cohort was infection. The aetiology of death differed significantly between the NASH and non-NASH cohorts (Table 4). To minimize the risk for type I error, only three categories of aetiology of death were analysed: infection; cardiac events, and all others. Infection-related (38% vs. 26%; P= 0.05) and cardiac (19% vs. 7%; P= 0.05) causes of death were more common in the NASH than the non-NASH cohort. Detailed information on the 21 deaths in the NASH cohort is provided in Table 5.
Table 4.
Causes of death in non-alcoholic steatohepatitis (NASH) and non-NASH patients
| Variable | NASH patients (n= 129) | Non-NASH patients (n= 775) | P-value |
|---|---|---|---|
| Total deaths, n (%) | 21 (16%) | 167 (22%) | 0.16 |
| Aetiology of deatha, n (%) | 0.05 | ||
| Infection | 8 (38%) | 43 (26%) | |
| Cardiac event | 4 (19%) | 12 (7%) | |
| Malignant (non-HCC) | 2 (10%) | 20 (12%) | |
| Recurrent HCC | 2 (10%) | 12 (7%) | |
| Pulmonary | 0 | 1 (1%) | |
| Recurrent hepatitis C | 0 | 19 (11%) | |
| Chronic rejection/graft failure | 0 | 14 (8%) | |
| Neurologic cause | 1 (5%) | 12 (7%) | |
| Renal failure | 1 (5%) | 8 (5%) | |
| Postoperative complications | 1 (5%) | 8 (5%) | |
| Other | 2 (10%) | 2 (1%) | |
| Unknown | 0 | 16 (10%) | |
Only three aetiology of death categories were analysed to reduce the risk for type I error: infection; cardiac event, and all other causes.
HCC, hepatocellular carcinoma.
Table 5.
Causes of death in non-alcoholic steatohepatitis patients
| Time post-transplant | Patient agea, ethnicity, gender | ||
|---|---|---|---|
| Infections | |||
| 1 | Meningitis | 4 months | 53 years, White, female |
| 2 | Pulmonary sepsis | 44 months | 59, White, male |
| 3 | Bacteraemia, MSOF | 1 month | 61, White, female |
| 4 | Pulmonary sepsis, ARF | 7 months | 68, White, female |
| 5 | Pulmonary sepsis | 3 months | 69, White, female |
| 6 | Fungaemia, MSOF | 16 days | 46, White, male |
| 7 | Bacteraemia, MSOF | 3 months | 51, White, male |
| 8 | Liver abscesses | 5 months | 67, White, male |
| Cardiac events | |||
| 9 | Sudden death | 1 month | 63, White, female |
| 10 | Acute myocardial infarction | Intraoperative death | 65, White, female |
| 11 | Sudden death | 71 months | 50, White, male |
| 12 | Sudden death | 2 months | 57, White, male |
| Neurologic cause | |||
| 13 | CVA | 10 months | 70, White, female |
| Malignancy | |||
| 14 | Lymphoma | 3 months | 47, White, female |
| 15 | Recurrent HCC | 34 months | 66, White, male |
| 16 | Recurrent HCC | 16 months | 67, White, male |
| 17 | Merkel cell tumour | 105 months | 60, White, female |
| Other | |||
| 18 | Postoperative complications, perforated atrium | 3 months | 61, White, female |
| 19 | Chronic renal failure | 68 months | 60, White, female |
| 20 | Massive gastrointestinal bleed | 42 months | 66, White, male |
| 21 | Massive gastrointestinal bleed | 2 months | 68, White, male |
Patient age in years at the time of transplantation.
ARF, acute renal failure; CVA, cerebrovascular disease; HCC, hepatocellular carcinoma; MSOF, multisystem organ failure.
Although the number of early post-transplant deaths caused by infectious or cardiac aetiologies was too limited to perform statistical analysis, some qualitative observations can be made. Infection-related death occurred within 4 months in five of eight (63%) patients. The average age of these patients was 58 years and was thus almost identical to the average age of the entire NASH cohort (57 years). By contrast, mean BMI was 38 kg/m2 in the NASH patients who suffered infection-related death compared with 34 kg/m2 in the entire NASH cohort. Furthermore, three of five (60%) NASH patients who suffered early post-transplant infection-related death were hospitalized and not ambulatory at the time of transplantation, whereas only 21% of the entire NASH cohort were hospitalized. Three of the four (75%) patients who suffered cardiac death died within 4 months of LT. One patient died intraoperatively of cardiac arrest; this patient had a normal echocardiogram and stress test, but had a history of coronary artery disease and had undergone placement of coronary stents 2 years prior to transplantation. No repeat arteriogram was performed prior to listing for transplant. The second patient died 1 month post-transplant, prior to discharge from hospital. This patient had been worked up and transplanted from the ICU. A preoperative echocardiogram revealed a normal ejection fraction and no wall motion abnormalities. No stress test had been performed because the patient was in the ICU and had no history of coronary artery disease. The final patient died 2 months postoperatively after discharge from hospital. This patient had no history of coronary artery disease and a normal echocardiogram. In addition, the MIBI was technically normal although the ejection fraction was mildly reduced on the MIBI (but normal on the echocardiogram). No invasive cardiac testing was performed.
