Abstract
Background
Stress worsens abdominal pain experienced by patients with irritable bowel syndrome (IBS), a chronic disorder of unknown origin with comorbid anxiety. We have previously demonstrated colonic hypersensitivity in Wistar-Kyoto rats (WKYs), a high-anxiety strain, that models abdominal pain in IBS. In low-anxiety rats, we have demonstrated that the central nucleus of the amygdala (CeA) regulates colonic hypersensitivity and anxiety induced by selective activation of either glucocorticoid receptors (GR) or mineralocorticoid receptors (MR), which is also mediated by the corticotropin releasing factor (CRF) type-1 receptor. The goal of the present study was to test the hypothesis that the CeA through GR, MR and/or CRF-1R regulates colonic hypersensitivity in WKYs.
Methods
One series of WKYs had micropellets of a GR antagonist, an MR antagonist or cholesterol (control) stereotaxically implanted onto the CeA. Another series were infused in the CeA with CRF-1R antagonist or vehicle. Colonic sensitivity was measured as a visceromotor response (VMR) to graded colorectal distension (CRD).
Key Results
The exaggerated VMR to graded CRD in WKYs was unaffected by GR or MR antagonism in the CeA. In contrast, direct CeA infusion of CRF-1R antagonist significantly inhibited the VMR to CRD at noxious distension pressures.
Conclusions & Inferences
Stress-hormones in the CeA regulate colonic hypersensitivity in the rat through strain-dependent parallel pathways. The colonic hypersensitivity in WKYs is mediated by a CRF-1R mechanism in the CeA, independent of GR and MR. These complementary pathways suggest multiple etiologies whereby stress hormones in the CeA may regulate abdominal pain in IBS patients.
Keywords: amygdala, corticotropin releasing factor, colon, visceral hypersensitivity, IBS, Wistar-Kyoto rat
Stress-induced anxiety is a major health concern and can facilitate a multitude of disorders including irritable bowel syndrome (IBS), which is a functional gastrointestinal (GI) disorder characterized by episodes of abdominal pain with diarrhea and/or constipation (1). Although the etiology of IBS is unknown and the impact of stress and anxiety on the GI tract is poorly understood, recent clinical evidence suggests that there may be hypothalamic-pituitary-adrenal (HPA) axis dysregulation involved in the pathophysiology of IBS (2, 3). To substantiate the HPA dysregulation theory of IBS, exaggerated adrenocorticotropic hormone (ACTH), cortisol, and colonic motility responses to exogenous corticotrophin releasing factor (CRF) have all been observed in IBS patients compared to healthy controls (2, 4).
A prominent feature of IBS symptomatology is altered visceral perception characterized by hypersensitivity of the colon to luminal distension (5, 6). In preclinical studies the Wistar-Kyoto (WKY) rat strain has been reported to be an effective model for studying anxiety and IBS (7-9). The WKY strain expresses high anxiety-like behavior compared to other strains (10, 11) in addition to colonic hypersensitivity to luminal distension demonstrated as an increased visceromotor response (VMR) to colorectal distension (CRD) (7). In WKY rats, there is also a strong positive correlation between increased central CRF and CRF type 1 receptor (CRF-1R) expression, with elevated anxiety–like behavior and colonic hypersensitivity (7, 12-14). WKY rats treated with a peripherally administered CRF-1R antagonist displayed colonic sensitivity that was similar to basal levels observed in the Fischer-344 (F344) rat strain that exhibit low-levels of anxiety-like behavior (11-13). Conversely, when F344 animals were administered CRF centrally via intracerebroventricular (i.c.v.) injection, colonic sensitivity resembled that seen in the WKY rats (12).
Cortisol is also altered in states of anxiety and in IBS patients who exhibit elevated release in response to stress and have greater basal systemic cortisol levels compared to healthy volunteers (2, 3). Cortisol acts upon key limbic regions, such as the amygdala, to facilitate behavioral and psychological stress responses including GI motility (15). Further, preclinical studies show that implants of corticosterone (CORT) micropellets in F344 rats localized to the dorsal margin of the central nucleus of the amygdala (CeA) increases anxiety-like behavior as well as visceral hypersensitivity to luminal distension, similar to levels found in the WKY strain (12, 16, 17). CORT acts upon glucocorticoid and mineralocorticoid receptors (GR/MR) (18), which are highly expressed in the amygdala (19, 20). Specific activation of both GR and MR in the CeA has been implicated in the development of anxiety and visceral hypersensitivity (17, 21-23), suggesting that corticosteroid receptor activation in the CeA is involved in the induction of anxiety and visceral hypersensitivity.
Although activation of GR/MR in the CeA induces anxiety-like behavior and visceral hypersensitivity in F344 rats, it is unknown whether the same mechanisms are present in WKY rats. Furthermore, although there is an increase in CRF activation involved in visceral hypersensitivity in WKY animals, the specific localization of CRF-mediated mechanisms remains unknown. Here we test the hypothesis that CRF and GR/MR activation of the CeA facilitates increased visceral hypersensitivity in the WKY strain.
Materials and Methods
Animals
Experiments were performed on weight matched male WKY or male F344 rats, 250-320 g (Charles Rivers Laboratory, Wilmington, MA, USA). All animals were single-housed to prevent post-surgery complications and maintained on a 12-hour light/dark cycle (lights on at 5:30 AM) at 21o C and 70% humidity with ad libitum access to food and water. Animals were acclimated to the animal facility for one week and to the experimenter and the laboratory for one week before experimentation. All manipulations were approved by the Oklahoma City Veterans Affairs Medical Center Animal Care and Use Committee in accordance with standards established by the Guide for Care and Use of Laboratory Animals (1996).
