Abstract
The protein kinase B-Raf is a critical component of the Ras/MAPK signaling pathway. An oncogenic B-Raf mutation that constitutively activates the kinase was identified in z50% of melanoma patients and in other cancers. A structure-guided drug discovery approach enabled the development of Zelboraf, a targeted inhibitor of oncogenic B-Raf. This drug has been used successfully in the clinic to treat metastatic melanoma patients harboring B-Raf mutations.
Analysis of the genetic and molecular basis of cancer carried on during the past four decades has provided a wealth of information about critical components of signaling pathways that are mutated or become deregulated in cancer. Because protein phosphorylation controls many fundamental cellular processes (such as cell proliferation, differentiation, cell survival, movement, and metabolism), it is not surprising that many protein kinases (Manning et al., 2002) are activated by gain-of-function mutations or by other cellular aberrations that occur in cancer (Futreal et al., 2004). Consequently, a variety of kinase inhibitors were identified and successfully applied in the past decade, engendering the paradigm of targeted therapy for cancers driven by protein kinases activated by oncogenic mutations or by overexpression. One of the best examples of targeted cancer therapy is treatment of melanoma patients harboring an oncogenic B-Raf mutation (V600E) with the kinase inhibitor Zelboraf (PLX 4032), whose genesis is described in this article.
Raf is an important component of the Ras–MAPK pathway
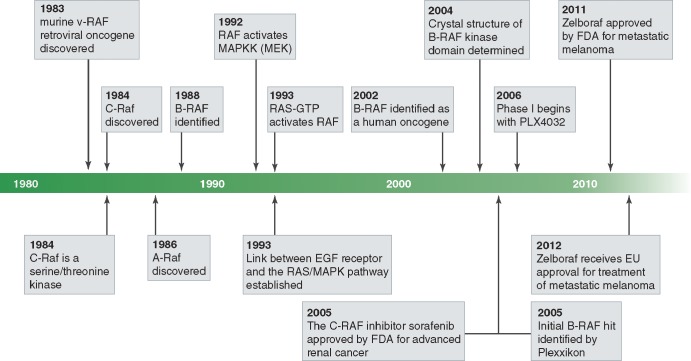
Raf was discovered in 1983 as a viral oncogene designated V-Raf (rapidly accelerated fibrosarcoma) that was isolated from 3611-MSV, a retrovirus that induces fibrosarcoma in mice (Rapp et al., 1983). An avian counterpart of the same oncogene designated V-Mil captured by the MH2 retrovirus was also discovered in 1983 (Coll et al., 1983). The cellular human homologue of V-Raf or V-Mil is called C-Raf, and the two other members of the gene family, designated A-Raf and B-Raf, were discovered in 1986 and 1988, respectively (Fig. 1; Huleihel et al., 1986; Ikawa et al., 1988). The three members of the Raf family are Ser/Thr kinases that are regulated by a variety of extracellular cues. Although the three Raf proteins are differentially expressed in a variety of tissues and cells, many tissues or cells express more than one Raf isoform.
Figure 1.
History of Raf protein kinases. Milestones from the initial discovery of Raf as a viral oncogene to the FDA and EMEA approval of a B-RAFV600E inhibitor, Zelboraf, for treatment of metastatic melanoma in the USA and EU, respectively.
