Abstract
Objective:
The effect of NIDCAP (Newborn Individualized Developmental Care and Assessment Program) was examined on the neurobehavioral, electrophysiological and neurostructural development of preterm infants with severe intrauterine growth restriction (IUGR).
Study Design:
A total of 30 infants, 27–33 weeks gestation, were randomized to control (C; N=17) or NIDCAP/experimental (E; N=13) care. Baseline health and demographics were assessed at intake; electroencephalography (EEG) and magnetic resonance imaging (MRI) at 35 and 42 weeks postmenstrual age; and health, growth and neurobehavior at 42 weeks and 9 months corrected age (9 months).
Results:
C and E infants were comparable in health and demographics at baseline. At follow-up, E infants were healthier, showed significantly improved brain development and better neurobehavior. Neurobehavior, EEG and MRI discriminated between C and E infants. Neurobehavior at 42 weeks correlated with EEG and MRI at 42 weeks and neurobehavior at 9 months.
Conclusion:
NIDCAP significantly improved IUGR preterm infants' neurobehavior, electrophysiology and brain structure. Longer-term outcome assessment and larger samples are recommended.
Keywords: NIDCAP, intrauterine growth restriction, preterm infants, APIB, EEG, MRI
Introduction
Experience in the newborn intensive care unit (NICU) alters development.1, 2 Of the 4.3 million annual births in the US, 12.7% are premature;3 of these, 30% were intrauterine growth restricted (IUGR).4 IUGR preterms show increased mortality and morbidity, along with brain changes and developmental disabilities (>50%).4, 5 The Newborn Individualized Developmental Care and Assessment Program (NIDCAP)6 reduces neurodevelopmental NICU sequelae, which was demonstrated in randomized controlled trials.2, 7 This study hypothesized that severely IUGR preterms (<5% in weight and head circumference for gestational age (GA)) receiving NIDCAP (experimental, E) show better neurobehavior, electrophysiology and brain structure than those receiving standard care (control, C).
Methods
Design
The longitudinal, two-group randomized controlled trial (blocked by gender and ethnicity) was approved by the study settings' Institutional Review Boards. Informed consent was obtained within 3 days postpartum using blinded randomization by pre-numbered, sealed envelope method. All study personnel, except experimental interveners, were blinded to group assignment.
Subjects
A total of 30 inborn, severe IUGR preterms were enrolled consecutively after admission to the 48-bed level-III NICU. Mothers' criteria included the following: Greater-Boston resident, ⩾14 years of age, no major medical/psychiatric illness or substance abuse, telephone access and speak English. Infant criteria: GA (fetal ultrasounds and/or GA assessment)8 26 4/7 to 33 3/7 weeks; IUGR diagnosis—umbilical artery Doppler-flow (reversed, decreased, or absent), and/or oligohydramnios/hypertension prompting delivery, birthweight and head-circumference/GA <5th %9 singleton or twin/co-twin appropriate growth for gestational age (AGA); 5-min Apgar ⩾7; no chromosomal/congenital anomalies, infections, or prenatal brain lesions; medically viable.
Of all, 58 infants met the criteria, during September 2005 to December 2008, 30 were enrolled (17C, 13E) and 28 declined (Supplementary Figure 1). Declining and participating subjects were comparable in IUGR criteria, GA, Apgar-5, gender and singleton/twin status.
Control and experimental experience
C infants received standard care and E infants received NIDCAP care6 (http://www.nidcap.org), implemented by two certified NIDCAP professionals, psychologist SB and infant developmentalist LL (Supplementary Table 1 details Standard (C) and NIDCAP (E) care). NIDCAP extended from randomization to 42 weeks. To assess the fidelity of NIDCAP implementation, before study onset three NICU professionals, knowledgeable of staff developmental care skill (DCS) levels, rated nurses' DCS on a 0- to 2-point scale: 0=little to no DCS; 1=moderate DCS; 2=high DCS. Score means were used in case of rater discrepancy. Nurse DCS scores per shift were assigned for all subjects' entire hospital stay; mean percent time (hours/week) of DCS scores per infant was calculated for group comparison. A blinded observer (SK) measured developmental care implementation (Template scores)10 for all subjects within 10 days of enrollment (baseline; mean age 31 weeks, 4 ±17 days) and Time 2 (34 weeks, 5±17 days). Significantly higher DCS score percent care time and Time 2 Template ratings were hypothesized for E infants compared with C infants.
