Abstract
While the most conspicuous response to low red/far-red ratios (R:FR) of shade light perceived by phytochrome is the promotion of stem growth, additional, less obvious effects may be discovered by studying changes in the stem transcriptome. Here, we report rapid and reversible stem transcriptome responses to R:FR in tomato (Solanum lycopersicum). As expected, low R:FR promoted the expression of growth-related genes, including those involved in the metabolism of cell wall carbohydrates and in auxin responses. In addition, genes involved in flavonoid synthesis, isoprenoid metabolism, and photosynthesis (dark reactions) were overrepresented in clusters showing reduced expression in the stem of low R:FR-treated plants. Consistent with these responses, low R:FR decreased the levels of flavonoids (anthocyanin, quercetin, kaempferol) and selected isoprenoid derivatives (chlorophyll, carotenoids) in the stem and severely reduced the photosynthetic capacity of this organ. However, lignin contents were unaffected. Low R:FR reduced the stem levels of jasmonate, which is a known inducer of flavonoid synthesis. The rate of stem respiration was also reduced in low R:FR-treated plants, indicating that by downsizing the stem photosynthetic apparatus and the levels of photoprotective pigments under low R:FR, tomato plants reduce the energetic cost of shade-avoidance responses.
Due to the optical properties of green leaves, canopy shade is characterized by low red/far-red ratios (R:FR) of the light. One of the most conspicuous responses of plants exposed to low, compared with high, R:FR is the promotion of stem growth (Smith, 1982; Casal and Smith, 1989; Ballaré, 1999; Morelli and Ruberti, 2002; Franklin and Whitelam, 2005; Casal, 2012). A taller stem exposes the leaves to higher and better light strata within the canopy. Conversely, leaf growth may increase, decrease, or remain unchanged when the plants receive low R:FR.
In sparse canopies, the horizontal leaves are exposed to the high R:FR of full sunlight while the vertical stems can receive low R:FR, caused by selective reflection of far-red light by the green foliage. This provides an early warning signal of the presence of neighbors (Ballaré et al., 1987). At later stages of canopy development, the leaves become mutually shaded and exposed to low R:FR. The low R:FR reaching only the stem causes a promotion of stem growth that is rapidly reversed to the prestimulation values upon return to high R:FR. However, when the low R:FR also reaches the leaves, the promotion is more persistent and only returns to the prestimulation values 24 h after the plants are exposed to high R:FR (Casal and Smith, 1988). Therefore, there is a positive correlation between the threat indicated by the shade signals and the persistence of the growth response.
In Arabidopsis (Arabidopsis thaliana) seedlings grown under continuous white light for 7 d (at which point they were producing their first true leaves), transfer to low R:FR significantly promotes the expression of cell wall-, cell elongation-, cell division-, and auxin-associated genes (Devlin et al., 2003). In Arabidopsis plants grown for 19 d under continuous white light before transfer to darkness, a pulse of far-red light given at the end of white light promoted the expression of auxin- and brassinosteroid-responsive genes both in the leaf petioles and blades, despite the contrasting growth responses of these parts of the leaf to far-red light (Kozuka et al., 2010). Conversely, several xyloglucan endotransglucosylase hydrolase (XTH) genes showed increased expression in the petiole but not in the blade, which correlates with the growth response (Kozuka et al., 2010).
Previous studies have described organ-specific transcriptome responses observed when dark-grown seedlings of Arabidopsis (Ma et al., 2005; López-Juez et al., 2008) or soybean (Glycine max; Li et al., 2011) are exposed to light for the first time. Despite the large stem-growth response to R:FR, our knowledge of stem transcriptome responses to R:FR is very limited. Here, we used light-grown tomato (Solanum lycopersicum) seedlings to investigate the stem transcriptome responses to R:FR. By comparing the stem (first internode) and the first pair of true leaves, we identified shared and organ-specific responses. We relate the differential stem and leaf transcriptome responses to organ-selective hormonal and physiological responses.
