Abstract
Aims
To assess the impact of relief of pulmonary stenosis (PS) and pulmonary regurgitation (PR) by percutaneous pulmonary valve implantation (PPVI) on biventricular function during exercise stress.
Methods and results
Seventeen patients, who underwent PPVI for PS or PR, were included. Magnetic resonance imaging was performed at rest and during supine exercise stress pre- and within 1-month post-PPVI, using a radial k − t SENSE real-time sequence. In patients with PS (n = 9), there was no reserve in right ventricular (RV) ejection fraction (EF) in response to exercise prior to PPVI (48.2 ± 12.1% at rest vs. 48.4 ± 14.8% during exercise, P = 0.87). Post-PPVI, reserve in RVEF in response to exercise was re-established (53.4 ± 15.0% at rest vs. 59.6 ± 17.3% during exercise, P = 0.003) with improvement in left ventricular stroke volume (LVSV) (45.4 ± 6.2 mL/m2 at rest vs. 52.8 ± 8.8 mL/m2 during exercise, P = 0.001). In patients with PR prior to PPVI (n = 8), LVSV during exercise increased (43.0 ± 8.5 vs. 54.3 ± 6.6 mL/m2, P < 0.001) due to reduction in PR fraction during exercise (29.2 ± 5.2 vs. 13.6 ± 6.1%, P < 0.001). After PPVI, LVSV increased from rest to exercise (48.4 ± 8.8 vs. 57.2 ± 8.1 mL/m2, P < 0.001) due to improved RVEF (45.5 ± 8.3 vs. 50.4 ± 6.9%, P = 0.001). There was a significantly higher increase in LVSV at exercise from pre- to post-PPVI in PS patients than in PR patients (ΔLVSV 8.2 ± 4.1 vs. Δ2.9 ± 4.1 mL/m2, P = 0.01). The reduction in the RV outflow tract gradient correlated significantly with the improvement in LVSV during exercise (r = −0.73, P < 0.001).
Conclusion
Percutaneous pulmonary valve implantation in patients with PS leads to restoration of reserve in RVEF during exercise stress. In patients with PR, SV augmentation improves only mildly post-PPVI. Improvement in SV augmentation during exercise stress after PPVI is dependent mainly on afterload reduction.
Keywords: Exercise physiology, Right ventricular outflow tract dysfunction, Percutaneous pulmonary valve implantation, Congenital heart disease, Exercise stress magnetic resonance imaging
Introduction
Percutaneous pulmonary valve implantation (PPVI) is becoming an increasingly important management strategy in patients with right ventricular outflow tract (RVOT) dysfunction secondary to congenital heart disease. Previous studies have demonstrated symptomatic improvements following PPVI in patients with both pulmonary stenosis (PS) and pulmonary regurgitation (PR).1 Furthermore, cardiac magnetic resonance (CMR) studies have demonstrated improved biventricular effective stroke volume (SV) and cardiac output (CO) in both groups.1–6 However, these improvements in cardiovascular function do not translate into improved exercise capacity (i.e. peak oxygen uptake) in patients with PR. This is in contrast to patients with PS in whom there is a marked increase in peak oxygen uptake (VO2). This discrepancy has important clinical implications: if the success of PPVI is assessed by improved exercise capacity alone, one might suggest that PPVI is not useful in patients with PR. Of course this disregards the CMR and symptomatic data, but it is a valid position with the current available data. Thus, resolving this discrepancy is important for better understanding of the patient groups that will benefit from PPVI. This requires an accurate assessment of cardiac performance during exercise stress.
Gated cardiac magnetic resonance (CMR) is the best available method of assessing biventricular function at rest. However, it has previously been difficult to use CMR during exercise due to motion artefacts and difficulties in breath holding. We have recently demonstrated the utility of rapid high resolution real-time CMR in assessing ventricular function during active exercise.7 Using this novel technique, it should be possible to better investigate the biventricular response to exercise before and after PPVI.
The aim of this study was to use real-time CMR to assess possible differences in biventricular response to exercise in patients with PS and PR before and after PPVI. A better understanding of cardiac physiology during exercise stress in these patients should inform on how to judge procedural success of this intervention, and should help define endpoints for future study that address the timing of pulmonary valve replacement.
Methods
Patient population and study protocol
In this prospective study, 17 consecutive children and young adults were studied. Patients were included when they had a clinical indication for PPVI in the context of significant RVOT obstruction or regurgitation as published previously.8 In addition, patients had to fulfill morphological requirements for PPVI.8 Exclusion criteria were unfavourable morphology for PPVI,8,9 occluded central veins, active infection, contraindications to CMR imaging, or exercise testing.
Valve implantation was performed under general anaesthesia with pressure measurements being carried out before and immediately after PPVI as previously described.3,8 All patients were also assessed using echocardiography and cardiopulmonary exercise testing (CPEX) within 1 month prior to PPVI, and within 1 month of PPVI as previously described.10
Percutaneous pulmonary valve implantation, in order to separate patients with predominant PS (PR fraction ≤ 25%, n = 9) from those with predominant PR (PR fraction >25%, n = 8).
The Institution's ethics committee approved the study. Written consent was obtained from patients and parents/guardians as appropriate.
Resting and exercise stress cardiac magnetic resonance imaging protocol
All examinations were performed using a four-element phased array coil setup on a 1.5 T MR scanner (Avanto, Siemens Medical Solutions, Erlangen, Germany). Ventricular volumetric assessment was performed at rest and during supine exercise.