Study time points and measures
Infants were assessed with regard to demographics at intake; health and anthropometrics at intake, 42 weeks and 9 months; electroencephalography (EEG) and magnetic resonance imaging (MRI) at 35 weeks (in-NICU) and 42 weeks (outpatient); and neurobehavior at 42 weeks and 9 months.
Demographics, health and anthropometrics
Demographics, health and anthropometric indices were obtained from medical charts; chest roentgenograms and head sonograms by study radiologist review; and 42 weeks and 9 months health and anthropometric indices, including Pediatric Complication Scale Scores,11 by chart review, parent interview and direct measurement.
Neurodevelopment
Significantly better E infant neurobehavior, neurophysiology and neurostructure outcomes were hypothesized at 35 weeks, 42 weeks and 9 months, compared with C infants.
Neurophysiology
At 35 and 42 weeks, the infants were studied with 20-channel, Laplacian-referenced EEG, yielding a minimum of 240 s artifact-free quiet sleep.12 Spectral coherence between pairs of electrodes estimates cortical connectivity between brain areas. A total of 40 coherence factors, derived from an independent normative sample (N=312),12 were generated on the study sample's 35- and 42-week data. Age-normed difference factors were used for subsequent analyses.
Neurostructure
Infants underwent MRI without sedation during natural sleep. A neonatologist monitored electrocardiography and pulse oximetry throughout scanning with a 1.5T Signa MRI (General Electric, Waukesha, WI, USA) at 35 and 42 weeks for the derivation of quantitative measures of brain structure. Using previously validated methods,12 the volumes of cortical and subcortical gray matter (cGM; subcGM), myelinated and unmyelinated white matter (mWM; umWM) and cerebrospinal fluid within the cranium, were determined to produce five measures. For regional comparisons of the five tissue volumes, they were subdivided into 16 anatomical landmark-specified13 regions of interest within the brain (Supplementary Figure 2). The resulting 80 measures were factor analyzed before statistical analysis. A total of 25 infants (13 C; 12 E) had motion-free data sets.
Diffusion MRI (DMRI) was used to determine the measures related to WM development and microstructural integrity.14 Average mean diffusivity (MD) and fractional anisotropy (FA) measures were calculated per subject for 13 regions of interest of developmental significance: Anterior (A) and posterior (P) limbs of the right (R) and left (L) internal capsule (IC) (RIC-A; RIC-P; LIC-A; LIC-P); genu, splenium and body of the corpus callosum (CC); R and L cingulum; R and L frontal WM (FWM); and R and L occipital WM. All 26 measures (FA and MD across 13 regions of interest) were factor analyzed before analysis. At both time points, 10 C infants and 8 E infants had artifact-free data.
The following MRI sequences (parameters) were used: 3D-SPGR (TR30/TE9, flip-angle 45, 0.78 × 0.78 mm in-plane, 1.5 mm contiguous slices, 10 min); T2-weighted fast-spin echo (TE140, ETL 15, approximately 0.78 × 0.78 mm in-plane, 2 mm contiguous slices, 6 min); and Echo Planar diffusion-weighted images (B=750s mm−2, 31 directions, 6 baselines, 2NEX, 0.86 × 0.86 mm in-plane, 2.5 mm contiguous slices, 11 min).
Neurobehavior
All infants were studied at 42 weeks with the APIB (Assessment of Preterm Infants' Behavior)15 and Prechtl (Neurological Examination of the Fullterm Newborn Infant).16 A total of 22 APIB and 12 Prechtl summary variables,17 and an additional 8 APIB/Prechtl factor scores, generated from a normative sample (312 infants)12 were analyzed. At 9 months, 29 infants were assessed with the Bayley Scales of Infant Development, Second Edition (Bayley-II).18 Mental and Psychomotor Developmental Indices, age-equivalent difference scores, sensitive to delay, and Behavior Rating Scale (BRS) sub-domain scores (four) were used for analyses. All infants were studied neurobehaviorally by a group-blind psychologist, H.A.