Mean follow-up was 55 months in the NASH cohort and 64 months in the non-NASH cohort. There was no statistical difference in 1-, 3- and 5-year overall survival between the NASH (90%, 88% and 85%, respectively) and non-NASH (92%, 86% and 80%, respectively) cohorts. However, the NASH cohort suffered more early postoperative deaths, after which the survival curves crossed and were relatively equal (Fig. 2). A cross-sectional analysis of deaths at 4 months postoperatively revealed a higher incidence in the NASH cohort compared with the non-NASH cohort (8.5% vs. 4.2%; P= 0.04).
Figure 2.
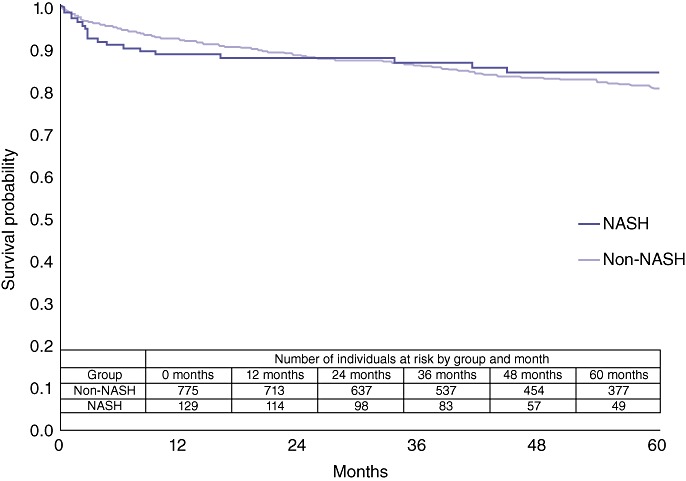
Comparison of overall survival in non-alcoholic steatohepatitis (NASH) and non-NASH liver transplant patients (P= 0.6518)
Subgroup survival analyses were performed between patients with, respectively, NASH and Laennec's cirrhosis, and between patients with, respectively, NASH and cryptogenic cirrhosis. There was no statistical difference in overall survival between the NASH and the Laennec's cirrhosis cohorts (P= 0.87) (Fig. 3). By contrast, patients with cryptogenic cirrhosis had superior post-transplant survival compared with the NASH cohort (P= 0.04) (Fig. 4).
Figure 3.
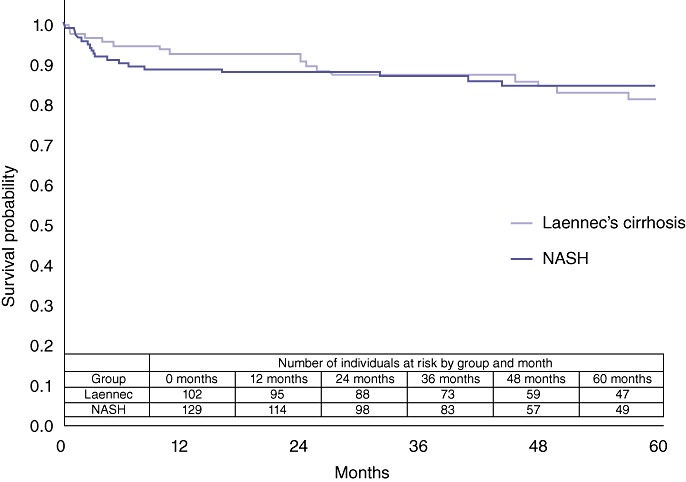
Comparison of overall survival in non-alcoholic steatohepatitis (NASH) and Laennec's cirrhosis liver transplant patients (P= 0.8655)
Figure 4.
