Abstract
Background
We used cardiovascular magnetic resonance (CMR) to investigate the association between epicardial adipose tissue (EAT) thickness and silent myocardial ischemia, as well as coronary artery stenosis, in asymptomatic type 2 diabetic patients.
Methods
The study included 100 type 2 diabetic subjects (51 male and 49 female; mean age: 56 ± 7 years). Silent myocardial ischemia, as determined by CMR, was defined as evidence of inducible ischemia or myocardial infarction. Signal reduction or stenosis of ≥ 50% in the vessel diameter was used as the criteria for significant coronary artery stenosis on coronary magnetic resonance (MR) angiography.
Results
EAT thickness was positively correlated with body mass index (BMI), waist-to-hip ratio, systolic blood pressure, postprandial glucose, fasting/postprandial triglyceride (TG), serum glycated hemoglobin (HbA1c) level, and homeostasis model assessment of insulin resistance (HOMA-IR) score. Significant coronary artery stenosis was found in 24 patients, while 14 patients had silent myocardial ischemia in CMR (1 with silent myocardial infarction, 11 with inducible ischemia, and 2 with both). EAT thickness was greater in patients who had coronary artery stenosis (13.0 ± 2.6 mm vs. 11.5 ± 2.1 mm, p = 0.01), but did not differ between the subjects with or without silent myocardial ischemia on CMR images (12.8 ± 2.1 vs. 11.7 ± 2.3 mm, p = 0.11). Multivariate logistic regression analysis indicated that EAT thickness was an independent indicator for significant coronary artery stenosis after adjusting for traditional risk factors (OR 1.403, p = 0.026).
Conclusions
Increased EAT thickness assessed by CMR is an independent risk factor for significant coronary artery stenosis in asymptomatic type 2 diabetes. However, EAT thickness was not associated with silent myocardial ischemia.
Keywords: Epicardial adipose tissue, Cardiovascular magnetic resonance, Silent ischemia, Coronary artery stenosis, Type 2 diabetes
Background
Epicardial adipose tissue (EAT) is a metabolically active visceral fat deposit found around the heart, between the pericardium and myocardium [1]. Transthoracic echocardiography (TTE) and multi-slice computed tomography (MSCT) scanning have been conventional methods for quantifying EAT [2]. Growing evidence suggests that EAT has clinical relevance in that it produces several proatherogenic molecules and influences the development and progression of coronary artery disease (CAD) [1,3,4]. Bettencourt et al. showed that EAT volume assessed by MSCT is positively and independently related to coronary atherosclerotic burden [5]. Similarly, Harada et al. reported that EAT volume is significantly increased in patients with acute coronary syndrome [6]. However, EAT images do not provide information on the functional status of myocardial cells such as viability, ischemia, and infarction.
Over the past several years, cardiac magnetic resonance (CMR) has been increasingly used for the assessment of patients with ischemic heart disease [7]. CMR offers a functional analysis of myocardial first-pass perfusion and delayed gadolinium enhancement for detection of myocardial infarction, as well as luminal assessment of the coronary artery. Recently, several trials have evaluated the association between epicardial or pericardial fat assessed by CMR and vascular function [8] or inflammatory markers [9] in subjects with obesity and metabolic syndrome. In the present study, we evaluated patients with diabetes mellitus, another important risk factor for CAD [10]. Specifically, we used CMR to assess the association between EAT thickness and myocardial ischemia, as well as coronary artery stenosis, in asymptomatic type 2 diabetic patients.
Methods
Subjects
The study included 100 Korean asymptomatic diabetic patients who were enrolled in a prospective trial that evaluated the role of endothelial progenitor cell count in CAD [11]. Study methods were described in detail in previous reports. None of the participants had a history of cardiovascular disease (angina, myocardial infarction (MI), cerebrovascular diseases (CVD), or peripheral artery disease (PAD)), malignancy, or severe renal or hepatic disease. They answered the Rose questionnaire [12] and reported no chest pain or equivalent symptoms. The Institutional Review Board of Yonsei University College of Medicine approved this study, and all subjects provided informed consent in accordance with the ethical committee and the Korean Good Clinical Practice guidelines.
The patients’ records were reviewed to verify the duration of diabetes. The body mass index (BMI) was calculated as weight divided the square of height (kg/m2), and waist circumference was measured at the midpoint between the lateral iliac crest and the lowest rib. Hip circumference was measured at the maximal protrusion of the greater trochanter, and waist-to-hip ratio (WHR) was calculated as the ratio of waist-to-hip circumference. Blood pressure was measured twice with a mercury sphygmomanometer on the right upper arm after resting for at least 10 minutes in sitting position, taking the average values of systolic and diastolic blood pressures. Metabolic syndrome was defined according to criteria of the Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program (NCEP’s). The modified ATP III definition of metabolic syndrome requires the presence of at least three or more of the following five components: 1) elevated waist circumference ≥ 90 cm (male), ≥ 80 cm (female); 2) elevated triglyceride (TG) > 150 mg/dL (1.695 mmol/L); 3) reduced high-density lipoprotein (HDL) cholesterol < 40 mg/dL (male), < 50 mg/dL (female); 4) hypertension or elevated blood pressure ≥ 130/85 mm Hg; or 5) diabetes or elevated fasting plasma glucose ≥ 5.6 mmol/L [13].