During the early nineties, an extensive body of research showed that Raf kinases are an important node of a cellular signaling pathway that relays information from the cell surface into the cytoplasm and to the nucleus to regulate gene transcription and a variety of other pleiotropic cellular responses. The pathway starts at the cell membrane with activation of the EGF receptor (EGFR) or other receptor tyrosine kinases (RTKs) that recruit and activate the Grb2–Sos complex by direct or indirect mechanisms. Grb2 is a small adapter protein that forms a complex with the Ras guanine nucleotide exchange factor (GEF) Sos, thereby linking RTK stimulation at the cell membrane to activation of the small GTPase Ras inside the cell (Lowenstein et al., 1992). Activated GTP-bound Ras at the cell membrane induced by EGF stimulation or by oncogenic mutations forms a physical complex with a Raf protein that functions as the first kinase of a three-kinase cascade involving the two protein kinases designated MAPKK (MEK) and MAPK (ERK; Cobb, 1999). The Raf–Mek–ERK cascade (also denoted the MAPK pathway) is highly evolutionarily conserved from yeast to man (Fig. 2 A). Moreover, the MAPK pathway plays an important role in the control of cell proliferation, differentiation, metabolism, and other critical cellular processes. Importantly, aberrant activation of the MAPK pathway caused by gain-of-function mutations in key elements of this signaling pathway (e.g., Ras) or loss-of-function mutations in critical negative regulators of the signaling pathway (e.g., NF1) have been implicated as important oncogenic drivers of many cancers (Dhillon et al., 2007). As a result, investigators in academia, biotechnology, and pharmaceutical industries have invested significant effort over the past few decades to pharmacologically target critical components of this pathway. The first C-Raf inhibitor, designated Sorafenib, was approved in 2001 by the FDA for the treatment of advanced renal cancer. Sorafenib is a small-molecule ATP inhibitor that blocks the proliferation of RAS-driven cancer cell lines in culture and tumor growth in mouse xenograft models. However, later work proposed that the success of this drug in advanced renal cancers was primarily due to its antiangiogenic effects through inhibition of the RTK VEGF receptor-2 rather than inhibition of Raf kinases (Escudier et al., 2007).
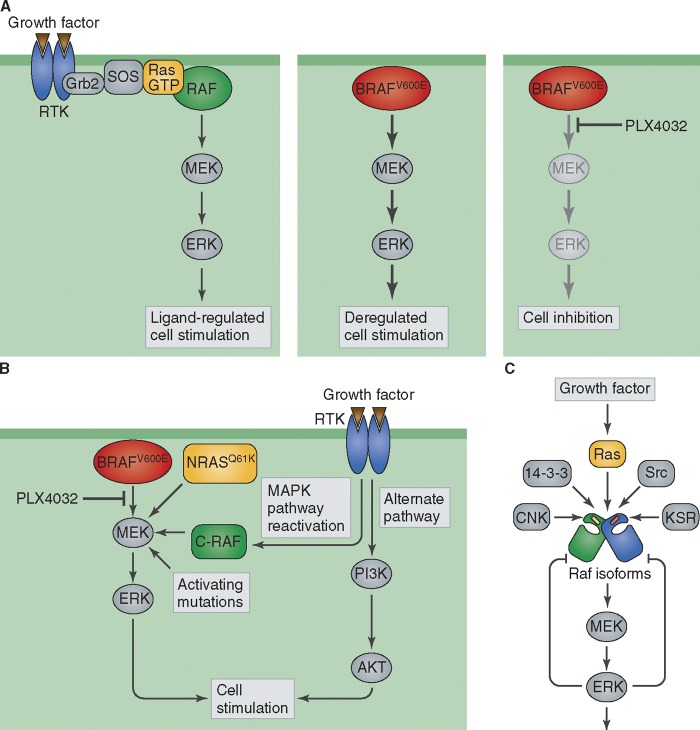
Figure 2.
B-RAF kinase inhibition by Zelboraf, and molecular mechanisms underlying resistance to Zelboraf treatment. (A) Raf kinases function as a critical node of a signal transduction pathway initiated at the cell membrane by RTK stimulation, leading to activation of the MAPK pathway. The constitutively stimulated kinase activity of B-RAF V600E oncogenic mutant in melanoma is blocked by Zelboraf (PLX4032) treatment, resulting in inhibition of tumor growth. (B) Molecular mechanism of resistance to Zelboraf treatment in melanoma. Drug resistance was shown to be mediated by different mechanisms that bypass PLX4032 inhibition of oncogenic mutant B-RAF, including expression of activated NRAS Q61K and KRAS K177N, activation of RTKs that reactivate MAPK and induce PI3K/AKT stimulation, or by mutational activation of members of the MEK kinase family. (C) Various positive and negative signals that regulate Raf and the MAPK pathway. The activity of RAF kinases is regulated by homo- and herterodimerization of the three Raf isoforms, and by interactions with additional factors that exert either positive (Src, KSR, and CNK) or negative (ERK, 14-3-3) effects on the MAPK signaling pathway. Red and yellow bars in the Raf molecule represent the regulatory αc helix.