The supine bicycle exercise stress and real-time CMR imaging protocols were described and validated in detail previously7 and are here summarized briefly for convenience: biventricular volumes and function during rest and exercise were assessed using a real-time radial k − t SENSE sequence. The whole ventricular volume was covered using between 10 and 12 contiguous slices and each slice was acquired for 1.5 s to ensure at least one full cardiac cycle was captured. The spatial and temporal resolution were ∼2.3 × 2.3 mm and 35 ms, respectively. All other imaging parameters were published previously.7
Exercise was performed with a CMR compatible ergometer (MRI cardiac ergometer Up/Down, Lode BV, Groningen, Netherlands). Prior to the scan, participants were placed supine in the MR scanner with their feet strapped in the pedals of the ergometer. Exercise was performed with an up- and downwards movement of the pedals. This had the advantage of less movement restriction caused by the MR tunnel in comparison with a rotary motion. Exercise intensity in patients >14 years was increased every 60 s by 2 W (n = 14), in patients <14 years every 60 s by 1 W (n = 3). Images were acquired at rest and every 3 min. A stepwise increase in workload every 60 s was chosen to allow 30 s to reach a steady state and 30 s for image acquisition with radial k − t real-time. Care was taken to ensure that the revolutions per minute (r.p.m.) ranged from 50 to 70 in order to assure that workload was independent of r.p.m. (hyperbolic ergometry).
Prior to PPVI, patients were encouraged to exercise until exhaustion. Post PPVI, patients were encouraged to exercise to the highest exercise level reached before PPVI. Cardiac magnetic resonance images acquired at the same exercise intensities pre- and post-PPVI were analysed. All exercise data were acquired during free breathing and under continuation of exercise.
By applying this technique in healthy volunteers, we could demonstrate a very small difference in right ventricular (RV) and left ventricular (LV) SV as a measure of accuracy (SD of difference ± 3.43 mL or 4.3% of LV SV).
The difference in CO in the same subject during two different imaging sessions as a measure of reproducibility was found to be ±0.6 L/min for the RV CO and ±0.45 L/min for LV CO (4.0% of LV CO).7
Analysis of cardiac magnetic resonance images
End-diastolic and end-systolic phases for each ventricle were identified by a visual inspection of the largest (end-diastolic) and smallest (end-systolic) blood pool area for each slice and manually segmented. The endocardial border was traced using the Open Source OsiriX software. The papillary muscles and, if present, coarse trabeculae were excluded from the blood pool. Right ventricle and LV end-diastolic volume (EDV), and end-systolic volume (ESV) were calculated using the slice summation method. Stroke volume was the difference between EDV and ESV, and EF was SV divided by EDV expressed as a percentage.
Pulmonary regurgitation fraction (PRF) was indirectly calculated as follows: [(RVSV − LVSV)/RVSV) × 100]. Effective RVSV was assumed to be equal to left ventricular stroke volume (LVSV). To allow for this indirect measurement of effective RVSV and PRF, patients with relevant atrioventricular valve regurgitation, aortic regurgitation, or evidence for intracardiac shunting were not included in this study. All volume measurements were indexed for the body surface area and expressed in millilitre per metre square.
Statistical analysis
Data were tested for the normal distribution by the Kolmogorow–Smirnow test. Normally distributed data are expressed as mean ± SD, non-normally distributed data were summarized using the median and range. Two-paired samples were analysed with paired Student's t-test for normally distributed data, and with the Mann–Whitney U test for non-normally distributed data.
Correlation analysis was performed to study the relationship between changes in biventricular performance during exercise stress and changes in RVOT obstruction, peak VO2, and PRF. All statistical tests were two-sided and a P-value of <0.05 was considered statistically significant. Statistical testing and data analysis were performed with SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism version 5.0b (Graphpad Software, San Diego, CA, USA).
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Patient characteristics
The patient characteristics are shown in Table 1. The mean age of the patient population was 19.2 ± 6.1 years. The majority of patients (11/17) had tetralogy of Fallot or a variant morphology. Right ventricular to PA conduits were present in all but one patient, who presented with a failed bioprothesis in the pulmonary position. All patients were symptomatic (NYHA functional class >1, see Table 1 for details). Percutaneous pulmonary valve implantation was successful in all patients.
Table 1.
Patient characteristics
| Sex | Age (years) | Weight (kg) | Primary diagnosis | RVOT anatomy | Outflow tract (mm) | No. of previous heart surgeries | Pre-procedure functional class |
|---|---|---|---|---|---|---|---|
| M | 28.3 | 93.4 | Aortic valve disease (Ross) | Homograft | 25 | 2 | 3 |
| F | 17.4 | 44.6 | Aortic valve disease (Ross) | Homograft | 18 | 3 | 2 |
| M | 17.8 | 66.9 | ToF | Bioprothesis | 21 | 2 | 2 |
| M | 14.8 | 70.6 | ToF | Homograft | 19 | 2 | 2 |
| M | 20.8 | 50.4 | Double outlet right ventricle | Hancock | 22 | 6 | 2 |
| M | 12.3 | 53.4 | ToF | Homograft | Unknown | 1 | 2 |
| M | 10.8 | 49.8 | ToF, Pulm atresia | Carpentier Edwards | 18 | 2 | 3 |
| M | 16.2 | 47.2 | Aortic valve disease (Ross) | Homograft | 19 | 1 | 2 |
| M | 17.2 | 65 | Aortic valve disease (Ross) | Homograft | 20 | 1 | 2 |
| F | 10 | 31.5 | Truncus arteriosus | Homograft | 16 | 5 | 3 |
| F | 21.7 | 65.4 | ToF, Pulm atresia | Homograft | 20 | 2 | 3 |
| M | 15.9 | 83 | ToF, Pulm atresia | Hancock | 22 | 3 | 2 |
| M | 21.9 | 50 | ToF | Homograft | 21 | 3 | 2 |
| M | 31.2 | 58 | ToF, Pulm atresia | Homograft | 22 | 3 | 2 |
| F | 18.6 | 56.2 | Truncus arteriosus | Homograft | 21 | 3 | 3 |
| M | 22.5 | 96 | Double outlet right ventricle | Homograft | 18 | 3 | 2 |
| M | 28.4 | 81.2 | ToF | Homograft | 22 | 4 | 2 |
| 19.2 ± 6.0 | 62.5 ± 17.8 | 20.3 ± 2.2 | 3 (1–6) | 2 (2–3) |
ToF, tetralogy of Fallot; Pulm atresia, pulmonary atresia; RVOT, right ventricular outflow tract; Ross, Ross procedure; last row represents summary data where applicable.