Laboratory methods were described in detail in previous reports [11]. Briefly, all blood samples were obtained in the morning after a 12-hour overnight fast, and we performed a standardized mixed meal stimulation test in all subjects using commercial liquid nutritional supplements (Ensure, Meiji Dairies Corporation, Tokyo, Japan; total 500 kcal, 17.5 g fat, 68.5 g carbohydrate, and 17.5 g protein) [14]. Blood samples were collected at 0 and 90 min after taking the liquid supplement (basal and stimulated levels, respectively) for glucose, TG, insulin, and C-peptide analyses. Plasma glucose was measured using the glucose oxidase method. Plasma TG, total cholesterol, HDL- cholesterol, blood urea nitrogen, creatinine, AST, and ALT levels were assayed using a routine Hitachi 7600 autoanalyzer (Hitachi Instruments Service, Tokyo, Japan). Low-density lipoprotein cholesterol (LDL- cholesterol) was calculated using the Friedewald equation. Serum glycated albumin (GA) was determined by an enzymatic method using an albumin-specific proteinase, ketoamine oxidase, albumin assay reagents (LUCICA GA-L, Asahi Kasei Pharma Co., Tokyo, Japan), and a Hitachi 7699 Pmodule autoanalyzer. The coefficient of variation (CV) was 1.43%. Serum glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography (HPLC) using a Variant II Turbo system (Bio-Rad Laboratories, Hercules, CA). The reference interval for HbA1c was between 4.0 and 6.0%, while that for GA was between 11.0 and 16.0%. Serum insulin and C-peptide levels were measured in duplicate using an immunoradiometric assay (IRMA) method (Beckman Coulter, Fullerton, CA). The homeostasis model assessment of insulin resistance (HOMA-IR) was computed as follows: fasting insulin (μIU/ml) × fasting glucose (mmol/ml)/22.5.
Cardiac magnetic resonance (CMR) examination
CMR was performed using a 3.0 Tesla magnetic resonance imaging (MRI) unit (Achieva 3.0 T TX, Philips Medical Systems, The Netherlands) with a 32-channel receiver coil. Myocardial ischemia was evaluated based on first-pass myocardial perfusion MR images acquired during adenosine stress (140 μg/kg/min for 3 minutes) and at rest with bolus injection of 0.01 mmol/kg of gadopentetate dimeglumine (Magnevist®, Bayer Schering Pharma, Berlin, Germany) for each session. After the perfusion study, whole heart coronary MR angiography (3-dimensional turbo field echo sequence with navigator gating) was performed without additional injection of the contrast medium. Cine MRI was performed with a balanced steady-state free-precession sequence along the cardiac short axis and horizontal long axis. Delayed enhancement MRI was obtained to assess myocardial viability using a 3-dimensional phase-sensitive inversion recovery sequence 10 minutes after the rest perfusion study.
Image analysis and epicardial adipose tissue (EAT) quantification
CMR images were independently evaluated by two experienced cardiac radiologists, who were not informed of the identity or any clinical information about the patients. After independent evaluation of the images, final conclusions were made by consensus interpretation. Abnormal findings on CMR included inducible ischemia, myocardial infarction, global left ventricular (LV) systolic dysfunction, and coronary artery stenosis. Inducible ischemia was defined as a perfusion deficit induced by adenosine stress on first-pass perfusion images that was reversed on resting perfusion images with no evidence of delayed myocardial hyper-enhancement on delayed enhancement images. Myocardial infarction was defined as an area with hyper-enhancement consistent with coronary distribution on delayed enhancement MRI. Silent myocardial ischemia by CMR was defined as an evidence of inducible ischemia or myocardial infarction. Global LV systolic dysfunction was defined as LV ejection fraction < 45% measured from cine MRI. Based on coronary MR angiography, the degree of stenosis was qualitatively defined as significant, suspected, minimal, or normal. Focal areas of marked signal loss, signal reduction, or stenosis of ≥ 50% in the vessel diameter were used as the criteria for significant stenosis [13,14].
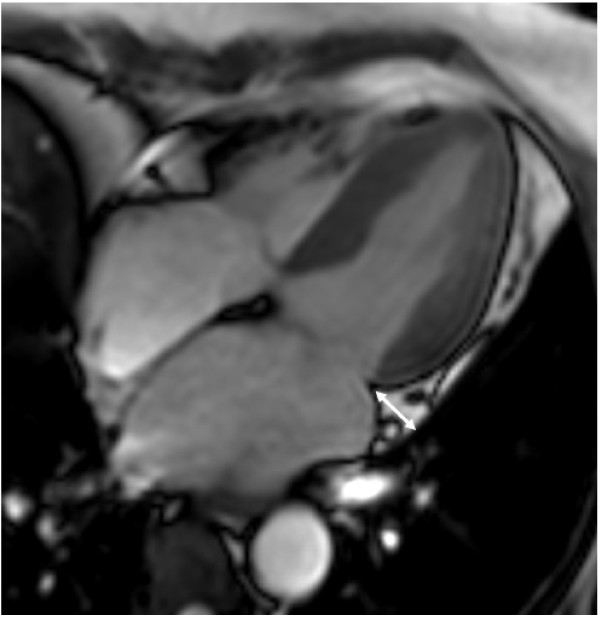
Fat thickness in the left atrioventricular groove was measured as previously mentioned in studies by other investigators [15,16]. The measurements were performed at the end-diastolic phase on the horizontal long-axis plane in cine MRI. Maximal EAT thickness was determined as measured from the myocardial surface to the pericardium (perpendicular to the pericardium) (Figure 1).
Figure 1.
Measurement of EAT thickness using cardiovascular magnetic resonance. Epicardial adipose tissue thickness in the left atrioventricular groove was measured in the horizontal long-axis plane in the end-diastolic phase. Maximal EAT thickness was determined from the myocardial surface to the visceral pericardium.
Statistical analysis
All statistical analyses were performed with PASW statistics software (version 18.0; SPSS Inc., Chicago, IL). Continuous variables with a normal distribution were expressed as mean ± SD, and discrete variables were expressed as percentages. The relationship between EAT thickness and other metabolic parameters was examined using Pearson’s correlation coefficient (R). Statistical comparisons between groups according to significant coronary artery stenosis or silent myocardial ischemia were performed using the Student’s t test or Mann–Whitney U test for continuous variables and a χ2 test for discrete variables. We then used multivariate logistic regression analysis to assess whether the associations between EAT thickness and the presence of significant coronary artery stenosis or silent myocardial ischemia were independent of age, gender, and traditional coronary risk factors. A P-value < 0.05 was considered significant.