Data analysis
The BMDP-2007 software (Statistical Solutions, Saugus, MA, USA) was used for statistical analyses. Variables and factor scores by domain were submitted to Multivariate analysis of variance (MANOVA), variable contributions to post-hoc ANOVA. The Brown–Forsythe test was used (F*-statistic), accounting for unequal variances and nonnormal distribution; Fisher's exact test was used to compare proportions; and Pearson χ2 test was used for categorical data. Two-tailed P<0.05 were considered significant. Power analysis indicated 85% power to detect a mean group difference of 1.5 points on the primary APIB variable assuming a pooled s.d. of 1.3 points (standardized effect size=1.5/1.3=1.15) using Student's t-test (version 7.0, nQuery Advisor, Saugus, MA, USA) for a sample size of 30 subjects (17 C and 13 E).
Discriminate function analysis with Wilks' lambda after jackknifing evaluated two-group classification success for 42 weeks spectral coherence, MRI segmentation and DMRI, and 42 weeks and 9 months neurobehavior factor scores. Canonical correlation explored the relationship between the 42-week behavioral, and the electrophysiological, MRI and medical/demographic background factors; Pearson product-moment correlation coefficients (r) explored the 42-week to 9-months behavioral factor relationship.
Results
Fidelity of experimental treatment
E infants received significantly more care than C infants from nurses who had moderate or high DCS (χ2=11.09, df=2, P=0.004; Supplementary Table 2). At baseline, Template rating results showed no significant group effect (P=0.45). Independent Time 2 Template rating results indicated overall significantly more developmentally appropriate environmental structuring, bedspace arrangement and direct infant care implementation for E infants compared with C infants (Supplementary Table 3a and 3b).
Medical/demographics
Groups were comparable on all medical and demographic background variables (P=0.45),19, 20, 21, 22 and all 42-week medical (P=0.91) and anthropometric outcome variables (P=0.72; Supplementary Table 4).
Neurobehavior
Ages (weeks post menstrual age) at the 42-week assessment were comparable (C: 43.28±1.54; E: 43.35±1.74; P=0.90). APIB scores showed significant group effects (P=0.029; Supplementary Table 5). E infants demonstrated significantly better motor regulation and self-regulation scores than the C infants, similar to earlier IUGR7 and AGA1 study findings. In contrast to AGA infants, IUGR C infants did worse than AGA C infants and IUGR E infants did better than AGA C infants yet worse than AGA E infants.
Prechtl scores did not differ (P=0.34). APIB/Prechtl factor scores showed better E- than C-group performance (P=0.006; Supplementary Table 6). Again, motor organization and self-regulation accounted for the difference. They also successfully (80%) differentiated the two groups (Wilks' Lambda = 0.577; P=0.0006). Thus, the 42-week neurobehavioral assessment again documented E infants as significantly more competent than C infants.