Haemodynamics and functional responses (total population)
Percutaneous pulmonary valve implantation was successful in all patients, resulting in a significant reduction in RV systolic pressure (55.5 ± 3.1 vs. 36.7 ± 1.8 mmHg; P < 0.001) and the pulmonary artery to RV pullback gradient (31.2 ± 3.9 vs. 13.5 ± 2.0 mmHg; P < 0.001).
On CPEX, exercise capacity was found to be markedly decreased in this patient population (peak VO2 25.6 ± 6.0 mL/min/kg). Following PPVI, peak VO2 increased (in the group as a whole) to 27.7 ± 6.2 mL/min/kg (P = 0.04 from pre- to post-PPVI). The peak heart rate (b.p.m.) on CPEX pre- and post-PPVI was 170.6 ± 13.6 and 169.6 ± 13.5 b.p.m., respectively (P = 0.9).
The changes in CMR parameters at rest and during exercise within the total population are summarized in Table 2.
Table 2.
Results of magnetic resonance imaging at rest and during exercise stress within the total population
| pre-PPVI rest vs. exercise |
post-PPVI rest vs. exercise |
Δ pre- vs. post-PPVI |
||||
|---|---|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| RV EDV, mL/m2 | 113.8 ± 41.0 | 115.2 ± 37.5 | 102.7 ± 33.0 | 105.5 ± 30.2 | −11.1 ± 13.7** | −9.7 ± 12.0** |
| RV ESV, mL/m2 | 61.3 ± 33.3 | 60.9 ± 33.4 | 55.2 ± 30.7 | 50.6 ± 28.6* | −6.1 ± 6.9** | −10.3 ± 12.4** |
| RV SV, mL/m2 | 52.5 ± 15.4 | 54.3 ± 13.5 | 47.5 ± 7.2 | 54.9 ± 8.2* | −5.0 ± 11.0 | 0.5 ± 7.8 |
| RV EF, % | 48.1 ± 11.1 | 49.3 ± 11.6 | 49.5 ± 12.7 | 55.3 ± 14.1* | 1.4 ± 7.1 | 5.9 ± 9.6** |
| PRF, % | 17.3 ± 13.3 | 8.6 ± 7.0* | 1.9 ± 2.5 | 1.3 ± 3.8 | −15.4 ± 11.6** | −7.3 ± 6.3** |
| LV EDV, mL/m2 | 71.1 ± 18.1 | 73.6 ± 22.7 | 77.4 ± 19.2 | 81.2 ± 22.4 | 6.4 ± 6.3** | 7.6 ± 6.7** |
| LV ESV, mL/m2 | 29.0 ± 14.9 | 24.4 ± 18.4* | 30.6 ± 13.8 | 26.3 ± 17.9 | 1.6 ± 4.2 | 1.9 ± 3.8 |
| Effective LV/RV SV, mL/m2 | 42.1 ± 8.4 | 49.2 ± 9.9* | 46.8 ± 7.5 | 54.9 ± 8.6* | 4.7 ± 5.2** | 5.7 ± 4.8** |
| LV EF, % | 60.9 ± 11.3 | 69.5 ± 12.7* | 61.7 ± 7.9 | 69.6 ± 10.5* | 0.9 ± 5.2 | 0.1 ± 4.2 |
| Heart rate, b.p.m. | 78.0 ± 9.7 | 132.9 ± 13.6* | 78.5 ± 10.2 | 120.5 ± 13.0* | 0.6 ± 4.9 | −12.4 ± 13.5** |
| CO LV, mL/min m2 | 3.3 ± 0.7 | 6.5 ± 1.3* | 3.6 ± 0.6 | 6.6 ± 1.4* | 0.3 ± 0.4** | 0.1 ± 0.5 |
RV, right ventricle; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction; PRF, pulmonary regurgitation fraction; LV, left ventricle; CO, cardiac output.
*A significant difference (P < 0.05) between parameters at rest vs. during exercise.
**A significant difference (P < 0.05) between parameters pre-PPVI vs. post-PPVI.
Haemodynamic and functional responses (pulmonary stenosis group)
Pre-percutaneous pulmonary valve implantation
The mean RVOT gradient in the PS group was 43 ± 11 mmHg and the mean PRF was 8.1 ± 4.2%. During exercise CMR, there were no significant changes in RV volumes or RVEF. However, there was a marked increase in left ventricle cardiac output (LVCO) (3.1–6.0 L/min, P < 0.05) during exercise, which was mainly driven by an increased heart rate (76–136 b.p.m., P < 0.05) (Table 3).