Results
Baseline characteristics of the study participants
Table 1 shows the baseline characteristics of the study participants. The final population consisted of 100 subjects (51 males and 49 females; mean age 56 ± 7 years). Their mean duration of diabetes was 8.4 ± 6.7 years. The levels of serum HbA1c and GA were 7.0 ± 0.9% and 16.7 ± 3.9%, respectively. The mean body mass index was 25.3 ± 3.2 kg/m2 (range: 18.7 – 35.6 kg/m2), and the mean WHR was 0.91 ± 0.06 (range: 0.78 – 1.10). Of these 100 patients, 78 had metabolic syndrome. A total of 24 patients had significant coronary artery stenosis defined as stenosis ≥ 50% on coronary MR angiography, and 14 patients had silent myocardial ischemia on CMR images (1 with silent myocardial infarction, 11 with inducible ischemia, and 2 with both). Ten patients had both silent myocardial ischemia and significant coronary artery stenosis. The average thickness of the layer of EAT at the left atrioventricular groove was 11.9 ± 2.3 mm (range: 6.8 – 17.9 mm, 12.0 ± 2.1 mm in males and 11.8 ± 2.5 mm in females). The mean LV ejection fraction was 68 ± 3%, and all patients had preserved LV systolic function (range: 56 – 77%). The mean LV mass was 83 ± 21 g (range: 43 – 165), which was significantly greater in males than in females (94 ± 19 g vs. 71 ± 16 g, p<0.001). None of the women was a smoker in this study.
Table 1.
Baseline characteristics of study participants
| Total (n = 100) | Male (n = 51) | Female (n = 49) | p value | |
|---|---|---|---|---|
| Age (years) |
56 ± 7 |
55 ± 7 |
57 ± 7 |
0.277 |
| Body-mass index (kg/m2) |
25.3 ± 3.2 |
25.1 ± 3.2 |
25.6 ± 3.3 |
0.434 |
| Waist circumference (cm) |
86.2 ± 8.0 |
87.7 ± 7.2 |
84.6 ± 8.5 |
0.059 |
| Waist-to-hip ratio |
0.91 ± 0.06 |
0.91 ± 0.05 |
0.90 ± 0.06 |
0.303 |
| Systolic blood pressure (mmHg) |
130 ± 13 |
133 ± 13 |
127 ± 12 |
0.013* |
| Diastolic blood pressure (mmHg) |
76 ± 9 |
79 ± 9 |
73 ± 9 |
0.002** |
| DM duration (years) |
8.4 ± 6.7 |
8.5 ± 7.1 |
8.2 ± 6.3 |
0.803 |
| Fasting glucose (mmol/L) |
6.7 ± 1.7 |
6.9 ± 1.8 |
6.6 ± 1.5 |
0.398 |
| Postprandial glucose (mmol/L) |
12.5 ± 3.8 |
11.8 ± 3.9 |
13.1 ± 3.7 |
0.104 |
| HbA1c (%) |
7.0 ± 0.9 |
7.0 ± 1.0 |
7.1 ± 0.8 |
0.801 |
| GA (%) |
16.7 ± 3.9 |
16.8 ± 4.3 |
16.6 ± 3.4 |
0.782 |
| Total cholesterol (mmol/L) |
4.3 ± 0.8 |
4.2 ± 0.8 |
4.5 ± 0.8 |
0.056 |
| LDL cholesterol (mmol/L) |
2.5 ± 0.8 |
2.4 ± 0.6 |
2.6 ± 0.9 |
0.115 |
| HDL cholesterol (mmol/L) |
1.1 ± 0.2 |
1.1 ± 0.2 |
1.2 ± 0.2 |
0.101 |
| Triglyceride (mmol/L) |
1.5 ± 0.7 |
1.4 ± 0.6 |
1.6 ± 0.8 |
0.139 |
| HOMA-IR |
4.9 ± 7.8 |
3.0 ± 0.2 |
6.6 ± 1.5 |
0.032* |
| Cardiovascular magnetic resonance |
|
|
|
|
| EAT thickness (mm) |
11.9 ± 2.3 |
12.0 ± 2.1 |
11.8 ± 2.5 |
0.738 |
| LV ejection fraction (%) |
68 ± 3 |
67 ± 3 |
69 ± 3 |
0.026* |
| LV mass (g) |
83 ± 21 |
94 ± 19 |
71 ± 16 |
<0.001*** |
| |
|
n (%) |
|
|
| Hypertension |
45 (45.0) |
20 (39.2) |
25 (51.0) |
0.184 |
| Smoking Hx |
18 (18.0) |
18 (36.7) |
0 (0) |
<0.001*** |
| Cardiovascular magnetic resonance |
|
|
|
|
| Silent myocardial ischemia |
14 (14.0) |
7 (13.7) |
7 (14.3) |
0.581 |
| Silent infarction |
3 (3.0) |
2 (3.9) |
1 (2.0) |
0.515 |
| Inducible ischemia |
13 (13.0) |
6 (11.8) |
7 (14.3) |
0.469 |
| Both |
2 (2.0) |
1(1.9) |
1(2.0) |
0.742 |
| Significant stenosis | 24 (24.0) | 14 (27.5) | 10 (20.4) | 0.278 |
Data are presented as mean ± s.d. or n(%). DM diabetes mellitus; HbA1c glycated hemoglobin; GA glycated albumin; LDL low-density lipoprotein; HDL high-density lipoprotein; HOMA-IR homeostasis model assess of insulin resistance; EAT epicardial adipose tissue; LV left ventricle.
* p<0.05, **p<0.01, ***p<0.001.