RESULTS
Shared and Specific Stem and Leaf Transcriptome Responses to Low R:FR
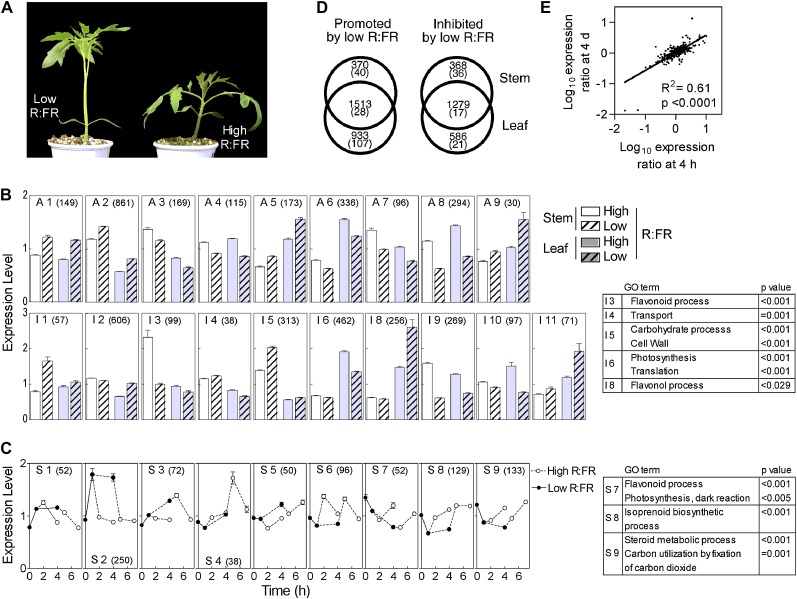
Plants of tomato were grown for 24 d under continuous white light. When the first internode (the stem between the cotyledonary node and the first leaf pair node) was 6.4 ± 0.4 mm (mean ± se), one-half of the plants were exposed to supplementary far-red light, reaching the leaves and the stem from both sides, to lower the R:FR of the horizontally propagating light from 4.6 (white light alone) to 0.05 (white light plus far-red light). Four days later (Fig. 1A), the first internode and the first pair of leaves were harvested separately. Total RNA was extracted from these samples, processed, and hybridized to tomato Affymetrix microarrays. Microarray data were filtered by presence criteria and subjected to factorial ANOVA with R:FR (high or low) and organ (stem or leaves) as main factors. The genes showing significant effects of treatments (P < 0.05, q < 0.003) were divided into three groups according to the significance of the effects of R:FR and its interaction with organ (Supplemental Table S1). The first group included genes showing significant effects of R:FR (P < 0.05, q < 0.04) and no R:FR by organ interaction. Four clusters of this gene group showed promotion by low R:FR (A1, A2, A5, A9) and five showed reduced expression in response to low R:FR (A3, A4, A6, A7, A8; Fig. 1B; Supplemental Table S1). Most genes of this first group also showed significant effects of organ, which were additive to the R:FR effects. The second group included genes showing differential responses to R:FR in the stem and leaves (significant interaction; P < 0.05, q < 0.09). Two clusters of this gene group showed promotion of expression by low R:FR in the stem (I1, I5), three clusters showed promotion of expression by low R:FR in the leaves (I2, I8, I11), two clusters showed inhibition of expression by low R:FR in the stem (I3, I9), and three clusters showed inhibition of expression by low R:FR in the leaves (I6, I7, I10; Fig. 1B; Supplemental Table S1). A few genes showed opposite responses (quantitatively modest) in stem and leaves (cluster I4). Some genes showed reduced expression in response to R:FR in both organs, but the effects were significantly higher in the stem (cluster I9). The third, residual group of genes showed no interaction, no significant effects of R:FR, and significant effects of organ and were not further analyzed (Supplemental Table S1).
Figure 1.
Shared and specific responses of the stem and leaves of tomato to low R:FR. A, Tomato plant grown under low R:FR for 4 d compared with the high-R:FR control. B, Clusters of genes with stem and/or leaf expression affected by 4 d of low compared with high R:FR. Clusters A1 to A9 show additive effects of R:FR and organ, and clusters I1 to I11 show interaction between R:FR and organ. C, Clusters of genes with early stem expression responses to low R:FR (S1–S9). In B and C, data are means and se of all the genes represented in the cluster (the number of genes is indicated in parentheses); clusters with less than 30 genes are not included (Supplemental Table S1), and overrepresented GO terms are indicated. D, Number of genes with expression promoted or inhibited by 4 d of low, compared with high, R:FR in the stem and/or leaves (the number of genes with at least 2-fold change is given in parentheses). E, Correlation of the log10 low-R:FR/high-R:FR expression ratio of the genes significantly affected after 4 h and 4 d by low R:FR. [See online article for color version of this figure.]
The total number of genes showing statistically significant responses to low R:FR in the same direction in both organs was larger than the number of genes responding significantly more in the leaves or the stem (Fig. 1D). However, the latter picture was inverted when the analysis was restricted to those genes with statistically significant responses with a magnitude of at least 2-fold. In other words, organ-independent changes are more numerous, but the largest changes in gene expression are organ specific (Fig. 1D).
