Abstract
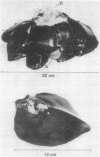
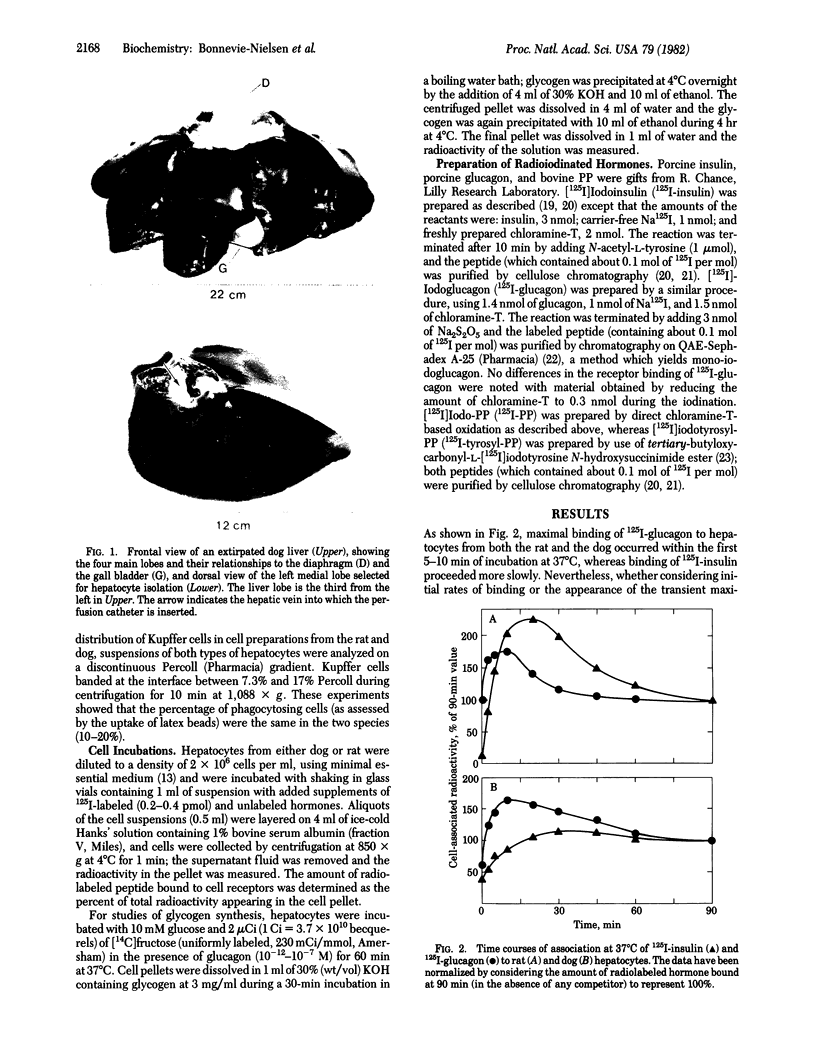
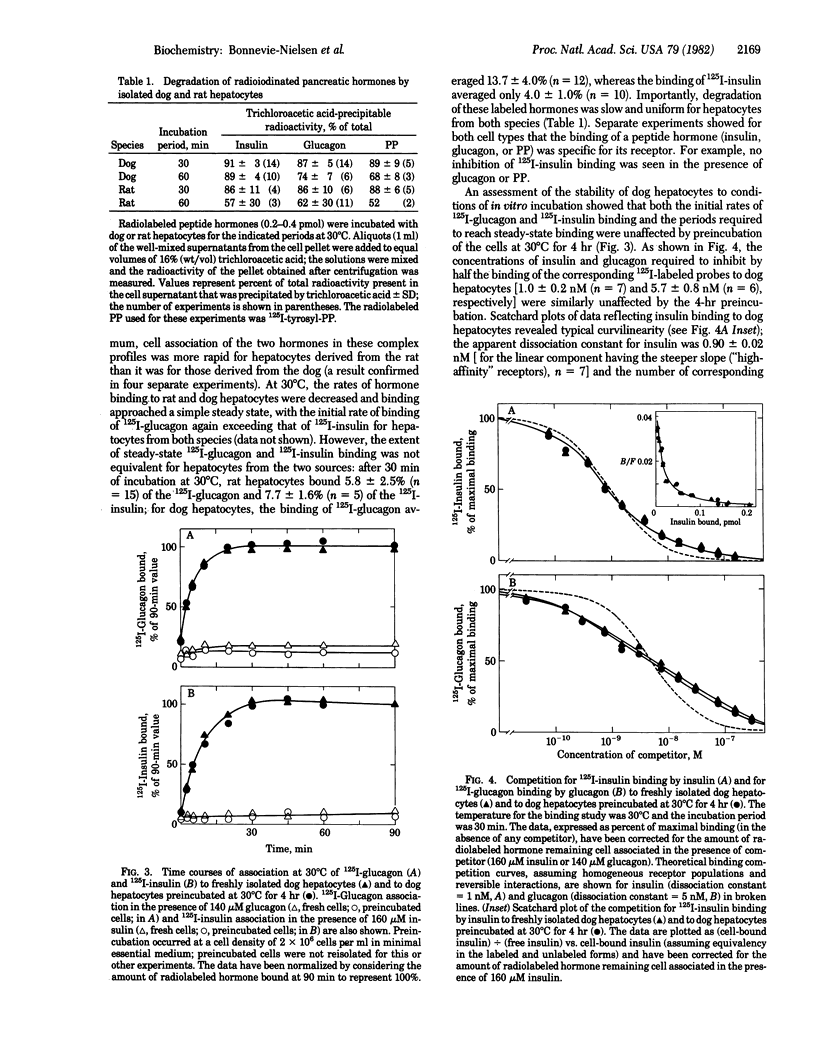
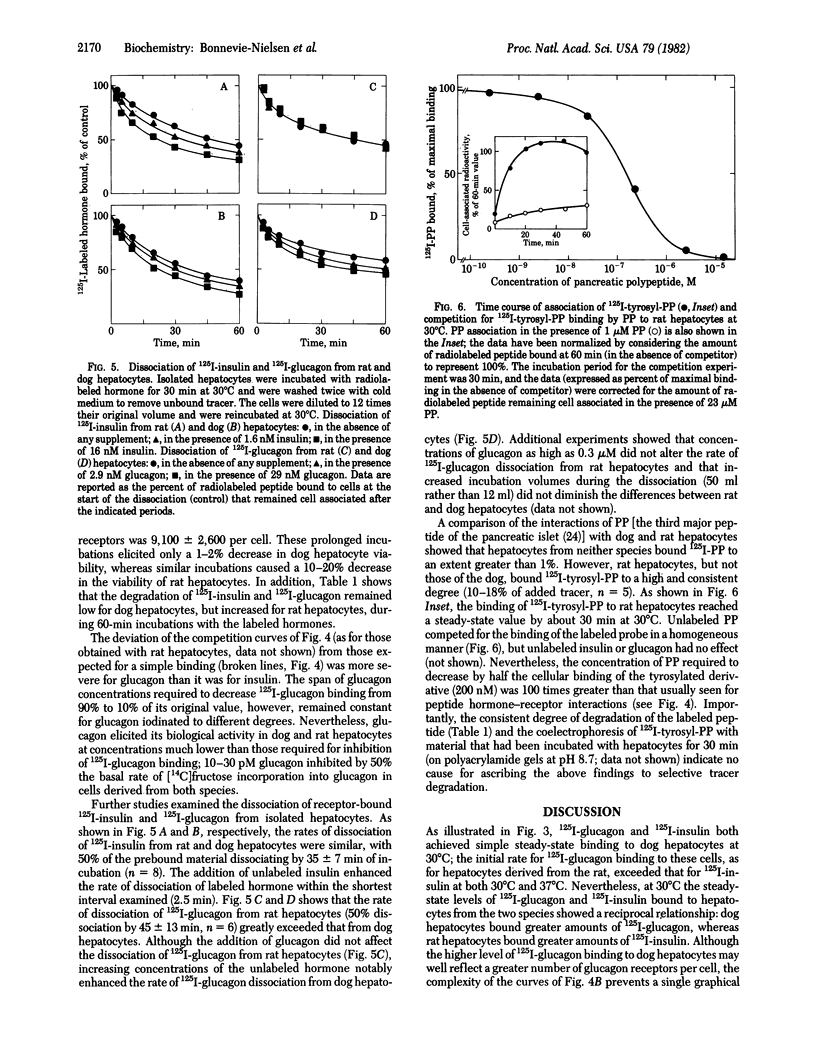
In order to evaluate potential differences in the kinetics of peptide hormone-receptor interactions in hepatocytes of different species, we developed a simple procedure for the isolation of canine hepatocytes. Cells (obtained by collagenase perfusion of an extirpated dog liver lobe) were isolated with uniform high viability and yield. In addition, isolated dog hepatocytes tolerated incubation for at least 4 hr in defined medium with only a slight decrease in viability and with no change in the kinetics of [125I]iodoinsulin or [125I]iodoglucagon binding to cell-surface receptors. Comparisons of peptide hormone interactions with isolated dog and rat hepatocytes showed that (i) [125I]iodoglucagon associated with specific membrane receptors more rapidly than did [125I]iodoinsulin, for both rat and dog hepatocytes and at both 30 degrees C and 37 degrees C; (ii) the steady-state binding of [125I]iodoglucagon at 30 degrees C was greater than that of [125I]iodoinsulin in dog hepatocytes, but the reverse relationship held in rat hepatocytes; (iii) the rate of dissociation of [125I]iodoinsulin from hepatocytes of both species was enhanced by the presence of the unlabeled hormone, whereas the rate of dissociation of receptor-bound [125I]iodoglucagon was enhanced by the presence of unlabeled glucagon only in hepatocytes derived from the dog; and (iv) [125I]iodopancreatic polypeptide bound to neither rat nor dog hepatocytes, although the [125I]iodotyrosylated peptide bound to rat hepatocytes with an unusually high apparent dissociation constant. While confirming essential findings of pancreatic hormone binding to isolated hepatocytes, this comparison suggests that both qualitative and quantitative aspects of hormone-target cell interactions can show interspecies variability.