Subjects with any anatomical (stenosis ≥ 50%) or functional abnormality had a consultation with a specialized cardiologist. Among 28 patients recommended to undergo invasive coronary angiography, 20 patients were submitted for invasive coronary angiography, and 8 were submitted for revascularization (7 percutaneous coronary interventions and 1 coronary artery bypass graft).
Correlation of EAT thickness with metabolic parameters
EAT thickness was positively and independently correlated with metabolic parameters including BMI, waist circumference, waist-to-hip ratio, systolic blood pressure, postprandial glucose, fasting/postprandial triglyceride, HbA1c level, and HOMA-IR. However, EAT thickness was not correlated with either LDL-cholesterol or HDL-cholesterol levels (Table 2).
Table 2.
Metabolic parameters that correlated independently with EAT thickness based on simple regression analysis
| |
Simple regression analysis |
|
|---|---|---|
| r | P value | |
| Age |
0.042 |
0.677 |
| Body-mass index |
0.26 |
0.009** |
| Waist circumference |
0.297 |
0.003** |
| Waist-to-hip ratio |
0.404 |
< 0.001*** |
| Systolic blood pressure |
0.223 |
0.027* |
| Diastolic blood pressure |
0.175 |
0.085 |
| Fasting glucose |
0.105 |
0.298 |
| Postprandial glucose |
0.197 |
0.049* |
| Fasting TG |
0.256 |
0.01* |
| Postprandial TG |
0.272 |
0.009** |
| Fasting insulin |
0.193 |
0.058 |
| HbA1c |
0.324 |
< 0.001*** |
| GA |
0.19 |
0.065 |
| LDL cholesterol |
−0.012 |
0.904 |
| HDL cholesterol |
−0.115 |
0.254 |
| HOMA-IR | 0.21 | 0.047* |
HbA1c glycated hemoglobin; GA glycated albumin; LDL low-density lipoprotein; HDL high-density lipoprotein; HOMA-IR homeostasis model assess of insulin resistance; EAT epicardial adipose tissue; LV left ventricle.
* p<0.05, **p<0.01, ***p<0.001.
Association of EAT thickness with significant coronary artery stenosis or silent myocardial ischemia
We divided the subjects into two different classes: 1) subjects with or without significant coronary artery stenosis, and 2) subjects with or without silent myocardial ischemia. As shown in Table 3, patients with significant coronary artery stenosis had significantly higher age, WHR, systolic blood pressure, total cholesterol, and GA than those without significant coronary artery stenosis. EAT thickness was significantly higher in the patients with stenosis than without stenosis (13.0 ± 2.6 mm vs. 11.5 ± 2.1 mm, p = 0.01). When subjects were divided based on presence of myocardial ischemia, only BMI was statistically different between the patients with and without silent myocardial ischemia (27.3 ± 3.8 kg/m2vs. 25.0 ± 3.0 kg/m2, p = 0.033) (Table 4A). In a subgroup analysis based on gender, female patients with myocardial ischemia had a higher EAT thickness than those without ischemia (14.1 mm (IR 2.5) vs. 11.5 mm (IR 3.4), p = 0.031). Similar EAT thickness was found between males with ischemia and without ischemia (11.9 mm (IR 4.2) vs. 12.5 mm (IR 2.9), p = 0.947) (Table 5). Multivariate logistic regression analysis revealed that EAT thickness was an independent indicator of significant coronary artery stenosis as assessed by coronary MR angiography, after adjusting for traditional risk factors (OR 1.403, p = 0.026) (Table 6).
Table 3.
A comparison of significant coronary artery stenosis using coronary MR angiography
| Significant coronary artery stenosis (n = 24) | No significant coronary artery stenosis (n = 76) | p value | |
|---|---|---|---|
| Age (years) |
60 ± 6 |
55 ± 7 |
0.002** |
| Body-mass index (kg/m2) |
26.1 ± 3.6 |
25.1 ± 3.1 |
0.240 |
| Waist-to-hip ratio |
0.93 ± 0.05 |
0.90 ± 0.06 |
0.015* |
| Systolic blood pressure (mmHg) |
137 ± 13 |
128 ± 12 |
0.009** |
| Diastolic blood pressure (mmHg) |
76 ± 9 |
77 ± 9 |
0.492 |
| DM duration (years) |
9.4 ± 6.6 |
8.0 ± 6.7 |
0.261 |
| Smoking history (n, %) |
4 (16.7) |
14 (18.9) |
0.536 |
| Fasting glucose (mmol/L) |
7.0 ± 1.9 |
6.6 ± 1.6 |
0.617 |
| Postprandial glucose (mmol/L) |
13.0 ± 3.1 |
12.3 ± 4.0 |
0.26 |
| HbA1c (%) |
7.1 ± 0.9 |
7.0 ± 0.9 |
0.731 |
| GA (%) |
17.8 ± 3.4 |
16.4 ± 4.0 |
0.039* |
| Total cholesterol (mmol/L) |
4.7 ± 0.7 |
4.3 ± 0.8 |
0.038* |
| LDL cholesterol (mmol/L) |
2.8 ± 0.9 |
2.4 ± 0.7 |
0.104 |
| HDL cholesterol (mmol/L) |
1.1 ± 0.2 |
1.1 ± 0.2 |
0.753 |
| Triglyceride (mmol/L) |
1.6 ± 0.7 |
1.4 ± 0.7 |
0.212 |
| HOMA-IR |
7.0 ± 11.7 |
4.3 ± 6.2 |
0.22 |
| Cardiovascular magnetic resonance |
|
|
|
| EAT thickness (mm) |
13.0 ± 2.6 |
11.5 ± 2.1 |
0.01** |
| LV ejection fraction (%) |
69 ± 4 |
68 ± 3 |
0.488 |
| LV mass (g) | 90 ± 24 | 81 ± 19 | 0.065 |
Data are presented as mean ± s.d. or n(%). DM diabetes mellitus; HbA1c glycated hemoglobin; GA glycated albumin; LDL low-density lipoprotein; HDL high-density lipoprotein; HOMA-IR homeostasis model assess of insulin resistance; EAT epicardial adipose tissue; LV left ventricle.