Table 3.
Results of magnetic resonance imaging at rest and during exercise stress within the pulmonary stenosis and pulmonary regurgitation group
| pre-PPVI rest vs. exercise |
post-PPVI rest vs. exercise |
Δ pre- vs. post-PPVI |
||||
|---|---|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| Pulmonary stenosis (n = 9) | ||||||
| RV EDV, mL/m2 | 103.0 ± 52.0 | 104.0 ± 44.4 | 93.9 ± 38.4 | 96.4 ± 35.9 | −9.2 ± 16.1 | −7.6 ± 11.9** |
| RV ESV, mL/m2 | 56.9 ± 42.2 | 57.7 ± 32.8 | 48.3 ± 37.3 | 43.7 ± 35.4* | −8.6 ± 7.9** | −14.0 ± 13.1** |
| RV SV, mL/m2 | 46.1 ± 14.1 | 46.4 ± 11.3 | 45.6 ± 6.4 | 52.7 ± 8.9* | −0.6 ± 9.6 | 6.3 ± 4.2 |
| RV EF, % | 48.2 ± 12.1 | 48.4 ± 14.8 | 53.4 ± 15.0 | 59.6 ± 17.3* | 5.2 ± 7.1** | 11.3 ± 9.8** |
| PRF, % | 8.6 ± 10.8 | 4.2 ± 4.4 | 0.5 ± 0.8 | 1.4 ± 3.8 | −8.1 ± 10.1** | −2.8 ± 4.2** |
| LV EDV, mL/m2 | 66.1 ± 16.7 | 66.0 ± 17.4 | 73.7 ± 14.1 | 75.9 ± 13.2 | 7.6 ± 4.8** | 9.9 ± 6.8** |
| LV ESV, mL/m2 | 24.8 ± 12.3 | 21.4 ± 12.8* | 28.3 ± 11.2 | 23.1 ± 10.1* | 3.5 ± 3.2** | 1.7 ± 3.9 |
| Effective LV/RV SV, mL/m2 | 41.3 ± 8.8 | 44.6 ± 10.4* | 45.4 ± 6.2 | 52.8 ± 8.8* | 4.1 ± 3.8** | 8.2 ± 4.1** |
| LV EF, % | 63.9 ± 11.4 | 69.6 ± 12.7* | 62.7 ± 8.6 | 70.4 ± 9.6* | −1.2 ± 4.6 | 0.9 ± 4.4 |
| Heart rate, b.p.m. | 76.6 ± 10.0 | 136.5 ± 15.3* | 76.0 ± 9.9 | 117.8 ± 13.8* | −0.6 ± 6.2 | −18.7 ± 12.3** |
| CO LV, mL/min * m2 | 3.1 ± 0.6 | 6.0 ± 1.4* | 3.4 ± 0.5 | 6.2 ± 1.4 | 0.3 ± 0.3 | 0.2 ± 0.4 |
| Pulmonary regurgitation (n = 8) | ||||||
| RV EDV, mL/m2 | 126.5 ± 24.5 | 127.9 ± 24.8 | 112.7 ± 24.1 | 115.7 ± 19.5 | −13.8 ± 12.0** | −12.2 ± 12.6** |
| RV ESV, mL/m2 | 65.3 ± 20.1 | 64.6 ± 20.9 | 63.0 ± 20.5 | 58.4 ± 17.7 | −2.2 ± 4.0 | −6.2 ± 10.7 |
| RV SV, mL/m2 | 61.3 ± 14.0 | 63.3 ± 9.9 | 49.7 ± 7.8 | 57.3 ± 6.9* | −11.6 ± 10.7** | −6.0 ± 5.4** |
| RV EF, % | 48.9 ± 9.9 | 50.4 ± 8.4 | 45.5 ± 8.3 | 50.4 ± 7.9* | −3.9 ± 3.6 | 0.0 ± 4.5 |
| PRF, % | 29.2 ± 5.2 | 13.6 ± 6.1* | 0.3 ± 0.0 | 1.9 ± 3.1 | −29.2 ± 5.1** | −11.7 ± 5.4** |
| LV EDV, mL/m2 | 76.7 ± 19.2 | 82.1 ± 25.9 | 81.7 ± 23.9 | 87.1 ± 129.5 | 4.9 ± 7.7 | 5.1 ± 5.9** |
| LV ESV, mL/m2 | 33.7 ± 19.9 | 27.7 ± 23.7* | 33.2 ± 16.7 | 29.9 ± 24.3 | −0.4 ± 4.5 | 2.1 ± 3.9 |
| effective LV/RV SV, mL/m2 | 43.0 ± 8.5 | 54.3 ± 6.6* | 48.4 ± 8.8 | 57.2 ± 8.1* | 5.4 ± 6.6** | 2.9 ± 4.2 |
| LV EF, % | 57.5 ± 10.8 | 69.5 ± 13.5* | 60.7 ± 7.4 | 68.7 ± 11.9* | 3.2 ± 5.0 | −0.8 ± 4.2 |
| Heart rate, b.p.m. | 79.5 ± 9.8 | 128.9 ± 10.9* | 81.4 ± 10.3 | 123.1 ± 12.5* | 1.9 ± 2.8 | −5.8 ± 11.9 |
| CO LV, mL/min m2 | 3.4 ± 0.7 | 7.0 ± 1.2* | 3.8 ± 0.6 | 7.1 ± 1.3* | 0.5 ± 0.5** | 0.0 ± 0.7 |
RV, right ventricle; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction; PRF, pulmonary regurgitation fraction; LV, left ventricle; CO, cardiac output.