Neurophysiology
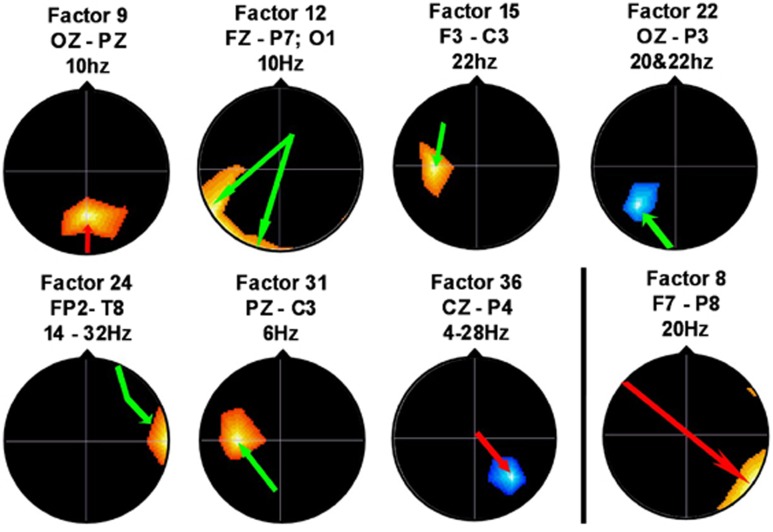
At the 35- and 42-week EEG assessment, ages also were comparable (34.20±1.62 (C); 34.92±2.22 (E); P=0.33; 43.28±1.54 (C); 43.35±1.74 (E); P=0.90). Analysis of co-variance (age at study) of the 40 coherence factors showed five significant group differences: factors 9, 12, 15, 22 and 36 (Supplementary Table 7; Figure 1), more than expected by chance. Supplementary Figure 312 aids interpretation. For factors 12, 15 and 22, E infants showed decreased coherence (green arrows), likely representing the pruning of C-group hyper-connectivities; for factors 9 and 36, increased coherence was observed (red arrows), likely representing enhanced association-cortex connectivities. Supplementary Table 7 explains factor coherence loadings, group difference direction, frequencies and electrode locations.
Figure 1.
EEG coherence measures at 42 weeks postmenstrual age: C=17; E=13. Head images correspond to coherence factors, top view, scalp left to image left; index electrode and frequencies above head. Color regions: location, magnitude and sign (red=positive; blue=negative) of maximally loading coherence on factor. Color arrows: direction of statistical association/difference (from E-group perspective; red=increased; green=decreased).
Four factors successfully (80%) differentiated C infants from E infants (Wilks' Lambda = 0.489; P<0.001), i.e., coherence factors 12, 36, 24 and 31 (Figure 1). Results indicate a general E-group connectivity decrease (5/7 factors), also found in the earlier IUGR study,7 in contrast to AGA study findings,1 which demonstrated E-group connectivity increase. NIDCAP largely appeared to reduce IUGR preterms' presumably undesirable over-connectivities, absent in AGA infants.
Neurostructure
Ages at the 35- and 42-week MRI assessments were comparable (34.63±1.58 (C); 35.47±1.39 (E); P=0.15; 43.47±1.48 (C); 43.10±1.30 (E); P=0.50).
Tissue segmentation
C and E groups showed comparable total intracranial capsule tissue volumes that increased significantly over time, as did the five separate total tissue volumes; however, by 42 weeks E infants showed a significantly smaller increase than C infants in umWM volume (Supplementary Table 8).
A total of 11 regional tissue factors accounted for 91.57% of total variance (Supplementary Table 9). The 11 factor scores revealed significant group, time and group-by-time interaction effects (Supplementary Table 10 for factors 1–5; factors 6–11 showed no effects). Factor 1, cerebrospinal fluid, significantly increased in both groups; factor 2, umWM, significantly increased for C infants but decreased for E infants, whereas factor 3, cGM, increased for both groups, yet significantly more so in E infants than in C infants. Factors 2 and 11 successfully (74.1%) differentiated C infants from E infants (Wilks' Lambda = 0.567; P<0.001). The findings identified more mature (less umWM, more cGM) brain structural development for E infants than for C infants.
DMRI
Five DMRI factors accounted for 81.2% of variance. MD in all 13 regions of interest loaded on factor-1; FA in 6 regions (LIC-A-P; RIC-A-P; CC splenium and genu) on factor-2. The remaining three factors were largely FA factors (factor 3: occipital WM-R-L, CC body, FWM-R; factor 4: Cingulum R-L; factor 5: FWM-R-L, CC body-MD). The five factors significantly differed between the groups and changed over time. E infants' MD (factor 1), already lower than that of C infants at 35 weeks, decreased significantly more by 42 weeks than did that of C infants. In turn, FA (factors 2 and 3) increased over time, yet without significant group difference, perhaps because of the smaller DMRI sample size (Supplementary Table 11).