Stereotaxic Implantation of Micropellets
Implantation of micropellets on the dorsal margin of the CeA was performed as described previously (16, 17, 24, 25). Briefly, rats were anesthetized with an intraperitoneal (i.p.) injection of 100 mg kg−1 Ketamine (Hospira, Lake Forest, IL, USA) and 10 mg kg−1 Xylazine (Ben Venue Laboratories, Bedford, OH, USA). Using aseptic technique, bilateral micropellets (30 μg each – see Experimental Design for additional detail) were placed at bregma −2.5 mm, medial/lateral ±4.2 mm and anteroposterior −7 mm from dura. Rats were then allowed to recover for 7 days before assessment of colonic sensitivity by colonic distension.
At the end of the experiment, post-mortem verification of micropellet placement was conducted as previously described (16, 17, 24, 25). Whole brains were rapidly removed following euthanasia and frozen with isopentane chilled at -80o C. Frozen brains were subsequently sectioned at 50 μm in a cryostat (Bright-OTF, Hacker Instruments and Industries Inc., Fairfield, NJ, USA), with sequential sections thaw mounted onto glass slides. Pellet localization was performed with the aid of a stereotaxic atlas (26). Four rats had micropellets that either damaged the CeA or were outside the 750 μm diffusion radius (17, 27) and were not used in the final data analysis.
Stereotaxic Implantation of Cannula for CeA Infusions
Implantation of Cannula
Implantation of bilateral indwelling cannula targeting the CeA was performed with modification of the procedure for implantation of a single intracerebroventricular cannula as previously described (12, 25). Custom cannula systems that included a matched injector and dummy cannula were purchased from Plastics One (Roanoke, VA, USA). The custom cannula (25-gauge surgical steel) extended 7.0 mm (CeA) below and 1 mm above the threaded pedestal. The matched injector extended 1 mm beyond the internal end of the cannula. The dummy cannula was flush with the internal end of the cannula. The cannula was placed flush to the skull at bregma −2.5 and medial/lateral ±4.2 mm for bilateral CeA infusions. Two stainless steel mounting screws (Plastics One) were placed on opposite sides of the cannula, which was held to the skull with Cerebond adhesive (Plastics One). Animals were allowed a 7-day recovery before colonic sensitivity assessment. One rat did not recover from the implantation surgery.
Administration of Drugs
Drug was administered immediately after instrumentation for colonic sensitivity assessment (see Colonic Sensitivity Assessment below) while rats were anesthetized with 2% isoflurane. Rats were positioned within the anesthesia mask so that the cannula was accessible. The dummy cannula was unscrewed and the matched injector was placed into the cannula. A total of 0.5 μl of vehicle (saline) or drug per side (1.0 μl total volume, see Experimental Design for additional detail) was manually injected with a microsyringe (Hamilton Co., Reno, NV, USA) over 60 sec, and the injector was left in place for an additional 120 sec to allow for the injection to disperse. The injector was then removed and the dummy cannula was retuned to the cannula and screwed into place. The rat was then placed in its home cage to continue the experimental protocol.
Localization of Cannula Placement
At the end of the experiment, following euthanasia, the cannulae were carefully removed and the brain was placed in a dissecting matrix (Kent Scientific Corp., Torrington, CO, USA). Location of the cannulae were confirmed by morphologic examination of the 1 mm slice containing the wound track with aid of the stereotaxic atlas (26).
Colonic Sensitivity Assessment
Instrumentation
Instrumentation for colonic sensitivity assessment was performed as previously described (16, 17, 24, 25). In brief, rats were fasted for 16-18 h before colonic sensitivity assessment. In the morning of the experiment, rats were brought to the laboratory and anesthetized with 2% isoflurane (Aerrane, Baxter Healthcare, Deerfield, IL, USA). A superficial skin incision was made on the abdomen and a strain-gauge force transducer (RB Products, Stillwater, MI, USA) was attached to the external abdominal oblique with sutures of 3-0 nylon (Ethicon Inc., Somerville, NJ, USA). A colonic balloon (6 cm) was inserted 11 cm past the anus into the colon and secured to the base of the tail with tape. Topical analgesic balm (Myoflex, Novartis Consumer Healthcare, Parsippany, NJ, USA) was applied to the skin incision, anesthesia was stopped, and the rats were placed in their home cage for the colorectal distension series.
Colorectal Distension
After a 30 min recovery period, conscious, freely moving rats underwent graded colonic distension as previously described (16, 17, 24, 25). Briefly, the colonic balloon catheter was attached to a Distender Series IIR Barostat (G & J Electronics Inc., Toronto, Ontario, Canada) and the strain-gauge transducer was attached to a Grass Model 7 polygraph (Astro-Med Inc., West Warwick, RI, USA). Abdominal muscle contractions in response to balloon distension were recorded as signals from the transducer, visualized with PolyView software (Astro-Med Inc.). Each isobaric distension series consisted of a 10 min recording period at 0 mmHg, followed by 10 min inflation at 15, 30, 45, or 60 mmHg separated by 10 min rest periods (0 mmHg) presented in a random order.