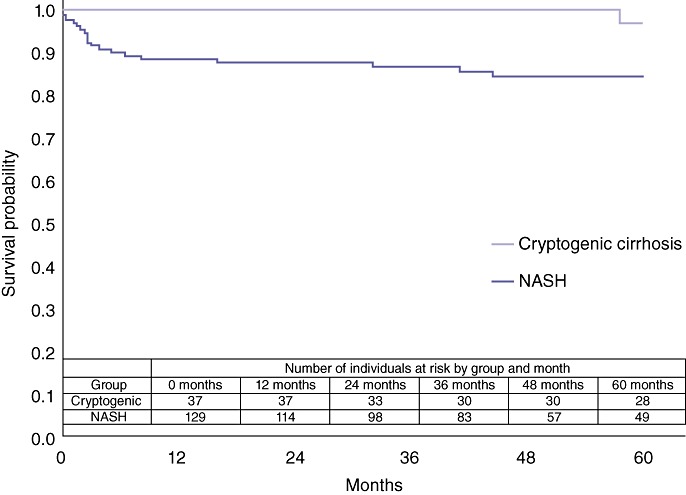
Comparison of overall survival in non-alcoholic steatohepatitis (NASH) and cryptogenic cirrhosis liver transplant patients (P= 0.0393)
The University of Pittsburgh LT group reported a ‘high-risk’ NASH cohort in which survival was much worse than in NASH patients without high-risk characteristics.25 The high-risk cohort was defined by age >60 years, BMI >30 kg/m2, and the presence of both diabetes and hypertension.25Figure 5 compares survival in the 20 NASH patients with the high-risk phenotype and the 109 NASH patients without the high-risk phenotype. Survival was worse in the high-risk NASH cohort (P= 0.02), which included three (15%) patients who died within 30 days of transplant.
Figure 5.
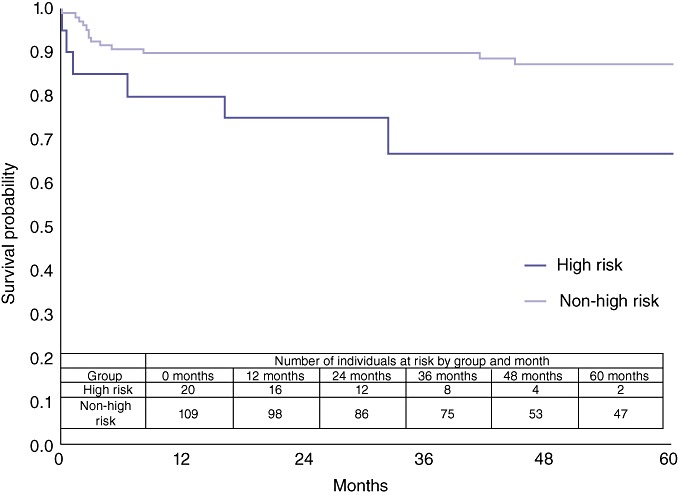
Comparison of overall survival in ‘high-risk’ non-alcoholic steatohepatitis (NASH) and non-high-risk NASH liver transplant patients (P= 0.0191)
Multivariable analysis of risk factors predicting death in patients who survived beyond 4 months post-transplant
In order to examine whether the distribution of survival times differed by disease status after adjusting for baseline characteristics, Cox proportional models were developed. The results are not reported because the key assumption of proportionality of hazard ratios between the NASH and non-NASH groups was violated. Although the model assumption was violated, differences between the survival distributions did not approach significance.
Discussion
This case series demonstrates equivalent 1-, 3- and 5-year survival in NASH and non-NASH patients following LT. However, very early postoperative deaths (at <4 months following transplantation) were more common in patients with NASH (8.5% vs. 4.2%). Although the number of deaths within 4 months post-transplant in the NASH cohort was small (11 of 129 patients), detailed examination of the five infection-related deaths that occurred at <4 months postoperatively reveals that more of the patients who died had been hospitalized prior to LT (60% vs. 21%) and had a higher BMI (38 kg/m2 vs. 34 kg/m2). Most of the hospitalized patients were clinically observed to be unable to mobilize in the early postoperative period. These early post-transplant, infection-related deaths may have been avoided with aggressive postoperative pulmonary hygiene and mobilization, but this can be very challenging in bed-bound, morbidly obese patients. Clinical practice at this centre has thus evolved to offer LT for morbidly obese patients only if they are ambulatory. Findings in a detailed examination of the three cardiac deaths that occurred within 4 months post-transplant strongly argue for more invasive cardiac testing in any NASH candidate with a history of coronary artery disease or anything but a perfectly normal echocardiogram and stress test.