Regional investigation showed significantly greater MD decrease for E infants, indicative of better development, throughout internal capsule (RIC-A-P; LIC-A-P; P=0.001; 0.01; 0.01; 0.02); CC (CC genu and body; P=0.02; 0.001); and occipital lobe (occipital WM-R-L; P=0.001; P=0.01). Factor 1 (MD, all regions) nearly successfully (60.9%) differentiated C infants from E infants (Wilks' Lambda =0.88; P=0.106).
Overall neurodevelopmental group classification
Four factors, identified by discriminate function analysis among the three neurodevelopmental domains, successfully (88.9. %) differentiated C infants from E infants (Wilks' Lambda = 0.297; P<0.0004): MRI factor 2 (umWM), APIB/Prechtl factor 8 (self-regulation), coherence factor 31 (6 Hz (theta) mid-parietal to left-central regions) and APIB/Prechtl factor 2 (motor system disorganization).
Health, growth and neurobehavior at 9 months corrected age
Ages (months corrected age) were comparable (17C: 9.78±1.02; 12E: 9.70±0.85; P=0.82).
Health
At 9 months, E infants were significantly healthier (MANOVA, P=0.047) than C infants, with better Pediatric Complication Scale Scores11 (C: 91.94±21.64; E: 111.42±22.28; P=0.03), fewer medications (P=0.05), severe illnesses (C: 0.88±.99; E: 0.17±0.39; P=0.01), emergency room visits (P=0.05) and less gastro-esophageal reflux (P=0.05). All children received some mother's milk by gavage feeding, bottle or breastfeeding. Mother's milk feeding durations (days) were comparable across groups (C: 55.47±27.03; E: 57.54±38.27; P=0.87).
Anthropometrics
Groups were comparable in 9 months anthropometrics (P=0.46).
Neurobehavior
E infants scored significantly better on the Bayley-II (Supplementary Table 12). The E-group mental developmental indices exceeded that of the C group on average by seven points. C infants scored 1.5 months below age-expectancy (significant delay), E infants scored at age expectancy. Neither psychomotor developmental index nor psychomotor developmental index age expectancy showed group differences. E infants scored significantly better on the BRS in Emotional Regulation, Motor Quality and BRS Total Score. Two Bayley-II factors (mental and motor) explaining 82.13% of the variance demonstrated significant group differences (P=0.03), and successfully (69.0%) differentiated C infants from E infants (Wilks' Lambda = 0.787; P<0.011). E infants scored significantly better than C infants on the mental factor (P=0.01), yet not on the motor factor (P=0.51). Thus, at 9 months, E infants showed significantly better health and neurobehavior. C (11) and E (15) infants comparably attended Early Intervention Programs (P=0.561).
Relationship of neurobehavior with EEG, MRI and medical background
Canonical correlation between APIB/Prechtl and spectral coherence factors (N=30) was significant (P<0.00004). The higher the left frontal to right parietal coherence (Coherence factor-8 (Figure 1)), the better pull-to-sit and attention performance (behavior factors-6 and -7), in keeping with AGA-preterm findings.1 Canonical correlation between APIB/Prechtl and regional segmentation factors (N=27) was also significant (P<0.01). The higher the cerebrospinal fluid level (segmentation-factor-11; Supplementary Table 11), the poorer the motor system regulation performance (behavior factor 8). The canonical correlation between the APIB/Prechtl and DMRI factors (N=23) neared significance (P<0.07). The higher the mean diffusivity (diffusion factor 1 (MD, all regions)), the poorer the motor system organization (behavior factor 1) and pull-to-sit performance (behavior factor 6; Figure 2). Moreover, APIB/Prechtl factor 8 (motor system regulation) correlated positively with Bayley factor 1 (mental developmental index; P<0.01). Medical and demographic background factors (four factors accounted for 72% of total variance) showed no correlation with any of the 42-week or 9-month neurodevelopmental factors.