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Blix P. M., Rubenstein A. H., Tager H. S. Iodotyrosylation of peptides using tertiary-Butyloxycarbonyl-L-[125I]iodotyrosine N-hydroxysuccinimide ester. Anal Biochem. 1980 Mar 15;103(1):70–76. doi: 10.1016/0003-2697(80)90238-9. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Tager H. S. [(125I]IodotyrosylB1]insulin. Semisynthesis, receptor binding, and cell-mediated degradation of a B chain-labeled insulin. J Biol Chem. 1981 Apr 25;256(8):4042–4049. [PubMed] [Google Scholar]
- Berhanu P., Olefsky J. M. Effects of insulin and insulin-like agents on the glucose transport system of cultured human fibroblasts. Diabetes. 1981 Jun;30(6):523–529. doi: 10.2337/diab.30.6.523. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Barazzone P., Freychet P., Le Cam A., Orci L. Intracellular localization of 125I-labeled insulin in hepatocytes from intact rat liver. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2803–2807. doi: 10.1073/pnas.76.6.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L. Peptide hormone receptors. Annu Rev Physiol. 1977;39:529–557. doi: 10.1146/annurev.ph.39.030177.002525. [DOI] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Siegel J. A., Olefsky J. M. Insulin-stimulated glucose transport in human adipocytes. Am J Physiol. 1979 Jun;236(6):E621–E625. doi: 10.1152/ajpendo.1979.236.6.E621. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- De Meyts P., Van Obberghen E., Roth J. Mapping of the residues responsible for the negative cooperativity of the receptor-binding region of insulin. Nature. 1978 Jun 15;273(5663):504–509. doi: 10.1038/273504a0. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Gill R. D., Hart I. C. Properties of insulin and glucagon receptors on sheep hepatocytes: a comparison of hormone binding and plasma hormones and metabolites in lactating and non-lactating ewes. J Endocrinol. 1980 Feb;84(2):237–247. doi: 10.1677/joe.0.0840237. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Sonne O. Binding and receptor-mediated degradation of insulin in adipocytes. J Biol Chem. 1978 Nov 10;253(21):7857–7863. [PubMed] [Google Scholar]