Another explanation for the increased number of deaths observed in the NASH cohort at 4 months postoperatively concerns the increased risk factors in this group compared with the non-NASH cohort. The NASH patients were older (57 years vs. 48 years), more likely to be obese (68% vs. 28%), had a higher laboratory MELD score (23 vs. 21) and their organs came from older donors (42 years vs. 36 years). These risk factors may impact perioperative mortality.27,28 After the first 4 months post-LT, however, no differences in survival were observed between NASH and non-NASH patients. The decreased survival after LT for NASH thus appears to be maximal in the early postoperative period, as suggested by other case series.24,25 The excellent longterm survival is surprising given the prevalences of obesity, diabetes, hypertension and renal insufficiency, all of which are risk factors associated with decreased patient survival in recent Organ Procurement and Transplant Network (OPTN) annual reports.27
Subgroup comparisons between patients with, respectively, NASH and Laennec's liver disease revealed equivalent survival probabilities. However, patients with cryptogenic cirrhosis achieved a remarkable 5-year survival of 96%, which was significantly higher than the 85% survival observed in the NASH cohort. Separating patients with the NASH phenotype from the cryptogenic cirrhosis cohort left a group of patients with outstanding post-transplant survival, which suggests that the comorbidity of metabolic syndrome adversely impacts transplant outcomes. Of issue is an escalating recognition that many cryptogenic cirrhosis patients are likely to have NASH-related cirrhosis.29 It is difficult to even generate a clinically useful difference in the definitions of cryptogenic and NASH-related cirrhosis. Large outcome studies of LT in cryptogenic cirrhosis highlight the difficulties in making a distinction between cryptogenic and NASH cirrhosis when a patient's' first presentation of liver disease is decompensated cirrhosis.26
The University of Pittsburgh group25 reported 1-year mortality of 50% following LT in a cohort of 16 NASH patients with a ‘high-risk phenotype’ defined as age >60 years, BMI >30 kg/m2 and the presence of diabetes and hypertension. Cardiac events occurring within 24 h of LT were responsible for four of eight cardiac deaths. This study confirmed that survival outcomes in NASH patients who fulfil criteria for the high-risk phenotype were significantly worse than in the non-high-risk NASH cohort. Three of 20 post-transplant deaths in the high-risk NASH cohort occurred within the first 30 days. Survival at 30 days in the high-risk NASH cohort was 85%, which is comparable with the 5-year survival of 87% in the non-high-risk NASH cohort. It is important to note, however, that 5-year survival in the high-risk cohort was 72%, which is comparable with the 74% reported for 5-year post-transplant survival in UNOS-wide reports.27 It would be interesting and clinically useful to establish whether the high-risk phenotype is a function of NASH or of the age and comorbidity profile (BMI >30 kg/m2, diabetes and hypertension) as many transplant candidates with liver failure of non-NASH aetiology have these ‘high-risk’ characteristics. Unfortunately, the present data were too limited to perform such an analysis. Furthermore, many of the non-NASH cohort may in fact have NASH as an aetiology of liver disease (i.e. hepatitis C + NASH or alcohol + NASH). This institution's programme has increasingly postulated that NASH is probably a frequent cofactor in the development of end-stage liver disease, analogous to the hepatitis C + alcohol example.
Total operative time, warm ischaemia time and units of blood transfused did not significantly differ between NASH and non-NASH LT recipients in this series. The Pittsburgh group reported statistically shorter total operative time and warm ischaemia time in its NASH cohort compared with contemporaneous controls.25 The Miami transplant cohort reported equivalent retransplantation rates in NASH and Laennec's cirrhosis LT patients.24 Collectively, these data suggest that surgeons perform the technical aspects of LT in NASH patients at least as well as in non-NASH patients. Adverse outcomes observed in NASH patients are therefore likely to reflect intrinsic differences in NASH patients, manifestations of patient comorbidities and/or differences in perioperative care.
As with all retrospective, single-centre, database studies, this investigation has limitations. Firstly, the identification of patients with NASH resulted in the inclusion of all patients who did not have other forms of liver disease and had a pre-transplant biopsy consistent with NASH or pre-cirrhotic imaging demonstrating steatosis or a diagnosis of cryptogenic cirrhosis that fulfilled the NASH phenotype. Patients were included only if they fulfilled the criteria published in recent studies of transplant outcomes in NASH by Malik et al.25 and Bhagat et al.,24 which provide definitions of NASH that are supported by editorials in the transplant literature.29 Lipid panels and waist circumference were not included in the definition of NASH phenotype. The definition of the NASH cohort, however, is likely to underestimate the total NASH population transplanted at UAB because patients with any suggestion of another liver disease were excluded. Secondly, the overall number of mortality events was low, which significantly raises the possibility of a type II error. Furthermore, the identification of pre-transplant risk factors that may specifically predict a cardiac or sepsis-related cause of death was limited by the low number of events. In addition, risk factor analysis for overall survival could not be performed because the key assumption of proportionality of hazard ratios between the NASH and non-NASH groups was violated. Despite these shortcomings, this report represents one of the largest single-centre series of LT outcomes in NASH to date.