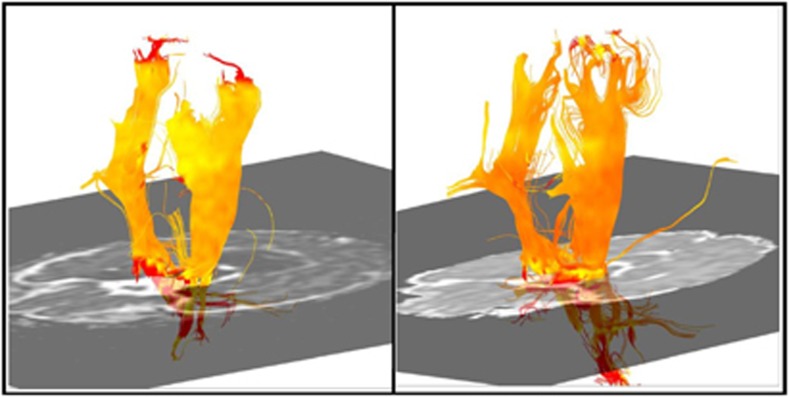
Figure 2.
MD in corticospinal tract (internal capsule) at 42 weeks postmenstrual age. Control infant on left, experimental infant on right. MD rendered onto trajectories of the corticospinal tract, and color-coded from red (low) to yellow (high). Brighter yellow: higher measure of MD; orange and red: lower measure of MD.
In summary, behavioral function at 42 weeks showed significant correlation to behavioral function at 9 months, surprisingly strong correlation with spectral coherence, somewhat lower correlation with MRI (smaller MRI-sample-size) and no correlation with medical/demographic background.
Discussion
This is the second study to report NIDCAP amelioration of IUGR preterm infants' brain function disadvantages,7 and the first study to report brain structure advantages. The results of both studies consistently favored E infants over C infants.
In the current study, NIDCAP infants compared with controls at 42 weeks again showed better self-regulation (increased self-regulation signals, including hand-to-face and hand-to-mouth, sucking, foot bracing and hand-grasping) and motor system functioning (improved reflexes, cuddling, crawling, modulation of movement, motor competence and decreased motor stress signals), and at 9 months better mental development, emotion regulation and motor quality. Spectral coherences again indicated the decrease of multiple connectivities between adjacent brain regions, whereas controls demonstrated excessive broad-spectrum cortical coupling,7 likely due to multiple pathological IUGR influences. Such pervasive aberrant connectivity is reminiscent of overabundant yet inefficient neuronal networks identified in learning-disabled children.23 Pruning of over-connectivities may facilitate neurodevelopmental differentiation and processing ease.
The NIDCAP-treated IUGR preterms also showed significantly decreased umWM volumes and increased cGM volumes, which substantiates their neurobehavior and spectral coherence improvements. Last trimester brain development involves continual growth of white and gray matter, with gradual umWM reduction and proportional increase in cGM.24 Acceleration of this process for the E group is further validated by the finding of significantly decreased mean diffusivities throughout internal capsules, the main cortical outflow regions; CC—genu and body—the major bi-hemispheric connective tract; and occipital regions, important visual and visual association cortical regions. Last trimester brain development also includes oligodendrocyte proliferation, pre-myelin ensheathment of axons and myelination in different brain regions at different rates.25, 26 Accelerated reduction in mean diffusivity is associated with improved long-term preterm infant outcome,27 substantiating the E infants' greater neurodevelopmental maturity and consistent with DMRI results for AGA preterm NIDCAP infants.1 In addition, the AGAs showed FWM improvement not replicated here, possibly because of the IUGR preterms' prenatal compromise. The NIDCAP IUGR preterms' occipital findings, not investigated in the AGA study,1 may reflect NIDCAP-recommended systematic protection from bright light, possibly resulting in enhanced visual cortical development. The NIDCAP effects on brain structure are consistent with reduction in cortical over-connectivities and improved behavioral function, all indicative of accelerated brain development. Thus, NIDCAP effects appear in keeping with species-expected environment effects on cortical development,28 which exceed mere environmental enrichment.29, 30 Although NIDCAP by no means is a womb substitute, it may be a faint approximation. The better 9-month mental and behavioral performance holds promise for continued functional improvement31, 32 foreshadowed by the 42-week markers of improved self-regulation and motor competence, and reduced over-coherence. The improved 9-month health status was unexpected. Perhaps NIDCAP facilitates gradual righting toward pre-compromise health potential.