B-Raf takes center stage in melanoma
In 2002, almost two decades after its discovery, B-Raf was shown to be a human oncogene. A V600E B-Raf mutation, which renders the kinase constitutively active, was identified in ∼50% of malignant melanomas, in 15% of thyroid tumors, in 8% of colon carcinoma, and in 4% of all solid tumors (Davies et al., 2002). Moreover, colon cancers harboring B-Raf mutations exhibit poor prognosis compared with colon cancers expressing wild-type (WT) B-Raf (Tol et al., 2009). The V600E B-Raf kinase mutation caused sustained ERK activation, transformed cells in vitro, and promoted tumor formation in xenograft mouse models. Knockdown of mutant B-Raf by RNA interference in cancer cells abrogated ERK activation and cell transformation. These exciting results suggested that selective pharmacological inhibition of B-Raf kinase activity may be clinically beneficial for treating cancers harboring B-Raf mutations (Davies et al., 2002; Solit et al., 2006).
Plexxikon’s powerful platform
The drug discovery platform developed by the biotechnology company Plexxikon offered a powerful strategy for discovering selective inhibitors for a variety of targets (Card et al., 2005), such as protein kinases. Briefly, Plexxikon’s platform involves an iterative approach starting with identification of low-affinity inhibitors followed by structural determination using crystallography to reveal the molecular basis underlying their inhibitory activity. Newly discovered chemical scaffolds are developed from the original hits using biochemical, structural, and computational approaches and further optimized into selective and potent inhibitors for a particular target protein such as the oncogenic B-Raf V600E mutant protein kinase (Tsai et al., 2008). Using this scaffold-based drug discovery approach, a 7-azaindole–containing scaffold was used to build and optimize increasingly potent and selective compounds. Co-crystallization of the chemical scaffolds with WT and V600E mutant B-Raf kinase domains led to the identification of specific inhibitors for B-Raf V600E, designated PLX4720 and PLX4032, which heralded the best pharmacokinetics in rodent models. PLX4720, for example blocks B-Raf V600E kinase activity with an IC50 of 13 nM, which is more than 10-fold lower than WT B-Raf (IC50 of 160 nM). Structural analyzes using co-crystallography demonstrated that unlike Sorafenib, which binds preferentially to an inactive configuration (termed “DFG-out”) of B-Raf kinase, PLX4720 binds preferentially to the active “DFG-in” configuration. Given these encouraging results, the compound was subject to preclinical testing in a large cohort of melanoma cell lines in culture, which revealed that only melanoma cells that harbored a B-Raf V600E were sensitive to PLX4720. Next, the efficacy of the compound was evaluated in a melanoma tumor mouse xenograft model with various melanoma cell lines harboring the B-Raf V600E mutation. Remarkably, PLX4720 caused the drastic regression of tumors and decreased ERK stimulation within tumors even when orally administered (Tsai et al., 2008). Initial clinical results were promising: in the phase I dose-escalation and subsequent extension trials, PLX4032 treatment induced a complete or partial response of 81% of metastatic melanoma patients harboring the B-Raf V600E mutation with no response scored in patients with WT B-Raf (Fig. 2 A). Notably, the dose escalation trial did not require B-Raf V600E screening while the extension trial selected only metastatic melanoma patients harboring the B-Raf V600E mutation. It was impressively shown that disease response was seen in all sites of metastasis within the B-Raf V600E cohort (Flaherty et al., 2010).