Rapid and Reversible Stem Transcriptome Responses to Low R:FR
A second microarray experiment was designed to investigate the early kinetics of stem transcriptome responses to low R:FR. Plants were grown under continuous white light, exposed to supplementary far-red light for either 1 or 4 h, and harvested 0, 1, or 3 h after returning to high R:FR (seven conditions, including the control never exposed to low R:FR). To minimize the impact of circadian rhythms, the plants were grown under continuous white light and harvested simultaneously. Microarray data were filtered by presence criteria and subjected to ANOVA (Supplemental Table S2). The genes showing significant effects of treatments (P < 0.01, q < 0.10) were used for cluster analysis. A total of 424 genes (clusters S1–S5) showed increased and 410 genes (clusters S6–S9) showed reduced expression in response to low R:FR (Fig. 1C; Supplemental Table S2). These clusters differed in the kinetics of response from those that responded rapidly to the transfer from high to low R:FR and showed a rapid reversal of the response after transfer to high R:FR (clusters S2, S8, and S6, the latter with transient overcompensation after the return to high R:FR) from those showing a gradual response to low R:FR and to subsequent high R:FR (clusters S7 and S9; Fig. 1C). Many genes were significantly affected by both 4 h and 4 d of low R:FR compared with high R:FR controls. These genes showed a strong correlation in the extent of relative response at both time points (Fig. 1E), supporting the robustness of the reported changes in gene expression.
Overrepresented Gene Ontology Terms
We searched for overrepresented Gene Ontology (GO) terms within each cluster (Fig. 1). The overview of both experiments revealed three major areas of transcriptome response in the stem: (1) cell wall carbohydrate process-related genes are overrepresented in cluster I5; (2) flavonoid process-related genes are overrepresented in clusters I3 and S7; and (3) photosynthesis-related genes and photosynthetic pigment-related genes (e.g. carotenoid metabolism genes within the GO terms “isoprenoid biosynthetic process” and “steroid metabolic process”) are overrepresented in clusters S7, S8, and S9. In subsequent sections, we investigate these functions in further detail. Transport-related genes were overrepresented in cluster I4, but the responses were small in magnitude. The leaves showed photosynthetic- and translation-related genes overrepresented in cluster I6 and flavonol-related genes overrepresented in cluster I8 (Fig. 1). The clusters describing organ-specific responses contain most of the genes with a significant effect of R:FR of at least 2-fold and yielded most of the overrepresented GO terms.
Low R:FR Differentially Affects Stem and Leaf Growth
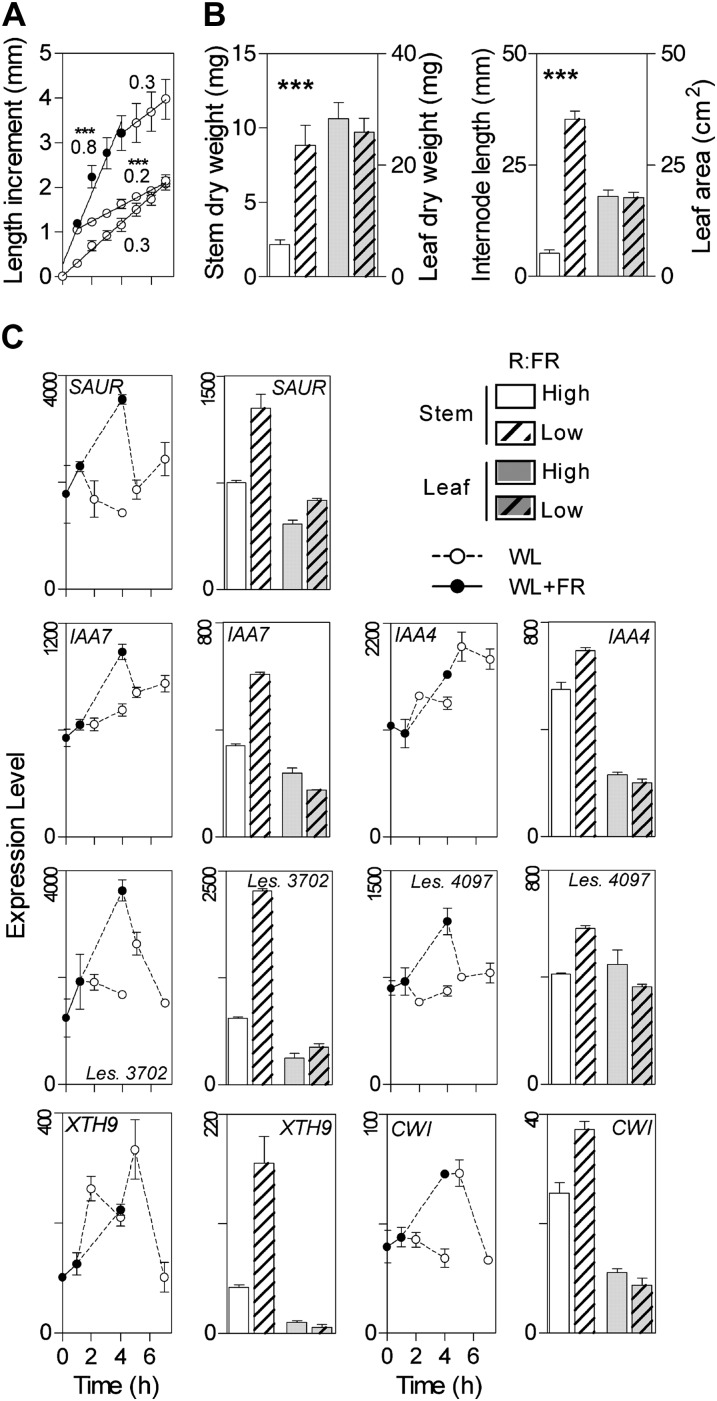