Experimental Design
Experimental series were performed as listed in Table 1. The first series, using rats that were not surgically manipulated, directly compared colonic sensitivity (see Colorectal Distension for additional detail) in high-anxiety WKY rats to that in low-anxiety F344 rats. The second series used WKY rats with bilateral micropellets on the CeA to examine the role of corticoid receptors in the CeA on the regulation of colonic hypersensitivity. All micropellets had a final mass of 30 μg, with 15 μg being composed of antagonist and the other 15 μg of cholesterol (CHOL). The doses of CHOL, spironolactone (SPIRO), and mifepristone (MIFE) were based on our previous studies in the F344 rat (17). Finally, in the last series, rats with bilateral CeA cannula were used to determine the role of specific CRF-1R in the CeA in modulating colonic sensitivity of the WKY rats. Rats, divided into two groups, received either CP 376395 or vehicle (saline) (0.5 μl/side). To ensure that the antagonist remained in solution, the CP 376395 solution had a pH between 5.0-5.5, and the saline vehicle was pH matched accordingly. The dose of CP 376395 (10 μg) was selected based on our previous studies in the F344 rat (25).
Table 1.
Experimental Groups.
| Surgery | Treatment | Dose | N |
|---|---|---|---|
| None | Naïve F344 | None | 8 |
| Naïve WKY | None | 8 | |
|
| |||
| Bilateral | CHOL | 30μg micropellets, 7 day-post surgery | 8 |
| Micropellets on | SPIRO | 15μg micropellets, 7 day-pot surgery | 8 |
| Dorsal Margin of | 30μg micropellets, 7 day-pot surgery | 6 | |
| CeA | MIFE | 15μg micropellets, 7 day-pot surgery | 8 |
| 30 μg micropellets, 7 day-post surgery | 6 | ||
|
| |||
| Bilateral | Saline | 0.5μl/side, intra-CeA, 30 min pre-dose | 6 |
| Cannulation of CeA | CP 376395 | 5.0 μg/side, intra-CeA, 30 min pre-dose | 5 |
CeA = central nucleus of the amygdala. CHOL = cholesterol. F344 = Fischer-344 rat. MIFE = mifepristone. SPIRO = spironolactone. WKY = Wistar-Kyoto rat.
Drugs and Chemicals
Cholesterol, mifepristone, spironolactone, saline, and isopentane were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). CP 376395 was purchased from Tocris Bioscience (Ellisville, MO, USA). Ketamine was obtained from the pharmacy at the Oklahoma City Veterans Affairs Medical Center. Xylazine, topical antibiotic cream, topical analgesic balm, and isoflurane were purchased from NLS Animal Health (Owings Mills, MD, USA).
Data Analysis
Data is presented as mean ± standard error of the mean (SEM) of the number of abdominal contractions per 10 min distension period. A two-factor repeated measures analysis of variance (RM-ANOVA) was used to determine the significance of main effects (distension pressure, strain/treatment) and interaction terms. A Bonferroni’s post-hoc test was used to determine significance between the mean number of abdominal contractions at a selected distension pressure between treatments. GraphPad Prism 5.0d for Macintosh (GraphPad Software, San Diego, CA, USA) was used for drawing graphs and all statistical analysis, with p < 0.05 being considered significant. Additional graph manipulation to produce composite, high-resolution images was performed using GIMP (GNU Image Manipulation Program, v2.6, Boston, MA, USA).
Results
Colonic hypersensitivity in WKY rats
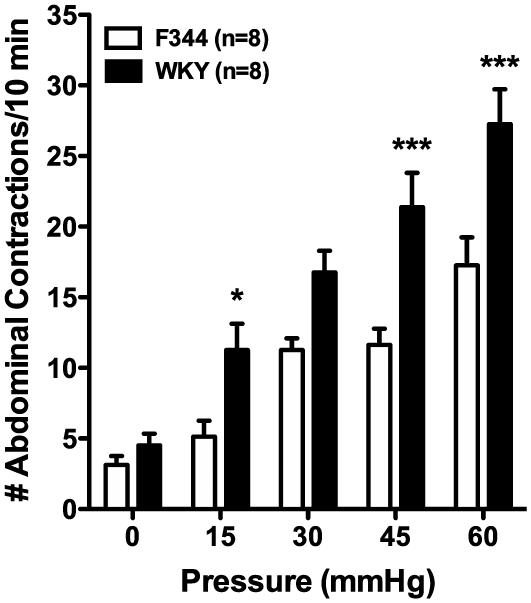
The first experiments were conducted to confirm colonic hypersensitivity to graded distension pressures in the WKY rat strain (7, 12). WKY rats exhibited an increased VMR response to CRD when compared to low anxiety F344 rats, a normosensitive comparator (7). These findings validate that the WKY rat represents a model of colonic hypersensitivity. The main effects of strain (F(1,14)=18.29, p < 0.001) and distension pressure (F(4,56)=56.37, p < 0.001) as well as the interaction between strain and pressure (F(4,56)=3.43, p<0.05) were significant. Post-hoc differences between the group means are shown in Figure 1. As illustrated, the VMR response was 54.5%, 32.8%, 45.6% and 36.7% greater in WKYs than in F344s at 15, 30, 45 or 60 mmHg respectively.
Figure 1. Verification of colonic hypersensitivity in the Wistar-Kyoto (WKY) rat.