How does PLX4032 work? Like other small molecule kinase inhibitors, PLX4032 binds to the nucleotide-binding pocket of activated B-Raf with greater affinity than endogenous ATP. Crystal structure of the catalytic domain of V600E B-Raf in complex with PLX4032 revealed an asymmetric kinase dimer in which the nucleotide-binding site of only one of the protomers is bound to PLX4032, resulting in a conformational change in the regulatory αC helix. This change may allosterically regulate the activity of the second unoccupied protomer within the V600E B-Raf dimer. Moreover, both the PLX4032-occupied and the free B-Raf protomers in the dimeric crystal structure exhibit DFG-in active kinase configurations. It is noteworthy that the crystal structures depict the dimeric structure of the kinase domain of mutant B-Raf lacking important regulatory regions (Bollag et al., 2010). These regulatory regions may play an important role in allosteric control mediated by the two other Raf isoforms as well as by the many known regulatory proteins that form a complex with B-Raf in cells, suggesting further complexity within this system (Fig. 2).
Subsequent phase II and phase III trials demonstrated that treatment of metastatic melanoma patients harboring the B-Raf V600E mutation was at least 10-fold more effective as compared with available standard of care dacarbazine (a DNA alkylating agent). Most significantly, in the phase III trial the patients in the dacarbazine arm of the study were able to switch to PLX4032, as it was apparent the treatment was far superior (Chapman et al., 2011; Sosman et al., 2012). In 2011 the FDA approved PLX4032 for treatment of metastatic melanoma. Following the FDA’s lead, PLX4032 was approved for metastatic melanoma in early 2012 by EMEA of the European Union.
Side effects and drug resistance
In many instances, drug studies unearth further complexities of the system. Analyses of the mechanism of action of PLX4032, or other Raf inhibitors by multiple groups (Fig. 2, B and C) revealed the unexpected capacity of this class of inhibitors to stimulate the MAPK pathway in certain cancer cells (Hatzivassiliou et al., 2010; Poulikakos et al., 2010). This “paradoxical activation” is thought to be responsible for the side effect of squamous cell carcinoma (keratoacanthoma type) seen in ∼30% of melanoma patients treated with PLX4032 (Flaherty et al., 2010). It is of note that like other intracellular signaling pathways, the Ras–MAPK signaling pathway is subject to multiple negative feedback mechanisms (e.g., ERK-dependent phosphorylation of Raf and Sos; Fig. 2 C). As B-Raf forms both homodimers and heterodimers with the two other Raf isoforms, it is thought that eliminating an inhibition by negative feedback together with cooperative interactions between dimeric Raf proteins may lead to aberrant activation of MAPK (Poulikakos et al., 2010). Recent studies demonstrate that upstream regulators of MAPK signaling, such as aberrant activation of EGFR or preexisting oncogenic activated Ras mutations in skin cells, will confer ERK activation and may lead to squamous cell carcinoma formation (Su et al., 2012).
Like patients treated with conventional chemotherapy or patients treated with other targeted cancer therapies, the majority of patients who exhibit an initial robust response toward PLX4032 treatment eventually develop acquired resistance. Several studies demonstrated that tumor heterogeneity, clonal evolution, tumor plasticity, and other mechanisms including epigenetic changes contribute toward and are responsible for drug resistance and tumor relapse (Fig. 2, A and B). The molecular mechanisms that have been identified suggest upstream activation of Ras and RTKs leading to MAPK pathway reactivation while also stimulating the alternative PI3K–AKT pathway as a major mode of acquired resistance (Nazarian et al., 2010; Villanueva et al., 2010). Also observed is that B-Raf can mediate resistance through alternative splicing and overexpression of the V600E mutant allele (Poulikakos et al., 2011; Shi et al., 2012). Finally, downstream MEK mutations were shown to confer resistance to PLX4032 (Wagle et al., 2011).
Important new insights about the molecular basis of drug resistance will be unveiled by elucidating most if not all of the additional genetic changes that take place in melanoma using state-of-the-art genomic technologies. This, together with functional genomic strategies involving synthetic lethal analysis using siRNA screening, will reveal additional epigenetic changes as well as critical signaling nodes that upon pharmacological intervention may lead, through combination therapies, to a more robust clinical response toward PLX4032 or other targeted therapies. Moreover, progress in the development of new cancer immunotherapies such as anti-CTLA4 antibodies (Waitz et al., 2012), which was approved by the FDA in 2011 for melanoma treatment, and anti-PD1 antibodies, has shown a durable clinical response for a population of melanoma and other cancer patients. This may herald the development of combination therapies with PLX4032 (or other RAF inhibitors) together with anti-CTLA4 or anti-PD1 antibodies in order to capture the pharmacological benefits of these two totally different novel cancer treatments, hopefully contributing toward tumor elimination and an eventual cure.