* p<0.05, **p<0.01, ***p<0.001.
Table 4.
A comparison of silent myocardial ischemia based on CMR findings
| Silent myocardial ischemia (n = 14) | No silent myocardial ischemia (n = 86) | p value | |
|---|---|---|---|
| Age (years) |
58 ± 8 |
56 ± 7 |
0.278 |
| Body-mass index (kg/m2) |
27.3 ± 3.8 |
25.0 ± 3.0 |
0.033* |
| Waist-to-hip ratio |
0.94 ± 0.06 |
0.90 ± 0.05 |
0.087 |
| Systolic blood pressure (mmHg) |
133 ± 9 |
130 ± 13 |
0.358 |
| Diastolic blood pressure (mmHg) |
74 ± 6 |
77 ± 10 |
0.305 |
| DM duration (years) |
10.1 ± 7.5 |
8.1 ± 6.5 |
0.396 |
| Smoking history (n, %) |
2 (14.3) |
16 (19.0) |
0.503 |
| Fasting glucose (mmol/L) |
6.3 ± 2.0 |
6.8 ± 1.6 |
0.072 |
| Postprandial glucose (mmol/L) |
13.4 ± 3.6 |
12.3 ± 3.9 |
0.200 |
| HbA1c (%) |
7.2 ± 0.8 |
7.0 ± 0.9 |
0.236 |
| GA (%) |
17.9 ± 3.9 |
16.5 ± 3.9 |
0.203 |
| Total cholesterol (mmol/L) |
4.4 ± 0.9 |
4.3 ± 0.8 |
0.941 |
| LDL cholesterol (mmol/L) |
2.7 ± 1.3 |
2.4 ± 0.7 |
0.602 |
| HDL cholesterol (mmol/L) |
1.2 ± 0.2 |
1.1 ± 0.2 |
0.453 |
| Triglyceride (mmol/L) |
1.3 ± 0.5 |
1.5 ± 0.7 |
0.295 |
| HOMA-IR |
4.4 ± 3.5 |
5.0 ± 8.2 |
0.649 |
| Cardiovascular magnetic resonance |
|
|
|
| EAT thickness (mm) |
12.8 ± 2.1 |
11.7 ± 2.3 |
0.111 |
| LV ejection fraction (%) |
69 ± 5 |
68 ± 3 |
0.353 |
| LV mass (g) | 87 ± 28 | 82 ± 19 | 0.739 |
Data are presented as mean ± s.d. or n(%). DM diabetes mellitus; HbA1c glycated hemoglobin; GA glycated albumin; LDL low-density lipoprotein; HDL high-density lipoprotein; HOMA-IR homeostasis model assess of insulin resistance; EAT epicardial adipose tissue; LV left ventricle.
* p<0.05, **p<0.01, ***p<0.001.
Table 5.
Subgroup analysis based on gender
| |
Male (n = 51) |
Female (n = 49) |
||||
|---|---|---|---|---|---|---|
| Silent myocardial ischemia (n = 7) | No silent myocardial ischemia (n = 44) | p value | Silent myocardial ischemia (n = 7) | No silent myocardial ischemia (n = 7) | p value | |
| Age (years) |
59 |
55 |
0.339 |
58.5 |
61 |
0.585 |
| Body-mass index (kg/m2) |
26.6 |
25.3 |
0.444 |
28.8 |
25.0 |
0.016* |
| Waist-to-hip ratio |
0.90 |
0.92 |
0.968 |
0.93 |
0.88 |
0.042* |
| Systolic blood pressure (mmHg) |
136 |
135 |
0.870 |
135 |
126 |
0.225 |
| Diastolic blood pressure (mmHg) |
81 |
77 |
0.203 |
73 |
73 |
0.932 |
| DM duration (years) |
6 |
6 |
0.725 |
12 |
5 |
0.183 |
| Fasting glucose (mmol/L) |
6.4 |
6.4 |
0.367 |
5.5 |
6.4 |
0.121 |
| Postprandial glucose (mmol/L) |
13.2 |
11.7 |
0.698 |
15.1 |
12.7 |
0.198 |
| HbA1c (%) |
6.9 |
6.8 |
0.989 |
7.4 |
6.95 |
0.069 |
| GA (%) |
15.6 |
15.5 |
0.942 |
18.6 |
15.5 |
0.096 |
| Total cholesterol (mmol/L) |
3.9 |
4.2 |
0.511 |
4.4 |
4.3 |
0.51 |
| LDL cholesterol (mmol/L) |
2.3 |
2.3 |
0.989 |
2.7 |
2.5 |
0.566 |
| HDL cholesterol (mmol/L) |
1.1 |
1.1 |
0.799 |
1.2 |
1.1 |
0.424 |
| Triglyceride (mmol/L) |
1.0 |
1.3 |
0.229 |
1.3 |
1.4 |
0.748 |
| HOMA-IR |
2.7 |
2.6 |
1.000 |
5.2 |
3.0 |
0.586 |
| Cardiovascular magnetic resonance |
|
|
|
|
|
|
| EAT thickness (mm) | 11.9 | 12.5 | 0.947 | 14.1 | 11.5 | 0.031* |
Data are presented as median. DM diabetes mellitus; HbA1c glycated hemoglobin; GA glycated albumin; LDL low-density lipoprotein; HDL high-density lipoprotein; HOMA-IR homeostasis model assess of insulin resistance; EAT epicardial adipose tissue.
* p<0.05, ** p≤0.01, *** p≤0.001.
Table 6.