NIDCAP appears to help compensate for in utero and in-NICU brain challenges for extreme IUGR preterms. Neither community early intervention services nor parents' socioeconomic status or ethnicity accounted for the beneficial effects. Another study reported promising preterm brain structure improvements in a mixed sample of preterms based on the in-NICU-administered MITP (Mother-Infant Transaction Program), which is theoretically anchored in the NIDCAP model of development.33
The neurobiological processes underlying NIDCAP effectiveness are speculative, which is a serious limitation of the study: NIDCAP may reduce iatrogenic NICU damage by resetting the NMDA (N-methyl-D-aspartate) axis, lowered because of pain and stressful NICU events, and causing inappropriate cell death and excitotoxic damage, lower sensory and pain thresholds, increased hyperreactivity and hypersensitivity,34, 35, 36 and release of brain-damaging free radicals, advanced oxidation protein products and total hydroperoxide. Furthermore, NIDCAP may prevent deleterious abrupt blood-flow velocity changes, associated with standard NICU care procedures and contributive to preterm infants' diffuse brain damage.37 Animal model, larger infant sample and longer-term outcome studies are recommended, as are studies that begin in the delivery room when blood-flow velocity changes are at their most extreme and IUGR-preterms at their most vulnerable.
Conclusion
Despite significant in utero compromise due to severe IUGR, preterm brain plasticity appears to prevail. NIDCAP, an individualized behavior observation-based intensive care and environment adaptation, significantly improved neurodevelopment in terms of behavior, functional brain connectivity and brain structure and health. Thus, NIDCAP might prove beneficial also for other prenatally brain- and organ-compromised infant populations.
Acknowledgments
We thank the study families and infants, as well as study EEG, MRI and NICU staff, for their participation and commitment. The study was supported by National Institutes of Health (NIH), grant R01 HD046855, a grant from the Irving Harris Foundation and a grant from the Weil Foundation to Heidelise Als, PhD; NIH grant R01 RR021885 to Simon K. Warfield, PhD as well as the Intellectual and Developmental Disabilities Research Center (CHIDDRC) grant P30HD018655 to Scott Pomeroy, MD, PhD, Children's Hospital Boston. Clinical Trial Registration—the trial registration number is NCT00914108.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Perinatology website (http://www.nature.com/jp)
Supplementary Material
References
- Als H, Duffy F, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, et al. Early experience alters brain function and structure. Pediatrics. 2004;113 (4:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- Peters K, Rosychuk R, Hendson L, Cote J, McPherson C, Tyebkhan J. Improvement of short- and long-term outcomes for very low birth weight infants: The Edmonton NIDCAP trial. Pediatrics. 2009;124:1009–1020. doi: 10.1542/peds.2008-3808. [DOI] [PubMed] [Google Scholar]
- Heron M, Sutton P, Xu J, Ventura S, Strobino D, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- Committee on Understanding Premature Birth and Assuring Healthy Outcomes Preterm birth: Causes, Consequences, and Prevention The National Academies Press: Washington, DC; 2007. p31. [PubMed] [Google Scholar]
- Sizonenko SV, Borradori-Tolsa C, Hüppi PS. [Intrauterine growth restriction: impact on brain development and function] Rev Med Suisse. 2008;4 (146:509–514. [PubMed] [Google Scholar]
- Als H.Program Guide—Newborn Individualized Developmental Care and Assessment Program (NIDCAP): An Education and Training Program for Health Care Professionals Copyright, NIDCAP Federation International: Boston; 1986. rev 2009. [Google Scholar]
- Als H, Duffy FH, McAnulty GB, Fischer CB, Kosta S, Butler SC, et al. Is the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) effective for preterm infants with intrauterine growth restriction. J Perinatol. 2011;31 (2:130–136. doi: 10.1038/jp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- Gairdner D, Pearson J. A growth chart for premature and other infants. Arch Dis Child. 1971;46:783–787. doi: 10.1136/adc.46.250.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Als H, Buehler D, Kerr D, Feinberg E, Gilkerson L.Profile of the Nursery Environment and of Care Components. Template Manual, Part I Children's Hospital: Boston; 1990, 1995. Rev. 1997. [Google Scholar]