Conclusion
The last decade saw a remarkable progress in genomic and proteomic technologies, raising hopes that research in these fields will make a major impact on biomedical research in general and on drug discovery specifically. Indeed, a variety of novel and importantly tractable somatic mutations were discovered in many cancers during the past decade. So far one of the first if not the best example of the benefits of genomic research on cancer therapy is the identification of the B-Raf V600E mutation in melanoma and other cancers. This together with the wealth of data accumulated since the early eighties about the viral oncogene Raf, followed by studies using various biochemical and genetic approaches establishing the critical role of Raf kinases in the RAS–MAPK signaling pathway, revealed the therapeutic importance of blocking this signaling node in cancer. Moreover, concomitant dramatic progress in the structure-guided scaffold-based drug discovery platform enabled the discovery and clinical development of Zelboraf for the treatment of metastatic melanoma.
Acknowledgments
Joseph Schlessinger thanks Peter Hirth and Gideon Bollag for the many excellent and productive discussions and for the team spirit at Plexxikon enabling the development of Zelboraf. Since August 2011, Plexxikon is a fully owned subsidiary of Diichi Sankyo. J. Schlessinger was a cofounder and served as the chairman of the Plexxikon board of directors until the company was acquired by Daiichi Sankyo. Illustrations were provided by Neil Smith, www.neilsmithillustration.co.uk.
Footnotes
Abbreviations used in this paper:
- Raf
- rapidly accelerated fibrosarcoma
- RTK
- receptor tyrosine kinase
- WT
- wild type
References
- Bollag G., Hirth P., Tsai J., Zhang J., Ibrahim P.N., Cho H., Spevak W., Zhang C., Zhang Y., Habets G., et al. 2010. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 467:596–599 10.1038/nature09454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card G.L., Blasdel L., England B.P., Zhang C., Suzuki Y., Gillette S., Fong D., Ibrahim P.N., Artis D.R., Bollag G., et al. 2005. A family of phosphodiesterase inhibitors discovered by cocrystallography and scaffold-based drug design. Nat. Biotechnol. 23:201–207 10.1038/nbt1059 [DOI] [PubMed] [Google Scholar]
- Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. ; BRIM-3 Study Group 2011. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364:2507–2516 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M.H. 1999. MAP kinase pathways. Prog. Biophys. Mol. Biol. 71:479–500 10.1016/S0079-6107(98)00056-X [DOI] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. 1983. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 2:2189–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. 2002. Mutations of the BRAF gene in human cancer. Nature. 417:949–954 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- Dhillon A.S., Hagan S., Rath O., Kolch W. 2007. MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290 10.1038/sj.onc.1210421 [DOI] [PubMed] [Google Scholar]
- Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M., Negrier S., Chevreau C., Solska E., Desai A.A., et al. ; TARGET Study Group 2007. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356:125–134 10.1056/NEJMoa060655 [DOI] [PubMed] [Google Scholar]
- Flaherty K.T., Puzanov I., Kim K.B., Ribas A., McArthur G.A., Sosman J.A., O’Dwyer P.J., Lee R.J., Grippo J.F., Nolop K., Chapman P.B. 2010. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363:809–819 10.1056/NEJMoa1002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futreal P.A., Coin L., Marshall M., Down T., Hubbard T., Wooster R., Rahman N., Stratton M.R. 2004. A census of human cancer genes. Nat. Rev. Cancer. 4:177–183 10.1038/nrc1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G., Song K., Yen I., Brandhuber B.J., Anderson D.J., Alvarado R., Ludlam M.J., Stokoe D., Gloor S.L., Vigers G., et al. 2010. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 464:431–435 10.1038/nature08833 [DOI] [PubMed] [Google Scholar]