*A significant difference (P < 0.05) between parameters at rest vs. during exercise.
**A significant difference (P < 0.05) between parameters pre-PPVI vs. post-PPVI.
Post-percutaneous pulmonary valve implantation
In the PS group, there was a significant reduction in RV systolic pressure (62.1 ± 12.6 vs. 39.6 ± 8.9 mmHg; P < 0.001) and the pulmonary artery to RV pullback gradient (42.8 ± 11.3 vs. 17.4 ± 9.7 mmHg; P < 0.001).
At rest, PPVI led to a fall in RVEDV and RVESV at rest. In addition, RVEF at rest was higher post-PPVI (P < 0.05). During exercise, RVESV fell significantly compared with rest and consequently RVSV and RVEF both increased during exercise. There was also an increase in LVEDV during exercise compared with rest. This is markedly different from the situation pre-PPVI when RV and LV volumes and function did not change. The magnitude of the increase in LVCO during exercise was similar pre- and post-PPVI. However, post-PPVI, this was driven by both LVSV and heart rate and therefore the peak heart rate was lower post-PPVI (Table 3 and Figure 1).
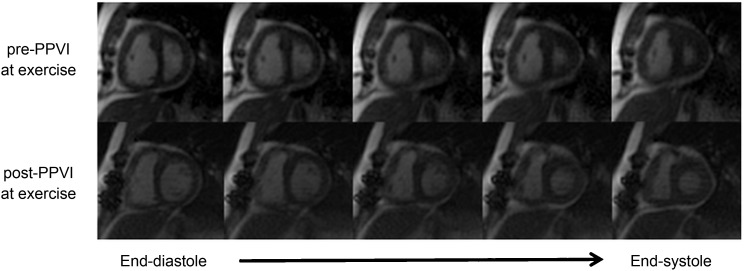
Figure 1.
Representative example of radial k − t real-time images in a patient with predominantly pulmonary stenosis acquired at the same exercise intensity pre-percutaneous pulmonary valve implantation (top row) and post-percutaneous pulmonary valve implantation (bottom row) showing five reconstructed phases including end-diastolic volume (far left) and end-systolic volume (far right). Post-percutaneous pulmonary valve implantation, the left ventricle appears to be better filled (higher end-diastolic volume), with improved right ventricular function. Note the visually better filled left ventricle (increased end-diastolic volume) and reduced right ventricular size.
Haemodynamic and functional responses (pulmonary regurgitation group)
Pre-percutaneous pulmonary valve implantation
The mean PRF in the PR group was 29 ± 10% and the mean RVOT gradient was 18.8 mmHg. During exercise, there were no significant changes in RV volumes or RVEF as measured by CMR. However, effective RVSV markedly increased due to >50% reduction in PRF at peak exercise. This resulted in better LV filling and ejection and a higher peak LVCO compared with the PS group (3.4–7.0 L/min in the PR group vs. 3.1–6.0 L/min in the PS group, P < 0.05). In the PR group, CO augmentation during exercise is driven by both heart rate and increased effective RVSV (Table 3).
Post-percutaneous pulmonary valve implantation
There was almost complete abolishment of PRF due to the insertion of competent valve (29–0.3%, P < 0.001). Percutaneous pulmonary valve implantation led to a reduction in RV volumes and RVEF at rest. During exercise, RVSV and RVEF increased significantly compared with at rest. However, augmentation in effective RVSV during exercise was similar pre- and post-PPVI. This is reflected by similar heart rate and CO responses pre- and post-PPVI (Table 3).
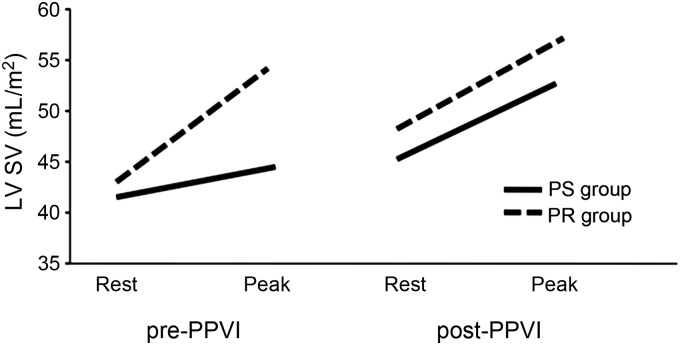
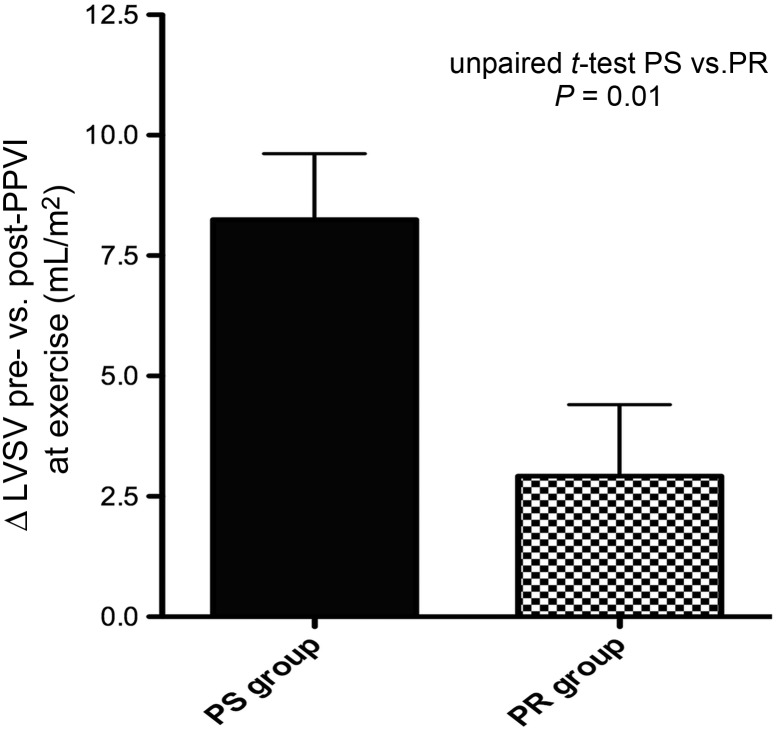
Although there was an increase in biventricular SV at peak exercise after PPVI in both the PS and the PR group, this increase was significantly higher after reduction RVOT obstruction than after reduction in PR (Δ8.2 ± 4.1 in the PS group vs. Δ2.9 ± 4.1 mL/m2 in the PR group, P = 0.01; Figures 2 and 3).