WKY rats (black bars, n=8) demonstrate an elevated number of abdominal contractions in response to colorectal distension compared to Fischer-344 (F344) rats (white bars, n=8). * p < 0.05, *** p < 0.001 vs. F344, two-factor RM-ANOVA, Bonferroni’s post-hoc test.
Role of CeA steroid receptors in the regulation of colonic hypersensitivity in the WKY rat
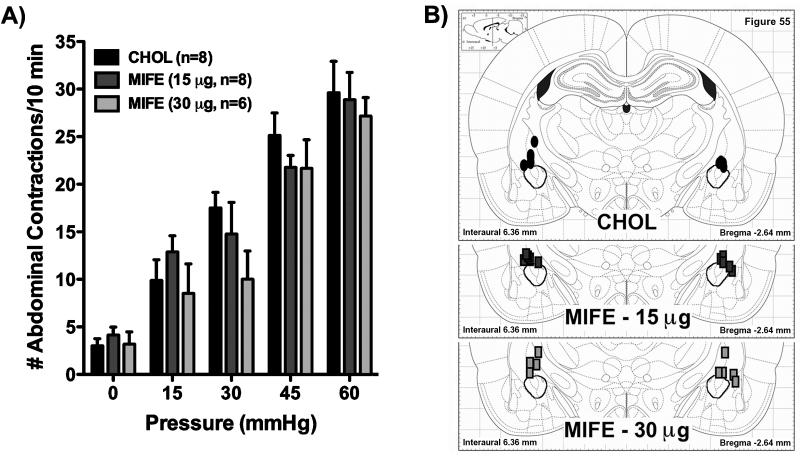
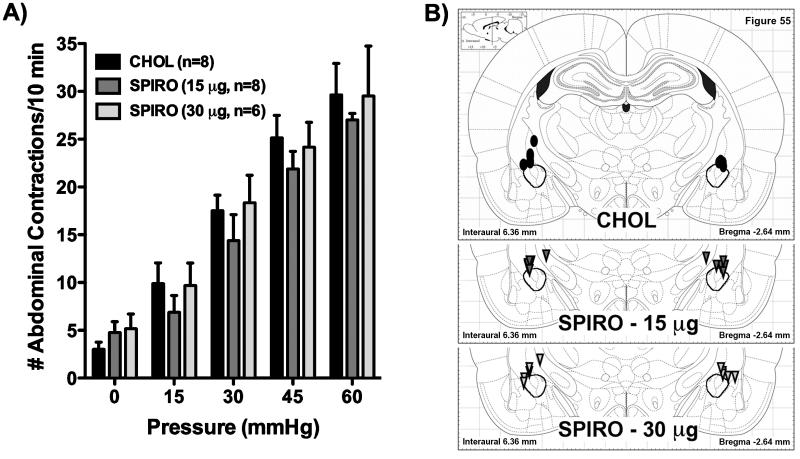
Experiments were performed to determine whether colonic hypersensitivity in WKY rats is mediated by steroid receptors in the CeA. In WKY rats we stereotaxically implanted onto the dorsal margin of the CeA micropellets of the selective GR antagonist, MIFE (15 or 30 μg), the selective MR antagonist, SPIRO (15 or 30 μg), or CHOL (30 μg). While there was a significant main effect of distension pressure (F(4,124)=128.11, p < 0.001), both the main effect of treatment (F(4,31)=0.73) and the interaction between treatment and pressure (F(16,124)=0.84) were not significant (p > 0.05). Figure 2A shows response to colonic distension for rats with MIFE implants on the CeA, with Figure 2B illustrating the location of the micropellets. Similarly, Figure 3A and 3B represent the colonic sensitivity data and the micropellet placement for rats with SPIRO implants. For both figures, there were no significant post-hoc differences between group means at any pressure (Bonferroni adjusted p = 1.00 for all pressures). In Figure 2A, 15 μg MIFE slightly increased the VMR by 30.4% at 15 mmHg and decreased VMR by 15.7%, 13.4% or 2.5% at 30, 45 or 60 mmHg, whereas 30 μg MIFE lowered the VMR by 13.9%, 42.9%, 13.8% or 8.3% at 15, 30, 45, or 60 mmHg compared to the CHOL control response. In Figure 3A, 15 μg SPIRO lessened the VMR at every pressure tested (30.4%, 17.9%, 12.9%, and 8.9%), while 30 μg SPIRO slightly reduced the VMR by 2.1%, 3.8% and 0.4% at 15, 45, or 60 mmHg and marginally increased the VMR by 4.8% at 30 mmHg, compared to CHOL.
Figure 2. Role of glucocorticoid-receptors in the CeA in the regulation of colonic hypersensitivity in the Wistar-Kyoto rat.
A) Bilateral implants of the selective GR-antagonist, MIFE, we implanted on the dorsal margin of the CeA. Neither the 15 μg pellets (dark grey bars, n=8) nor the 30 μg pellets (light grey bars, n=6) changed the response to colorectal distension when compared to the CHOL-implanted control group (black bars, n=8). p > 0.05 for all pressures, two-factor RM-ANOVA, Bonferroni’s post-hoc test. B) Location of implanted micropellets. For simplicity, all pellets are represented on single figure; however, the actual range for the pellets was from -2.16 to -3.48 mm from bregma. Black ovals represent CHOL pellets (30 μg), rectangles represent MIFE pellets (dark grey, 15 μg; light grey, 30 μg) (symbols are not drawn to scale). The stereotaxic plate is from The Rat Brain in Stereotaxic Coordinates, 5th edition (26) and the CeA has been outlined for emphasis.