- Huleihel M., Goldsborough M., Cleveland J., Gunnell M., Bonner T., Rapp U.R. 1986. Characterization of murine A-raf, a new oncogene related to the v-raf oncogene. Mol. Cell. Biol. 6:2655–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa S., Fukui M., Ueyama Y., Tamaoki N., Yamamoto T., Toyoshima K. 1988. B-raf, a new member of the raf family, is activated by DNA rearrangement. Mol. Cell. Biol. 8:2651–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein E.J., Daly R.J., Batzer A.G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E.Y., Bar-Sagi D., Schlessinger J. 1992. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 70:431–442 10.1016/0092-8674(92)90167-B [DOI] [PubMed] [Google Scholar]
- Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. 2002. The protein kinase complement of the human genome. Science. 298:1912–1934 10.1126/science.1075762 [DOI] [PubMed] [Google Scholar]
- Nazarian R., Shi H., Wang Q., Kong X., Koya R.C., Lee H., Chen Z., Lee M.K., Attar N., Sazegar H., et al. 2010. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 468:973–977 10.1038/nature09626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos P.I., Zhang C., Bollag G., Shokat K.M., Rosen N. 2010. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 464:427–430 10.1038/nature08902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos P.I., Persaud Y., Janakiraman M., Kong X., Ng C., Moriceau G., Shi H., Atefi M., Titz B., Gabay M.T., et al. 2011. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 480:387–390 10.1038/nature10662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U.R., Goldsborough M.D., Mark G.E., Bonner T.I., Groffen J., Reynolds F.H., Jr, Stephenson J.R. 1983. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc. Natl. Acad. Sci. USA. 80:4218–4222 10.1073/pnas.80.14.4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Moriceau G., Kong X., Lee M.K., Lee H., Koya R.C., Ng C., Chodon T., Scolyer R.A., Dahlman K.B., et al. 2012. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 3:724 10.1038/ncomms1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solit D.B., Garraway L.A., Pratilas C.A., Sawai A., Getz G., Basso A., Ye Q., Lobo J.M., She Y., Osman I., et al. 2006. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 439:358–362 10.1038/nature04304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosman J.A., Kim K.B., Schuchter L., Gonzalez R., Pavlick A.C., Weber J.S., McArthur G.A., Hutson T.E., Moschos S.J., Flaherty K.T., et al. 2012. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 366:707–714 10.1056/NEJMoa1112302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F., Viros A., Milagre C., Trunzer K., Bollag G., Spleiss O., Reis-Filho J.S., Kong X., Koya R.C., Flaherty K.T., et al. 2012. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 366:207–215 10.1056/NEJMoa1105358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol J., Nagtegaal I.D., Punt C.J. 2009. BRAF mutation in metastatic colorectal cancer. N. Engl. J. Med. 361:98–99 10.1056/NEJMc0904160 [DOI] [PubMed] [Google Scholar]
- Tsai J., Lee J.T., Wang W., Zhang J., Cho H., Mamo S., Bremer R., Gillette S., Kong J., Haass N.K., et al. 2008. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl. Acad. Sci. USA. 105:3041–3046 10.1073/pnas.0711741105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J., Vultur A., Lee J.T., Somasundaram R., Fukunaga-Kalabis M., Cipolla A.K., Wubbenhorst B., Xu X., Gimotty P.A., Kee D., et al. 2010. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 18:683–695 10.1016/j.ccr.2010.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle N., Emery C., Berger M.F., Davis M.J., Sawyer A., Pochanard P., Kehoe S.M., Johannessen C.M., Macconaill L.E., Hahn W.C., et al. 2011. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 29:3085–3096 10.1200/JCO.2010.33.2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitz R., Solomon S.B., Petre E.N., Trumble A.E., Fassò M., Norton L., Allison J.P. 2012. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 72:430–439 10.1158/0008-5472.CAN-11-1782 [DOI] [PMC free article] [PubMed] [Google Scholar]