Figure 2.
Changes in left ventricular stroke volume at rest and during exercise (peak) from pre- to post-percutaneous pulmonary valve implantation in patients with predominantly pulmonary stenosis and pulmonary regurgitation.
Figure 3.
The graph demonstrates a significantly higher increase in left ventricular stroke volume during exercise from pre- to post-percutaneous pulmonary valve implantation in patients with pulmonary stenosis as compared with pulmonary regurgitation.
Correlation analysis
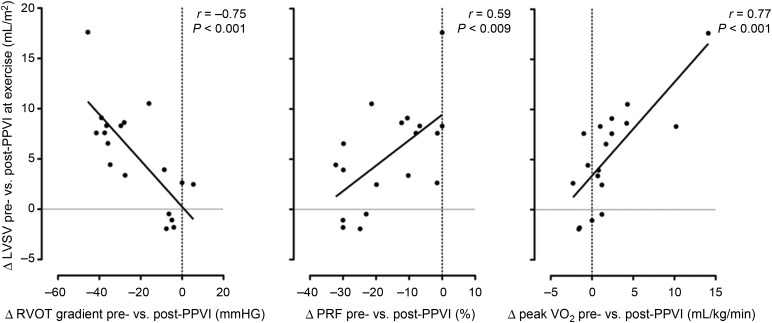
Both the PS group and the PR group had some mixed disease. Therefore, the whole group was also analysed using correlation analysis. The main significant correlations were: (i) improvement in LVSV augmentation post-PPVI was positively correlated with reduction in the RVOT gradient after PPVI (r = −0.75, P < 0.001; Figure 4, left panel); (ii) improvement in LVSV augmentation post-PPVI was negatively correlated with reduction in PRF (r = 0.59, P = 0.009; Figure 4, middle pane); and (iii) improvement in LVSV augmentation post-PPVI was positively correlated with improved peak VO2 (r = 0.77, P < 0.001; Figure 4, right panel).
Figure 4.
Δ, Change in parameter pre-percutaneous pulmonary valve implantation vs. post-percutaneous pulmonary valve implantation; at exercise, parameters assessed during peak exercise; peak oxygen uptake, peak VO2. Left panel: The reduction in resting right ventricular outflow tract gradient from pre- to post-percutaneous pulmonary valve implantation as assessed on echocardiography (Δ right ventricular outflow tract gradient pre- vs. post-percutaneous pulmonary valve implantation) at the time of exercise stress cardiac magnetic resonance imaging correlates significantly with the change of left ventricular stroke volume augmentation in response to exercise from pre- to post-percutaneous pulmonary valve implantation (Δ left ventricular stroke volume pre- vs. post-percutaneous pulmonary valve implantation at exercise; r = −0.75, P < 0.001). Middle panel: There is an inverse relationship between reduction in pulmonary regurgitation fraction from pre- to post-percutaneous pulmonary valve implantation as assessed at rest (Δ pulmonary regurgitation fraction pre- vs. post-percutaneous pulmonary valve implantation) and the change of left ventricular stroke volume augmentation in response to exercise from pre- to post-percutaneous pulmonary valve implantation (Δ left ventricular stroke volume pre- vs. post-percutaneous pulmonary valve implantation at exercise; r = 0.59, P = 0.009). This mean that patients with a high degree of pulmonary regurgitation fraction prior to percutaneous pulmonary valve implantation and a marked reduction in pulmonary regurgitation fraction post-percutaneous pulmonary valve implantation show less increase in left ventricular stroke volume during exercise from pre- to post-percutaneous pulmonary valve implantation. Right panel: The change in left ventricular stroke volume augmentation in response to exercise from pre- to post-percutaneous pulmonary valve implantation (Δ left ventricular stroke volume pre- vs. post-percutaneous pulmonary valve implantation at exercise) correlated significantly with the change in peak VO2 from pre-percutaneous pulmonary valve implantation to post-percutaneous pulmonary valve implantation as assessed on cardiopulmonary exercise testing (Δ peak VO2 pre- vs. post-percutaneous pulmonary valve implantation; r = 0.77, P < 0.001).
Discussion
The main findings of this study were: (i) patients with PR or PS are unable to augment total RVSV in response to exercise; (ii) patients with PR are still able to augment effective RVSV due to a reduction in PR, which leads to an increased LVSV; (iii) following PPVI, the RV is able to augment SV during exercise in both PR and PS patients; however, (iv) in the PR group, effective RVSV augmentation during exercise was similar pre- and post-PPVI.