Figure 3. Role of mineralocorticoid-receptors in the CeA in the regulation of colonic hypersensitivity in the Wistar-Kyoto rat.
A) Bilateral implants of the selective MR-antagonist, SPIRO, we implanted on the dorsal margin of the CeA. Neither the 15 μg pellets (dark grey bars, n=8) nor the 30 μg pellets (light grey bars, n=6) changed the response to colorectal distension when compared to the CHOL-implanted control group (black bars, n=8). p > 0.05 for all pressures, two-factor RM-ANOVA, Bonferroni’s post-hoc test. B) Location of implanted micropellets. For simplicity, all pellets are represented on single figure, however, the actual range for the pellets was from -2.16 to -3.48 mm from bregma. Black ovals represent CHOL pellets (30 μg), triangles represent SPIRO pellets (dark grey, 15 μg; light grey, 30 μg) (symbols are not drawn to scale). The stereotaxic plate is from The Rat Brain in Stereotaxic Coordinates, 5th edition (26) and the CeA has been outlined for emphasis.
Role of CeA CRF-1R in the regulation of colonic hypersensitivity in the WKY rat
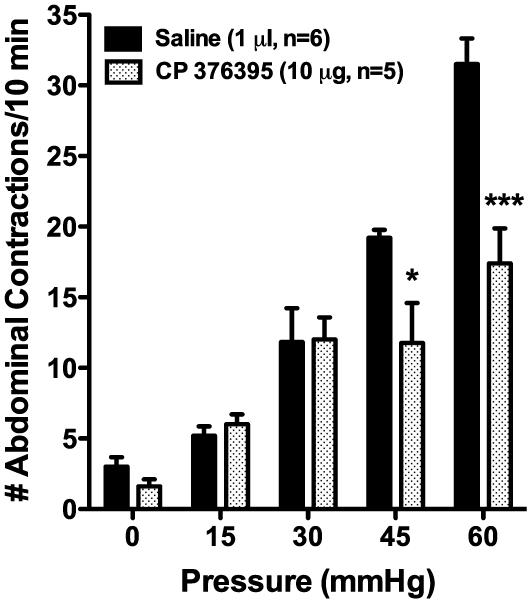
Having previously demonstrated that a peripherally administered CRF-1R antagonist could inhibit the hypersensitive response to CRD in WKY rats (12), the current study investigated whether direct administration of a selective CRF-1R antagonist to the CeA could inhibit the VMR to CRD in WKY rats. In WKY rats dosed with the selective CRF-1R antagonist, CP 376395 (10.0 μg, intra-CeA), there were significant main effects of distension pressure (F(4,36)=50.69, p < 0.001) and treatment (F(1,9)=5.28, p < 0.05) as well as a significant interaction term (F(4,36)=5.46, p < 0.01), when compared to rats injected with the vehicle control (saline). As illustrated in Figure 4, the pressures of 45 mmHg (33.8% inhibition) and 60 mmHg (44.8% inhibition) had significant post-hoc differences in the mean number of abdominal contractions to distension (CP 373395 vs. vehicle), whereas the response at 15 and 30 mmHg were statistically unchanged (15.4% and 1.4% increased, respectively).
Figure 4. Importance of CeA CRF-1R receptors in the regulation of colonic hypersensitivity in the Wistar-Kyoto rat.
WKY rats with bilateral chronic CeA cannulae were treated with vehicle (saline, 0.5 μl/side, 1.0 μl total, n=6) or CP 376395 (5.0 μg/side, 10 μg total, n=5) 30 min before the colorectal distension series. As shown, CP 376395 inhibited colonic hypersensitivity to noxious colonic distension. * p < 0.05, *** p < 0.001 vs. vehicle; two-factor RM-ANOVA, Bonferroni’s post-hoc test.
Discussion
This study aimed to delineate the specific roles of stress hormones within the CeA and their involvement in colonic hypersensitivity in the WKY rat, a model used extensively to study anxiety and visceral pain. In the current study we demonstrated that high-anxiety WKY rats are hypersensitive to colonic distension when compared to F344 rats, a low-anxiety rat strain. Stereotaxically delivering antagonists selective for GR and MR revealed that neither receptor in the CeA modulates colonic hypersensitivity in the WKY rat. We next examined the role of CRF-1Rs in the CeA on the regulation of colonic hypersensitivity. We found that site-specific infusion of CRF-1R antagonist to the CeA attenuated the characteristic hypersensitivity to colonic distension in the WKY rats. Thus, based on the results of this study, we identified that the CeA modulates the spontaneous colonic hypersensitivity exhibited by WKY rats through CRF-1Rs.
HPA dysfunction is implicated as an important contributing factor in the symptomatology of functional bowel disorders such as IBS (2, 3). Similar to reports in IBS patients, when compared to other rat strains, WKY rats demonstrate altered HPA activity in response to stressors, particularly hypersecretion of ACTH and prolonged plasma CORT levels (28-30). Likewise, in F344 with micropellets of CORT stereotaxically implanted on the dorsal margin of the CeA, which also increases anxiety-like behavior and induces colonic hypersensitivity, the plasma CORT response is protracted following an acute stressor (28). In the F344-implant model, our previous findings provide evidence for the important role of GR/MR activation, the receptors that bind CORT, in the modulation of anxiety-like behavior and colonic hypersensitivity at the level of the CeA (17, 24, 25). Moreover, a recent study found elevated basal GR mRNA expression in the amygdala of WKY rats, which suggests that amygdala GR activation may also be involved in the pathological phenotypes displayed by WKY rats (31). Based on the behavioral similarities between the F344-implant model and WKY rats, we investigated the importance of amygdala GR and MR activation in the regulation of colonic hypersensitivity in WKY rats. However, using the same experimental paradigm as in F344-implant model, we found that neither selective antagonism of GR nor MR in the amygdala had an inhibitory effect on colonic hypersensitivity in the WKY rat.