Association between EAT thickness and significant coronary artery stenosis based on multivariate logistic regression analysis
| OR | 95% CI for OR | P value for EAT effect | |
|---|---|---|---|
| Unadjusted |
1.340 |
1.073 - 1.674 |
0.01** |
| Adjusted for |
|
|
|
| Age, gender |
1.334 |
1.062 - 1.677 |
0.013* |
| Age, gender, BMI |
1.262 |
0.993 - 1.603 |
0.057 |
| Age, gender, BMI, HbA1c, LDL, systotlic blood pressure, smoking history | 1.403 | 1.042 - 1.890 | 0.026* |
BMI body mass index; HbA1c glycated hemoglobin; LDL low-density lipoprotein.
* p<0.05, ** p≤0.01, *** p≤0.001.
Discussion
Numerous studies have implicated epicardial adipose tissue (EAT) in the development and aggravation of CAD [1,3,4,17]. However, many questions still remain to be answered regarding the optimal approach to take for evaluating the significance of EAT, including its pathophysiological roles in ischemic heart disease, method for quantification, and clinical relevance on ischemic heart disease. Paracrine interaction between EAT and the coronary arteries might underlie the observed pathogenesis [3]. One possible mechanism might involve the increased activity of an adipokine associated with EAT. Adipose tissue secretes various adipokines, including leptin, adiponectin, resistin, TNF-a, and chemerin. Recent studies have shown that CAD in humans is associated with a decreased circulating adiponectin level [18] or increased expression of chemerin, at both the protein and mRNA levels, in EAT [19]. In addition, EAT also shows an association with metabolic syndrome, as shown by Yorgun et al.[20]. Park et al.[21] recently reported that EAT was a powerful predictor of metabolic syndrome and CAD in patients with BMI < 27 kg/m2, indicating that the measurement of EAT may be more useful in Asian populations with low BMI than in obese subjects.
In the present study, we measured the EAT thickness at the end-diastolic phase on the horizontal long-axis plane in cine MRI. Nelson et al. [22] validated the usefulness of measuring pericardial fat in cine sequences. Recently, Okayama et al. [23] reported a novel technique for improved quantification of EAT, especially in the subjects with elevated T2 signals including pericardial effusion or myocardial edema. However, we checked the left ventricular (LV) function, and found that it was preserved in all patients. None of our subjects showed pericardial effusion or myocardial edema, indicating that cine sequences provided optimal measurements of EAT in our study population.
Numerous studies have focused on the quantification of EAT, but the best imaging tool and the most optimal parameter for EAT measurement remain uncertain [24]. Many investigators have used echocardiography because of its easy availability and safety [25], while others have preferred to use CT scans [26]. However, echocardiography has the inherent problem of a limited acoustic window and variability between operators, while CT scans result in inevitable, harmful exposure to radiation. For these reasons, CMR is now gaining popularity as a promising method for evaluation of CAD, primarily because of its non-invasive nature and safe characteristics, especially in diabetic patients who face an increased risk of contrast-induced nephropathy [27].
Controversy still exists regarding which measurement of EAT best reflects its metabolic role. Wang et al. reported that EAT thickness of the left AV groove provides a more accurate assessment of metabolic risk [15] and confirmed this with consistent results [16]. Nevertheless, evidence to clarify the clinical relevance of EAT on ischemic heart disease remains insufficient. We addressed the discrepancy between CAD and myocardial ischemia, as well as the relevance of EAT on myocardial ischemia, by adopting the novel evaluation method of cardiac magnetic resonance (CMR), which offers functional analysis of myocardial ischemia as well as luminal assessment of the coronary artery. The diagnostic accuracy of CMR, is well known, especially with respect to combined stress perfusion and delayed enhancement infarction imaging. Klein et al.[28] and Seeger et al.[29] recently suggested a possible role for coronary MR angiography in CAD diagnosis.
The present study has four main findings. First, it verifies that EAT thickness measured by CMR is closely associated with significant CAD evaluated by coronary MR angiography. Second, it demonstrates that EAT is not associated with silent myocardial ischemia, as assessed by combined stress perfusion and delayed enhancement infarction in CMR. Third, it shows that higher EAT thickness is an independent risk factor for significant CAD, after adjusting for traditional risk factors such as age, gender, BMI, HbA1c, and blood pressure. Fourth, it shows a gender-based discrepancy in the association between EAT thickness and myocardial ischemia.
Clinical relevance of EAT on ischemic heart disease was indicated from our data, which showed that EAT measured by CMR was statistically related to insulin resistance-related metabolic parameters, such as BMI, waist circumference, waist-to-hip ratio, systolic blood pressure, postprandial glucose, fasting/postprandial TG, HbA1c level, and HOMA-IR score. This finding is consistent with data from previous studies that used echocardiography [30] or CT [31] for assessment. Similar to previous reports, EAT thickness was significantly associated with CAD, as evaluated by coronary MR angiography. Therefore, EAT might be postulated to have a major role in the progression of atherosclerosis in the coronary arteries. However, our results conflict with those reported by Tamarappoo et al.[32], who showed that pericardial fat volume measured by CT was significantly associated with myocardial ischemia assessed by single photon emission computed tomography (SPECT). In the present study, EAT was not associated with silent myocardial ischemia as assessed by CMR in this study. These conflicting results may be due to differences in the study participants, the anatomical site of EAT measurement, and imaging modality. The participants in our study were asymptomatic type 2 diabetic patients whose disease was being relatively well controlled (mean HbA1c = 7.0 ± 0.9%), which could have weakened the association between EAT and silent myocardial ischemia.