Cardiovascular response to exercise pre- and post-percutaneous pulmonary valve implantation
In normal subjects, CO increases during exercise to deal with the increased metabolic demands of the musculature. The increase in CO has chronotropic (increased heart rate) and inotropic elements (increased SV and EF), and its magnitude is related to the amount of work being performed.11 The inotropic effects are particularly important during exercise as right ventricular afterload increases and thus it is necessary for contractility to increase to maintain/increase RVSV. Left ventricular filling is entirely dependant of RVSV (in patients with no shunts) and reduced effective RVSV will lead to reduced LVEDV and LVSV. In PS patients, there was no increase in RVSV or RVEF during exercise pre-PPVI and augmentation of LVCO was driven entirely by heart rate. We speculate that RVSV augmentation is not possible in patients with PS because they are already working at high afterload due to the fixed obstruction. Thus, during submaximal exercise the RV is unable to augment contraction against the further increase in afterload. Furthermore, there may be reduced contractility reserve due to long standing pressure overload. This hypothesis is strengthened by the fact that after PPVI these patients had significantly improved RVSV (and LVSV) augmentation during exercise. Furthermore, the improvement in SV augmentation correlated with the reduction in the RVOT gradient in all patients, irrespective of the predominant physiological lesion. Thus, the removal of the fixed obstruction allows the normal exercise-induced increase in contraction to augment RVSV.
In the PS group of patients, left ventricular CO remained unchanged at the same exercise intensity pre- and post-PPVI. This is unsurprising as the CO augmentation is related to the amount of work being performed. However, after PPVI CO augmentation is driven by both heart rate and SV and as further increases in heart rate are possible, peak LVCO should be increased. An increased peak LVCO should be reflected by an increased peak VO2, which is found in these patients post-PPVI.2
Patients with PR do not show any change in RVSV and RVEF during exercise. However, effective RVSV (LVSV) did increase during exercise due to reduced PRF, which in turn led to improved LV filling and LVSV. This finding is in keeping with a previous report on exercise CMR in patients with free PR and tetralogy of Fallot.12 As speculated by the authors, reduction in PR during exercise can be attributed to increased systemic venous return, shortening of diastole, reduction in pulmonary vascular resistance (enhancing PA forward flow) and cardiorespiratory interactions during exercise. Thus in PR patients, CO augmentation is driven by both increased heart rate and SV. The reasons for the lack of change in RV volumes and function in submaximal exercise are mutlifactorial. However, it may simply be that because effective SV is increased (due to fall in PR) there is no need for a ventricular inotropic response at submaximal exercise. Others possible causes are a reduced RV compliance and impaired filling during a shortened diastole or the reduced RV intrinsic contractility reserve due to longstanding RV volume overload.
In patients with PR, there was improved augmentation of RVSV and RVEF after PPVI. However, augmentation of effective RVSV was of a similar magnitude before and after PPVI. This is in marked contrast to the situation in patients with PS and can be attributed to the fact that patients with PR have ‘physiological’ valve competence (i.e. reduced PRF) during exercise. Therefore, physical valve competence has no real benefit at during exercise. This lack of improvement in effective RVSV means that unlike PS patients there is no improvement in heart rate reserve post-PPVI. Thus, peak LVCO should remain unchanged explaining why these patients do not experience any improvement in peak VO2. Despite no improvement in peak VO2 post-PPVI in patients with PR, the changes in rest haemodynamics and the reduction in volume loading suggest that PPVI has important benefits to the patient as a whole.
Clinical implications
The results of this study shed light on the fundamentally different changes in physiology during exercise in patients with PS vs. PR. Furthermore, it provides insight into how to interpret CPEX data before and after RVOT interventions.
Cardiopulmonary exercise testing in patients with PS relates to RV pressure overload severity as demonstrated by the improvement in peak VO2 with reduction in RVOT gradient post-PPVI. The relationship between RV function, RVOT gradient, and CPEX supports its use as an indicator of PPVI procedural success.
In patients with PR, it is RV contractility, rather than PRF, that contributes to reduced exercise capacity. However, the multi-factorial nature of reduced exercise capacity in these patients limits the usefulness of CPEX data as a reflection of RV contractility. After PPVI, we do not believe that the change or lack of change in exercise capacity should be used to judge procedural success of the treatment. As shown previously, PPVI does reduce PR, but should not impact on RV contractility.2,4,10 Therefore, it might not be surprising that exercise performance remains unchanged post-PPVI, despite a successful procedure in terms of RV volume overload reduction.
Limitations of the study
Since patients with relevant atrioventricular valve regurgitation, aortic regurgitation, or evidence for intracardiac shunting were not included in this study, it seems reasonable to calculated PRF by subtracting LV SV from RV SV. However, some degree of, in particular, tricuspid regurgitation during exercise cannot be excluded and could have confounded the data. However, tricuspid regurgitation during exercise would have led to an overestimation of PRF and therefore attenuation of the demonstrated reduction in PRF during exercise.
The peak heart during exercise on the supine CMR compatible bicycle ergometer was lower than the peak heart rate reached during exercise on the conventional bicycle. Therefore, exercise within in the MR scanner has to be considered to be submaximal. Changes in cardiac physiology during submaximal exercise correlate well with changes in parameters of maximal exercise capacity; nevertheless, patterns of cardiac adaptation to submaximal exercise might differ from those seen at peak exercise.