In the remainder of the study, we refocused our investigation on CRF signaling within the CeA as a probable target for stress-hormone regulation of colonic hypersensitivity in light of evidence that WKY rats express a greater amount of CRF and CRF-1R mRNA in the CeA as well as and the paraventricular nucleus of the hypothalamus (PVN) compared with other rat strains (13, 14). Using CP 376395, a selective CRF-1R antagonist, we found direct administration to the CeA mitigated the colonic hypersensitivity to noxious CRD (45 and 60 mmHg) as measured by the decreased VMR at those pressures. This finding was in contrast to our previous study in the WKY where intraperitoneal administration of antalarim inhibited the VMR to CRD at 30 mmHg (12). Since antalarmin can cross the blood-brain barrier, the dose used in the previous study would have bound to CRF-1R receptors throughout the brain, spinal cord, and in peripheral tissues (32), suggesting that CRF-1Rs outside of the CeA regulate the response to CRD at 15 and 30 mmHg. While there are many nuclei that change activity in response to stress and/or distension (33), in particular, increases in neuronal activity within the locus coeruleus (LC) evoked by CRD or intracisternal CRF injections can be inhibited by a selective CRF-1R antagonist (34) and a study by Pearson et al. (35) demonstrated that WKY rats had increased expression of CRF within the LC. Additionally, a recent imaging study by Hubbard et al. (36) demonstrated that brain activation in IBS patients in response to the threat of abdominal pain was decreased in the amygdala and LC following administration of a selective CRF-1R antagonist. Thus, the LC is a likely additional nucleus that could regulate colonic hypersensitivity in the WKY. Additionally, although not modeled by the WKY rat, multiple CRF-1R antagonists have been demonstrated to inhibit stress-induced colonic hypersensitivity (reviewed most recently by Larauche et al., 2011 [37]), which agrees with the current results. Finally, while this study did not investigate the role of CRF type 2 receptors (CRF-2Rs) in the regulation of colonic sensitivity, studies of single-unit neuronal activity in the CeA suggest that CRF-2Rs in the CeA mediate inhibitory responses to nociceptive stimuli and therefore are not likely to contribute to the effects in this study (38, 39).
Our data also does not preclude the involvement of CRF or CRF-1R receptors within the enteric nervous system (ENS) in the initiation or maintenance of colonic hypersensitivity. Peripheral administration of CRF increases colonic secretion, permeability, and motor function in rodents (40). Anatomical studies have identified both CRF and CRF-1R expression in the rat colon (41), and the CRF present in colon can act directly on the ENS through activation of CRF-1R on colonic myenteric neurons (42). Confocal calcium imaging experiments showed that CRF activates at least one-fifth of the myenteric neurons in the guinea pig intestine through CRF-1R (43). The same experiments also demonstrated that activation of CRF-1R hyperexcites myenteric neurons by increasing intercellular calcium through cyclic adenosine monophosphate (cAMP) activation of voltage-gated calcium channels. Accordingly, because colonic CRF-1Rs are differentially expressed in response to an acute stress in WKY compared to Sprague-Dawley rats (9), an additional potential mechanism for colonic hypersensitivity in the WKY rat strain may be through the activation of CRF-1R found on the myenteric neurons of the colon (44). In recent studies, colonic hypersensitivity has been observed in WKY rats by visually assessing pain behaviors (45-47). This study quantified colonic hypersensitivity in freely moving rats using a reproducible, validated technique (7, 12, 16, 17, 24, 25). A limitation of this technique is the requirement for a minor surgical intervention to implant a transducer to measure the VMR, which may have indirectly affected colonic sensitivity. However since all the rats in this study were subjected to the same experimental design, the contribution of any acute surgical effect would be expected to be identical across all the treatments and thus unlikely to affect the interpretation or scientific merit of this study.
Although anxiety-like behavior was not directly addressed in this study, we can infer from our previous studies, as well as others, that the administered corticosteroid and CRF-1R antagonists would have decreased anxiety-like behavior in WKY rats. Similar to WKY rats, F344 rats implanted with CORT on the CeA display exaggerated anxiety-like behavior as observed on the elevated plus-maze (EPM) (12, 13, 52). In contrast, when F344 CORT-implant animals also received implants of the GR and MR antagonists MIFE and SPIRO, respectively, at the same concentration tested in this study, we demonstrated an inhibition of anxiety-like behavior (17). Furthermore, Wistar rats receiving i.c.v. administration of GR, MR and CRF antagonists also exhibited a decrease in anxiety-like behavior on the EPM (53-55). Nevertheless, because reliably measuring anxiety-like behavior in WKY rats in behavioral tests such as the EPM or open field is complicated by their well-documented passive avoidance behavior that increases their immobility in those tests (56-59), we did not include a measure for anxiety-like behavior in the present study.