Interestingly, EAT thickness was significantly associated with silent myocardial ischemia in the female population in our study. Some plausible explanations for this finding include hormonal influences on adipose tissue metabolism [33] or differential expression of adiponectin and leptin in EAT [34]. Fei et al.[35] showed age- and sex-matched changes in EAT adipokines in a rodent model, where aging had a more potent impact on EAT-derived mediators in female rats than in male rats. In our study population, the male group, which had a higher proportion of smokers, showed higher systolic / diastolic blood pressure and insulin sensitivity than did the female group. Compared to the male group, females showed significantly less left ventricular mass (94 ± 19 g vs. 71 ± 16 g, p<0.001) despite similar EAT thickness (12.0 ± 2.1 mm vs. 11.8 ± 2.5 mm) (Table 1). In the male group, no difference was observed in main metabolic parameters between subjects with and without myocardial ischemia, while in female group, subjects with myocardial ischemia were more obese and had more poorly controlled glucose when compared to those without ischemia. These differences may partly explain the gender discrepancy; however, no clear reasons were apparent that would explain why the discrepancy was manifested only for myocardial ischemia and not for coronary artery stenosis.
This study had some limitations. First, this was a retrospective analysis; therefore, we were unable to explain the causal relationship between EAT and CAD or myocardial ischemia. Second, we did not investigate any inflammation markers. The pathophysiological mechanism of CAD has been suggested to involve the release of inflammatory signals from epicardial or perivascular adipose tissue, which then promote atherogenesis in the coronary arteries [3]. Many studies have shown an association between inflammatory markers and EAT [36]. Third, we could not perform a volumetric analysis of epicardial fat. However, volumetric assessment is time consuming and requires a skilled observer with sufficient knowledge of cardiac anatomy [31]. Several recent studies have reported a correlation between the EAT thickness or EAT area and metabolic or cardiovascular parameters [37]. Lastly, we were unable to confirm the association between the results of CMR and luminal coronary stenosis because of the absence of angiographic data for all of the patients in this study.
Conclusions
We demonstrated that increased EAT thickness assessed by CMR was an independent risk factor for significant coronary artery stenosis in asymptomatic type 2 diabetes patients. However, this thickness was not associated with silent myocardial ischemia. In a subgroup analysis, EAT thickness was associated with silent myocardial ischemia in females but not in males. Further studies are warranted to discover why EAT is associated with coronary stenosis but not with myocardial ischemia, in asymptomatic type 2 diabetic patients. In addition, the possible gender-based differences in the role of EAT should be further evaluated.
Abbreviations
EAT, Epicardial adipose tissue; TTE, Transthoracic echocardiography; MSCT, Multi-slice computed tomography; CAD, Coronary artery disease; CMR, Cardiovascular magnetic resonance; MI, Myocardial infarction; CVD, Cerebrovascular diseases; PAD, Peripheral artery disease; BMI, Body mass index; WHR, Waist-to-hip ratio; HbA1c, Glycated hemoglobin; GA, Glycated albumin; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; HOMA-IR, Homeostasis model assessment of insulin resistance; MRI, Magnetic resonance imaging; LV, Left ventricular; SPECT, Single photon emission computed tomography.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HMK researched data and wrote/edited the manuscript. KJK reviewed the manuscript. HJL performed the radiologic analysis. HTY contributed discussion. JHM researched data. ESK contributed discussion. BSC contributed discussion. HCL contributed discussion. BWL reviewed/edited the manuscript. YJK performed the radiologic analysis and reviewed/edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Hyun Min Kim, Email: als81@yuhs.ac.
Kwang Joon Kim, Email: PREPPIE@yuhs.ac.
Hye-Jeong Lee, Email: IAMOTWO@yuhs.ac.
Hee Tae Yu, Email: heetyu@gmail.com.
Jae Hoon Moon, Email: jaemoon76@gmail.com.
Eun Seok Kang, Email: EDGO@yuhs.ac.
Bong Soo Cha, Email: bscha@yuhs.ac.
Hyun Chul Lee, Email: ENDOHCLEE@yuhs.ac.
Byung-Wan Lee, Email: bwanlee@yuhs.ac.
Young Jin Kim, Email: dryj@yuhs.ac.
Acknowledgments
This study was supported by the National Research Foundation of Korea Grant funded bythe Korean Government (MEST) (NRF-2010-0003277 and NRF-2009-0064591).