- Littman B, Parmelee AH.Manual for Pediatric ComplicationsInfant Studies Project, Department of Pediatrics, School of Medicine, ed:University of California: Los Angeles; 1974 [Google Scholar]
- Duffy FH, Als H, McAnulty GB. Infant EEG spectral coherence data during quiet sleep: Unrestricted Principal Components Analysis - Relation of factors to gestational age, medical risk, and neurobehavioral status. Clin Electroencephalogr. 2003;34 (2:54–69. doi: 10.1177/155005940303400204. [DOI] [PubMed] [Google Scholar]
- Mewes A, Huppi P, Als H, Rybicki F, Inder T, McAnulty G, et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118 (1:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion tensor MRI. J Magn Reson B. 1996;11:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Als H, Lester BM, Tronick EZ, Brazelton TB. Theory and Research in Behavioral Pediatrics. Plenum Press: New York; 1982. Manual for the assessment of preterm infants' behavior (APIB) pp. 65–132. [Google Scholar]
- Prechtl HFR.The Neurological Examination of the Full-term Infant: A manual for clinical use2nd edn.Lippincott: Philadelphia; 1977 [Google Scholar]
- Buehler DM, Als H, Duffy FH, McAnulty GB, Liederman J. Effectiveness of individualized developmental care for low-risk preterm infants: Behavioral and electrophysiological evidence. Pediatrics. 1995;96:923–932. [PubMed] [Google Scholar]
- Bayley N.Bayley Scales of Infant Development2nd edn.The Psychological Corporation: San Antonio; 1993 [Google Scholar]
- Richardson D, Corcoran J, Escobar G, Lee S.Canadian-NICU-Network, Kaiser-Permanente-Network, et al.SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores J Pediatr 200113892–100. [DOI] [PubMed] [Google Scholar]
- Gray CM, Engel AK, König P, Singer W. Synchronization of oscillatory neuronal responses in cat striate cortex: Temporal properties. Visual Neurosci. 1992;8:337–347. doi: 10.1017/s0952523800005071. [DOI] [PubMed] [Google Scholar]
- Littman B, Parmelee AH.Manual for Obstetric ComplicationsInfant Studies Project, Department of Pediatrics, School of Medicine, ed:University of California: Los Angeles; 1974 [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Working Paper: Yale University: New Haven; 1975. [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a ‘local to distributed' organization. PLoS Comput Biol. 2009;5 (5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori-Tolsa C, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, et al. Primary cortical folding in the human newborn: An early marker of later functional development. Brain. 2008;131 (8:2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan ML, Dyet LE, Boardman JP, Kapellou O, Allsop JM, Cowan F, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120 (3:e604–e6e9. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci USA. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage F. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, et al. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Westrup B, Böhm B, Lagercrantz H, Stjernqvist K. Preschool outcome in children born very prematurely and cared for according to the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) Acta Paediatrica. 2004;93 (4:498–507. doi: 10.1080/08035250410023548. [DOI] [PubMed] [Google Scholar]
- McAnulty G, Duffy F, Butler S, Bernstein J, Zurakowski D, Als H. Effects of the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) at age 8 years: Preliminary data. Clin Pediatr. 2010;49 (3:258–270. doi: 10.1177/0009922809335668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom J, Newnham C, Anderson PJ, Doyle LW, Gemmill AW, Lee K, et al. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res. 2010;67 (3:330–335. doi: 10.1203/PDR.0b013e3181cb8e2f. [DOI] [PubMed] [Google Scholar]
- Meany MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior. Biol Neonate. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- Kolber B, Boyle M, Wieczorek L, Kelley C, Onwuzurike C, Nettles S, et al. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30 (7:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Gauvreau K, O'Leary H, Moore M, Bassan H, Eichenwald E, et al. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:e1006–e1013. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.