The relatively small sample size represents an important limitation of this study. This is in particular the case for the subgroup analyses (predominantly PS vs. PR). Further, the heterogeneity of the patient population in terms of age and underlying pathology has to taken in account when interpreting the results. Finally, the majority of patients had undergone more than one open-heart surgery. Multiple open-heart surgery is likely to impact on recovery after RVOT interventions and might have prohibited a more favourable adaption to exercise stress before and in particular after relief of RV pressure or volume overload.
Conclusion
Percutaneous pulmonary valve implantation in patients with PS leads to restoration of reserve in RVEF during exercise stress. In patients with PR, SV augmentation improves only mildly post-PPVI. Improvement in SV augmentation during exercise after PPVI is mainly depending on afterload reduction.
These findings are of importance when CPEX and assessment of exercise capacity are used to guide clinical indications for RVOT interventions and to judge procedural success.
Funding
P.L. was funded by the European Union (Health e Child Initiative) (grant no. 027749). V.M. is funded by the British Heart Foundation (BHF) (grant no. FS/08/012/24454). A.M.T. is funded by the National Institute of Health Research (NIHR), UK (grant no. SRF/08/01/018).
Conflict of interest: P.B. is consultant to Medtronic and NuMed and has received honoraria and royalties for the device described.
References
- 1.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJ. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. doi:10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 2.Lurz P, Giardini A, Taylor AM, Nordmeyer J, Muthurangu V, Odendaal D, Mist B, Khambadkone S, Schievano S, Bonhoeffer P, Derrick G. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. Am J Cardiol. 2010;105:721–726. doi: 10.1016/j.amjcard.2009.10.054. doi:10.1016/j.amjcard.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 3.Coats L, Khambadkone S, Derrick G, Sridharan S, Schievano S, Mist B, Jones R, Deanfield JE, Pellerin D, Bonhoeffer P, Taylor AM. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113:2037–2044. doi: 10.1161/CIRCULATIONAHA.105.591438. doi:10.1161/CIRCULATIONAHA.105.591438. [DOI] [PubMed] [Google Scholar]
- 4.Coats L, Khambadkone S, Derrick G, Hughes M, Jones R, Mist B, Pellerin D, Marek J, Deanfield JE, Bonhoeffer P, Taylor AM. Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. Eur Heart J. 2007;28:1886–1893. doi: 10.1093/eurheartj/ehm181. doi:10.1093/eurheartj/ehm181. [DOI] [PubMed] [Google Scholar]
- 5.Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G, Mist B, Walker F, van Doorn C, Bonhoeffer P, Taylor AM. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118(14 Suppl):S182–S190. doi: 10.1161/CIRCULATIONAHA.107.756825. doi:10.1161/CIRCULATIONAHA.107.756825. [DOI] [PubMed] [Google Scholar]
- 6.Ghez O, Tsang VT, Frigiola A, Coats L, Taylor A, Van Doorn C, Bonhoeffer P, De Leval M. Right ventricular outflow tract reconstruction for pulmonary regurgitation after repair of tetralogy of Fallot. Preliminary results. Eur J Cardiothorac Surg. 2007;31:654–658. doi: 10.1016/j.ejcts.2006.12.031. doi:10.1016/j.ejcts.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Lurz P, Muthurangu V, Schievano S, Nordmeyer J, Bonhoeffer P, Taylor AM, Hansen MS. Feasibility and reproducibility of biventricular volumetric assessment of cardiac function during exercise using real-time radial k-t SENSE magnetic resonance imaging. J Magn Reson Imaging. 2009;29:1062–1070. doi: 10.1002/jmri.21762. doi:10.1002/jmri.21762. [DOI] [PubMed] [Google Scholar]
- 8.Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. doi: 10.1161/CIRCULATIONAHA.107.735779. doi:10.1161/CIRCULATIONAHA.107.735779. [DOI] [PubMed] [Google Scholar]
- 9.Schievano S, Coats L, Migliavacca F, Norman W, Frigiola A, Deanfield J, Bonhoeffer P, Taylor AM. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson. 2007;9:687–695. doi: 10.1080/10976640601187596. doi:10.1080/10976640601187596. [DOI] [PubMed] [Google Scholar]
- 10.Lurz P, Nordmeyer J, Giardini A, Khambadkone S, Muthurangu V, Schievano S, Thambo JB, Walker F, Cullen S, Derrick G, Taylor AM, Bonhoeffer P. Early versus late functional outcome after successful percutaneous pulmonary valve implantation—are the acute effects of altered right ventricular loading all we can expect? J Am Coll Cardiol. 2011;57:724–731. doi: 10.1016/j.jacc.2010.07.056. doi:10.1016/j.jacc.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Roest AA, Kunz P, Lamb HJ, Helbing WA, van der Wall EE, de Roos A. Biventricular response to supine physical exercise in young adults assessed with ultrafast magnetic resonance imaging. Am J Cardiol. 2001;87:601–605. doi: 10.1016/s0002-9149(00)01438-7. doi:10.1016/S0002-9149(00)01438-7. [DOI] [PubMed] [Google Scholar]
- 12.Roest AA, Helbing WA, Kunz P, van den Aardweg JG, Lamb HJ, Vliegen HW, van der Wall EE, de Roos A. Exercise MR imaging in the assessment of pulmonary regurgitation and biventricular function in patients after tetralogy of fallot repair. Radiology. 2002;223:204–211. doi: 10.1148/radiol.2231010924. doi:10.1148/radiol.2231010924. [DOI] [PubMed] [Google Scholar]