References
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F, Xara S, Leite-Moreira A, Nagel E, Gama V. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158:26–32. doi: 10.1016/j.ijcard.2010.12.085. [DOI] [PubMed] [Google Scholar]
- Harada K, Amano T, Uetani T, Tokuda Y, Kitagawa K, Shimbo Y, Kunimura A, Kumagai S, Yoshida T, Kato B. et al. Cardiac 64-multislice computed tomography reveals increased epicardial fat volume in patients with acute coronary syndrome. Am J Cardiol. 2011;108:1119–1123. doi: 10.1016/j.amjcard.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Oshinski JN, Delfino JG, Sharma P, Gharib AM, Pettigrew RI. Cardiovascular magnetic resonance at 3.0 T: current state of the art. J Cardiovasc Magn Reson. 2010;12:55. doi: 10.1186/1532-429X-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberg FL, Chen Z, Hua N, Bigornia S, Guo Z, Hallock K, Jara H, LaValley M, Phinikaridou A, Qiao Y. et al. The relationship of ectopic lipid accumulation to cardiac and vascular function in obesity and metabolic syndrome. Obesity (Silver Spring) 2010;18:1116–1121. doi: 10.1038/oby.2009.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KW, Tsai IC, Lee WJ, Lee IT, Lee WL, Lin SY, Wan CJ, Fu CP, Ting CT, Sheu WH. MRI measured epicardial adipose tissue thickness at the right AV groove differentiates inflammatory status in obese men with metabolic syndrome. Obesity (Silver Spring) 2012;20:525–532. doi: 10.1038/oby.2011.155. [DOI] [PubMed] [Google Scholar]
- Seon CS, Min KW, Lee SY, Nho KW, Park SH, Koo BK, Han KA. Cardiovascular Risk assessment with vascular function, carotid atherosclerosis and the UKPDS risk engine in Korean patients with newly diagnosed Type 2 Diabetes. Diab Metab J. 2011;35:619–627. doi: 10.4093/dmj.2011.35.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Kim KJ, Moon JH, Lee HJ, Chae MK, Chang HJ, Kang ES, Cha BS, Lee HC, Kim YJ, Lee BW. Association between EPCs count and rate of coronary revascularization in asymptomatic type 2 diabetic patients. Acta Diabetol. 2011. [Epub ahead of print] [DOI] [PubMed]
- Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- Denke MA, Pasternak RC. Defining and treating the metabolic syndrome: a primer from the adult treatment panel III. Curr Treat Options Cardiovasc Med. 2001;3:251–253. doi: 10.1007/s11936-001-0087-5. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee BW, Won HK, Moon JH, Kim KJ, Kang ES, Cha BS, Lee HC. Postprandial triglyceride is associated with fasting triglyceride and HOMA-IR in Korean subjects with Type 2 diabetes. Diabetes & metabolism journal. 2011;35:404–410. doi: 10.4093/dmj.2011.35.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TD, Lee WJ, Shih FY, Huang CH, Chang YC, Chen WJ, Lee YT, Chen MF. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab. 2009;94:662–669. doi: 10.1210/jc.2008-0834. [DOI] [PubMed] [Google Scholar]
- Wang TD, Lee WJ, Shih FY, Huang CH, Chen WJ, Lee YT, Shih TT, Chen MF. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis. 2010;213:279–287. doi: 10.1016/j.atherosclerosis.2010.07.055. [DOI] [PubMed] [Google Scholar]
- de Feyter PJ. Epicardial adipose tissue: an emerging role for the development of coronary atherosclerosis. Clin Cardiol. 2011;34:143–144. doi: 10.1002/clc.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, Dong N, Chen X. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2. doi: 10.1186/1475-2840-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Mi S, Zhang F, Gong F, Lai Y, Gao F, Zhang X, Wang L, Tao H. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:87. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgun H, Canpolat U, Hazirolan T, Ates AH, Sunman H, Dural M, Sahiner L, Kaya EB, Aytemir K, Tokgozoglu L, Increased epicardial fat tissue is a marker of metabolic syndrome in adult patients. Int J Cardiol. 2011. [Epub ahead of print] [DOI] [PubMed]
- Park JS, Ahn SG, Hwang JW, Lim HS, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Impact of body mass index on the relationship of epicardial adipose tissue to metabolic syndrome and coronary artery disease in an Asian population. Cardiovasc Diabetol. 2010;9:29. doi: 10.1186/1475-2840-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Worthley MI, Psaltis PJ, Carbone A, Dundon BK, Duncan RF, Piantadosi C, Lau DH, Sanders P, Wittert GA, Worthley SG. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J Cardiovasc Magn Reson. 2009;11:15. doi: 10.1186/1532-429X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama S, Ayako S, Somekawa S, Uemura S, Kubota Y, Saito Y. Feasibility of dual gradient-echo in-phase and opposed-phase magnetic resonance imaging for the evaluation of epicardial fat. Acta Radiol. 2011;52:723–729. doi: 10.1258/ar.2011.100454. [DOI] [PubMed] [Google Scholar]
- Sicari R, Sironi AM, Petz R, Frassi F, Chubuchny V, De Marchi D, Positano V, Lombardi M, Picano E, Gastaldelli A. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr. 2011;24:1156–1162. doi: 10.1016/j.echo.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- Sarin S, Wenger C, Marwaha A, Qureshi A, Go BD, Woomert CA, Clark K, Nassef LA, Shirani J. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008;102:767–771. doi: 10.1016/j.amjcard.2008.04.058. [DOI] [PubMed] [Google Scholar]
- Ishida M, Kato S, Sakuma H. Cardiac MRI in ischemic heart disease. Circ J. 2009;73:1577–1588. doi: 10.1253/circj.CJ-09-0524. [DOI] [PubMed] [Google Scholar]
- Klein C, Gebker R, Kokocinski T, Dreysse S, Schnackenburg B, Fleck E, Nagel E. Combined magnetic resonance coronary artery imaging, myocardial perfusion and late gadolinium enhancement in patients with suspected coronary artery disease. J Cardiovasc Magn Reson. 2008;10:45. doi: 10.1186/1532-429X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger A, Hennemuth A, Klumpp B, Fenchel M, Kramer U, Bretschneider C, Mangold S, May AE, Claussen CD, Peitgen HO, Miller S. Fusion of MR coronary angiography and viability imaging: feasibility and clinical value for the assignment of myocardial infarctions. Eur J Radiol. 2012;81:71–76. doi: 10.1016/j.ejrad.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der Graaf Y, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, Friedman JD, Hayes SW, Thomson LE, Slomka PJ. et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging. 2010;3:1104–1112. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14:2918–2924. doi: 10.2174/092986707782359972. [DOI] [PubMed] [Google Scholar]
- Iglesias MJ, Eiras S, Pineiro R, Lopez-Otero D, Gallego R, Fernandez AL, Lago F, Gonzalez-Juanatey JR. Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue. Findings in patients undergoing cardiac surgery. Rev Esp Cardiol. 2006;59:1252–1260. doi: 10.1157/13096596. [DOI] [PubMed] [Google Scholar]
- Fei J, Cook C, Blough E, Santanam N. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis. 2010;212:488–494. doi: 10.1016/j.atherosclerosis.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Lonn E, Lamy A, Singh N, Sharma AM. Epicardial fat thickness and coronary artery disease correlate independently of obesity. Int J Cardiol. 2011;146:452–454. doi: 10.1016/j.ijcard.2010.10.117. [DOI] [PubMed] [Google Scholar]