In summary, the findings in this study more precisely define the role of stress hormones within the CeA in the modulation of the colonic hypersensitivity found in the WKY strain of rat. Because the WKY is identified as a putative model of co-morbid anxiety and colonic hypersensitivity, the results of this study may further our understanding of one of the underlying mechanisms involved in the pathophysiology of IBS, namely a potential functional genomic alteration of the central CRF system. Further clinical genome wide association studies could be used to identify if specific polymorphisms within the CRF system are present within a sub-set of IBS patients.
Acknowledgements
This work was supported by a merit grant from the Department of Veterans Affairs, USA (to BG-VM). Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number F31DK089871. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- IBS
irritable bowel syndrome
- GI
gastrointestinal
- HPA
hypothalamic-pituitary-adrenal
- ACTH
adrenocorticotropic hormone
- CRF
corticotrophin releasing factor
- WKY
Wistar-Kyoto rat
- VMR
visceromotor response
- CRD
colorectal distension
- CRF-1R
CRF type 1 receptor
- F344
Fischer-344 rat
- i.c.v.
intracerebroventricular
- CORT
corticosterone
- CeA
central nucleus of the amygdala
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- i.p.
intraperitoneal
- CHOL
cholesterol
- SPIRO
spironolactone
- MIFE
mifepristone
- SEM
standard error of the mean
- RM-ANOVA
repeated measures analysis of variance
- PVN
paraventricular nucleus of the hypothalamus
- LC
locus coeruleus
- CRF-2R
CRF type 2 receptor
- ENS
enteric nervous system
- cAMP
cyclic adenosine monophosphate
- EPM
elevated plus-maze
Footnotes
Competing Interests The authors have no competing interests.
Author Contribution AJ & LT performed the research and analyzed the data; AJ, LT, JS & BG-VM wrote the paper; BG-VM designed the research study.
References
- 01.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 02.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 03.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 04.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 05.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 06.Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci. 1980;25:404–413. doi: 10.1007/BF01395503. [DOI] [PubMed] [Google Scholar]
- 07.Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav. 2000;69:379–382. doi: 10.1016/s0031-9384(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 08.McKernan DP, Nolan A, Brint EK, et al. Toll-like receptor mRNA expression is selectively increased in the colonic mucosa of two animal models relevant to irritable bowel syndrome. PLoS One. 2009;4:e8226. doi: 10.1371/journal.pone.0008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 09.O’Malley D, Julio-Pieper M, Gibney SM, Gosselin RD, Dinan TG, Cryan JF. Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto rat. Neurogastroenterol Motil. 2010;22:301–311. doi: 10.1111/j.1365-2982.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 10.Pare WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51:1051–1056. doi: 10.1016/0031-9384(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 11.Glowa JR, Hansen CT. Differences in response to an acoustic startle stimulus among forty-six rat strains. Behav Genet. 1994;24:79–84. doi: 10.1007/BF01067931. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood-Van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil. 2005;17:415–422. doi: 10.1111/j.1365-2982.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 13.Shepard JD, Myers DA. Strain differences in anxiety-like behavior: association with corticotropin-releasing factor. Behav Brain Res. 2008;186:239–245. doi: 10.1016/j.bbr.2007.08.013. 2008. [DOI] [PubMed] [Google Scholar]
- 14.Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol. 2011;14:666–683. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- 15.Lyubashina OA. Possible mechanisms of involvement of the amygdaloid complex in the control of gastric motor function. Neurosci Behav Physiol. 2004;34:379–388. doi: 10.1023/b:neab.0000018750.65372.18. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res. 2001;893:135–142. doi: 10.1016/s0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- 17.Myers B, Greenwood-Van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1622–G1629. doi: 10.1152/ajpgi.00080.2007. [DOI] [PubMed] [Google Scholar]
- 18.de Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- 19.Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of [3H]corticosterone receptors in rat brain. Brain Res. 1983;271:331–334. doi: 10.1016/0006-8993(83)90295-0. [DOI] [PubMed] [Google Scholar]
- 20.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 21.Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol. 2003;89:1343–1352. doi: 10.1152/jn.00834.2002. [DOI] [PubMed] [Google Scholar]
- 22.Qin C, Greenwood-Van Meerveld B, Foreman RD. Visceromotor and spinal neuronal responses to colorectal distension in rats with aldosterone onto the amygdala. J Neurophysiol. 2003;90:2–11. doi: 10.1152/jn.00023.2003. [DOI] [PubMed] [Google Scholar]
- 23.Venkova K, Foreman RD, Greenwood-Van Meerveld B. Mineralocorticoid and glucocorticoid receptors in the amygdala regulate distinct responses to colorectal distension. Neuropharmacology. 2009;56:514–521. doi: 10.1016/j.neuropharm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Myers B, Greenwood-Van Meerveld B. Divergent effects of amygdala glucocorticoid and mineralocorticoid receptors in the regulation of visceral and somatic pain. Am J Physiol Gastrointest Liver Physiol. 2010;298:G295–G303. doi: 10.1152/ajpgi.00298.2009. [DOI] [PubMed] [Google Scholar]
- 25.Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res. 2010;214:465–469. doi: 10.1016/j.bbr.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed Academic Press; Burlington: 2005. [Google Scholar]