- Jorgensen K. H., Larsen U. D. Purification of 125 I-glucagon by anion exchange chromatography. Horm Metab Res. 1972 May;4(3):223–224. doi: 10.1055/s-0028-1097092. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J Clin Invest. 1980 Oct;66(4):763–772. doi: 10.1172/JCI109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott D. M., Howard B. V., Bennett P. H. Stoichiometric binding and regulation of insulin receptors on human diploid fibroblasts using physiologic insulin levels. J Biol Chem. 1979 Sep 25;254(18):8762–8767. [PubMed] [Google Scholar]
- Olefsky J. M., Green A., Ciaraldi T. P., Saekow M., Rubenstein A. H., Tager H. S. Relationship between negative cooperativity and insulin action. Biochemistry. 1981 Jul 21;20(15):4488–4492. doi: 10.1021/bi00518a038. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M., Chang H. Interactions between insulin and its receptors after the initial binding event. Functional heterogeneity and relationships to insulin degradation. Diabetes. 1979 May;28(5):460–471. doi: 10.2337/diab.28.5.460. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding to monocytes and total mononuclear leukocytes from normal and diabetic patients. J Clin Endocrinol Metab. 1976 Jul;43(1):226–231. doi: 10.1210/jcem-43-1-226. [DOI] [PubMed] [Google Scholar]
- Pangburn S. H., Newton R. S., Chang C. M., Weinstein D. B., Steinberg D. Receptor-mediated catabolism of homologous low density lipoproteins in cultured pig hepatocytes. J Biol Chem. 1981 Apr 10;256(7):3340–3347. [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- Terris S., Hofmann C., Steiner D. F. Mode of uptake and degradation of 125I-labelled insulin by isolated hepatocytes and H4 hepatoma cells. Can J Biochem. 1979 Jun;57(6):459–468. doi: 10.1139/o79-059. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]