In conclusion, this study found that survival outcomes were equivalent between patients with and without NASH-related cirrhosis, except that early post-transplant deaths were increased in NASH patients and were largely infection-related or cardiac in nature. Patients with NASH who demonstrated the University of Pittsburgh high-risk phenotype had significantly worse survival outcomes. The technical measures of the LT operation were similar between NASH and non-NASH patients, which suggests that pre-existing patient comorbidities and/or differences in perioperative care accounted for the increased early postoperative mortality observed in NASH patients. Further research examining which risk factors place NASH and non-NASH patients at greatest risk for early post-transplant death should be examined to determine if these risk factors have the same impact in both groups.
Acknowledgments
The authors give special thanks to Amy Spear mph, Birmingham Transplant Outcomes Office, University of Alabama, for assistance in data retrieval.
This research was supported in part by a National Institutes of Health Short-Term Training Grant (T35 HL007473) awarded as a Summer Research Fellowship to CK.
Conflicts of interest
None declared.
References
- 1.Angulo P. Non-alcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Clark JM. The epidemiology of non-alcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl. 1):5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 4.Clark JM, Brancati FL, Diehl AM. Non-alcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM, Brancati FL, Diehl AM. The prevalence and aetiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark JM, Diehl AM. Non-alcoholic fatty liver disease: an under-recognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, Bhathal PS, O'Brien PE. Non-alcoholic fatty liver disease: predictors of non-alcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 8.Gholam PM, Kotler DP, Flancbaum LJ. Liver pathology in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg. 2002;12:49–51. doi: 10.1381/096089202321144577. [DOI] [PubMed] [Google Scholar]
- 9.Beymer C, Kowdley KV, Larson A, Edmonson P, Dellinger EP, Flum DR. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Arch Surg. 2003;138:1240–1244. doi: 10.1001/archsurg.138.11.1240. [DOI] [PubMed] [Google Scholar]
- 10.Liou I, Kowdley KV. Natural history of non-alcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl. 1):11–16. doi: 10.1097/01.mcg.0000168644.23697.31. [DOI] [PubMed] [Google Scholar]
- 11.McCullough A. The epidemiology and risk factors of NASH. In: Farrell G, Hall P, George J, McCullough A, editors. Fatty Liver Disease: NASH and Related Disorders. Oxford: Blackwell Publishing; 2005. pp. 23–37. [Google Scholar]
- 12.Harrison SA, Torgerson S, Hayashi PH. The natural history of non-alcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 13.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of non-alcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 14.Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, et al. Longterm outcomes of cirrhosis in non-alcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420–427. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- 15.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of non-alcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 17.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 18.Starley BQ, Calcagno CJ, Harrison SA. Non-alcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 19.Solga SF, Clark JM, Alkhuraishi AR, Torbenson M, Tabesh A, Schweitzer M, et al. Race and comorbid factors predict non-alcoholic fatty liver disease histopathology in severely obese patients. Surg Obes Relat Dis. 2005;1:6–11. doi: 10.1016/j.soard.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Laine F, Bendavid C, Moirand R, Tessier S, Perrin M, Guillygomarc'h A, et al. Prediction of liver fibrosis in patients with features of the metabolic syndrome regardless of alcohol consumption. Hepatology. 2004;39:1639–1646. doi: 10.1002/hep.20219. [DOI] [PubMed] [Google Scholar]
- 21.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with non-alcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 22.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 23.Afzali A, Berry K, Ioannou GN. Excellent post-transplant survival for patients with non-alcoholic steatohepatitis in the United States. Liver Transpl. 2012;18:29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- 24.Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to non-alcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 25.Malik SM, de Vera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782–793. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 26.Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Non-alcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or non-alcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov/. [Accessed 30 March 2011.]
- 28.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK longterm follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newsome PN. Recurrence of non-alcoholic fatty liver disease after liver transplantation: it is common, but does it affect outcome? Liver Transpl. 2010;16:420–422. doi: 10.1002/lt.22038. [DOI] [PubMed] [Google Scholar]