- 27.Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963:203–213. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- 28.Solberg LC, Olson SL, Turek FW, Redei E. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R786–R794. doi: 10.1152/ajpregu.2001.281.3.R786. [DOI] [PubMed] [Google Scholar]
- 29.Solberg LC, Baum AE, Ahmadiyeh N, et al. Genetic analysis of the stress-responsive adrenocortical axis. Physiol Genomics. 2006;27:362–369. doi: 10.1152/physiolgenomics.00052.2006. [DOI] [PubMed] [Google Scholar]
- 30.Pardon MC, Gould GG, Garcia A, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 31.Schaffer DJ, Tunc-Ozcan E, Shukla PK, Volenec A, Redei EE. Nuclear orphan receptor Nor-1 contributes to depressive behavior in the Wistar-Kyoto rat model of depression. Brain Res. 2010;1362:32–39. doi: 10.1016/j.brainres.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 32.Deak T, Nguyen KT, Ehrlich AL, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- 33.Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett. 1996;215:165–168. doi: 10.1016/0304-3940(96)12978-5. [DOI] [PubMed] [Google Scholar]
- 34.Kosoyan HP, Grigoriadis DE, Tache Y. The CRF(1) receptor antagonist, NBI-35965, abolished the activation of locus coeruleus neurons induced by colorectal distension and intracisternal CRF in rats. Brain Res. 2005;1056:85–96. doi: 10.1016/j.brainres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Pearson KA, Stephen A, Beck SG, Valentino RJ. Identifying genes in monoamine nuclei that may determine stress vulnerability and depressive behavior in Wistar-Kyoto rats. Neuropsychopharmacology. 2006;31:2449–2461. doi: 10.1038/sj.npp.1301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbard CS, Labus JS, Bueller J, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larauche M, Mulak A, Tache Y. Stress and visceral pain: From animal models to clinical therapies. Exp Neurol. 2011 May 6; doi: 10.1016/j.expneurol.2011.04.020. [Epub ahead of print] doi:10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji G, Neugebauer V. Differential effects of CRF-1R and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol. 2007;97:3893–3904. doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- 39.Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol. 2008;99:1201–1212. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- 40.Tache V, Perdue M. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 41.Yuan PQ, Wu SV, Wang L, Tache Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322–331. doi: 10.1016/j.peptides.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chatzaki E, Crowe PD, Wang L, Million M, Tache Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 43.Bisschops R, Vanden Berghe P, Sarnelli G, Janssens J, Tack J. CRF-induced calcium signaling in guinea pig small intestine myenteric neurons involves CRF-1 receptors and activation of voltage-sensitive calcium channels. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1252–G1260. doi: 10.1152/ajpgi.00349.2004. [DOI] [PubMed] [Google Scholar]
- 44.Miampamba M, Maillot C, Million M, Tache Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF(1) receptor in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G857–G865. doi: 10.1152/ajpgi.00434.2001. [DOI] [PubMed] [Google Scholar]
- 45.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 46.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brusberg M, Ravnefjord A, Martinsson R, Larsson H, Martinez V, Lindstrom E. The GABA(B) receptor agonist, baclofen, and the positive allosteric modulator, CGP7930, inhibit visceral pain-related responses to colorectal distension in rats. Neuropharmacology. 2009;56:362–367. doi: 10.1016/j.neuropharm.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Hong S, Zheng G, Wu X, Snider NT, Owyang C, Wiley JW. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011;140:627–637. e4. doi: 10.1053/j.gastro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibney SM, Gosselin RD, Dinan TG, Cryan JF. Colorectal distension-induced prefrontal cortex activation in the Wistar-Kyoto rat: implications for irritable bowel syndrome. Neuroscience. 2010;165:675–683. doi: 10.1016/j.neuroscience.2009.08.076. [DOI] [PubMed] [Google Scholar]
- 50.O’Mahony SM, Bulmer DC, Coelho AM, et al. 5-HT(2B) receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol Motil. 2010;22:573–578. e124. doi: 10.1111/j.1365-2982.2009.01432.x. [DOI] [PubMed] [Google Scholar]
- 51.McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil. 2010;22:1029–1035. e268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 52.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 53.Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF. Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther. 1994;269:564–572. [PubMed] [Google Scholar]
- 54.Korte SM, de Boer SF, de Kloet ER, Bohus B. Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneuroendocrinology. 1995;20:385–394. doi: 10.1016/0306-4530(94)00069-7. [DOI] [PubMed] [Google Scholar]
- 55.Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73:261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- 56.Pare WP. Passive-avoidance behavior in Wistar-Kyoto (WKY), Wistar, and Fischer-344 rats. Physiol Behav. 1993;54:845–852. doi: 10.1016/0031-9384(93)90291-m. [DOI] [PubMed] [Google Scholar]
- 57.Baum AE, Solberg LC, Churchill GA, Ahmadiyeh N, Takahashi JS, Redei EE. Test- and behavior-specific genetic factors affect WKY hypoactivity in tests of emotionality. Behav Brain Res. 2006;169:220–230. doi: 10.1016/j.bbr.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nosek K, Dennis K, Andrus BM, et al. Context and strain-dependent behavioral response to stress. Behav Brain Funct. 2008;4:23. doi: 10.1186/1744-9081-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marti J, Armario A. Forced swimming behavior is not related to the corticosterone levels achieved in the test: a study with four inbred rat strains. Physiol Behav. 1996;59:369–373. doi: 10.1016/0031-9384(95)02104-3. [DOI] [PubMed] [Google Scholar]