The growth rate of the stem showed a rapid promotion by low R:FR. When the plants were returned back to high R:FR, the rate of stem growth also returned rapidly to the prestimulation values (Fig. 2A). However, following a short (1-h) exposure to low R:FR, the stem growth rate showed an overcorrection upon the return to high R:FR, leading to a complete compensation of the promotion 3 h later. After 4 d of treatment, the stem showed the characteristic response to low R:FR while the leaf area and dry weight were unaffected (Fig. 2B). In accordance with the latter pattern, cell wall carbohydrate process genes are overrepresented in cluster I5, showing promotion by low R:FR in the stem and not in the leaves (Fig. 1B). Cell wall-associated genes are also promoted by low R:FR in Arabidopsis (Devlin et al., 2003). Cluster I5 includes several XTH genes, and XTH genes had previously been associated with the promotion of hypocotyl and petiole growth responses to low R:FR in Arabidopsis (Kozuka et al., 2010; Sasidharan et al., 2010). Some of these XTH genes showed rapid responses to R:FR (Fig. 2C). Auxin signaling genes are promoted by low R:FR in Arabidopsis (Devlin et al., 2003; Sessa et al., 2005; Kozuka et al., 2010), and this was also the case in the stem of tomato (Fig. 2C). These results collectively establish a base of coincidence with previous transcriptome reports in Arabidopsis, which contrasts with the tomato stem-specific patterns reported in subsequent sections.
Figure 2.
Promotion of stem growth by low R:FR. A, Stem length increment as affected by 1 or 4 h of low R:FR and the subsequent transfer back to high R:FR. The growth rate is indicated close to the corresponding regression line. B, Size (length or area) and dry weight of the stem and first pair of leaves in seedlings exposed to 4 d of low R:FR compared with the high-R:FR controls. C, Low R:FR promotes the expression of auxin response genes (IAA7, IAA4, Les. 3702, Les. 4097) and cell wall genes (XTH9, CELL WALL INVERTASE [CWI]). In A and B, data are means ± se of seven plant replicates, and significant effects of low compared with high R:FR are indicated (t test, *** P < 0.001). Data shown in B are from Supplemental Tables S1 and S2. WL, White light.
Some observations require clarification before continuing with the main analysis of this work. Previous results had shown that mustard (Sinapis alba; Casal and Smith, 1988) and tomato (Casal et al., 1995) plants exposed to low R:FR for more than 3 h have elevated rates of stem growth after transfer to high R:FR. This was confirmed under the current conditions, as upon transfer to high R:FR, the stem of plants previously exposed to 4 h of low R:FR grew more during the subsequent 20 h (1.7 ± 0.2 mm, P < 0.01, n = 42) than the plants previously exposed to only 1 h of low R:FR (1.1 ± 0.1 mm) or high R:FR controls (1.0 ± 0.1 mm). However, this long-term promotion was not observed immediately after the 4 h of low R:FR (Fig. 2A).
Low R:FR Differentially Affects Stem and Leaf Flavonoids
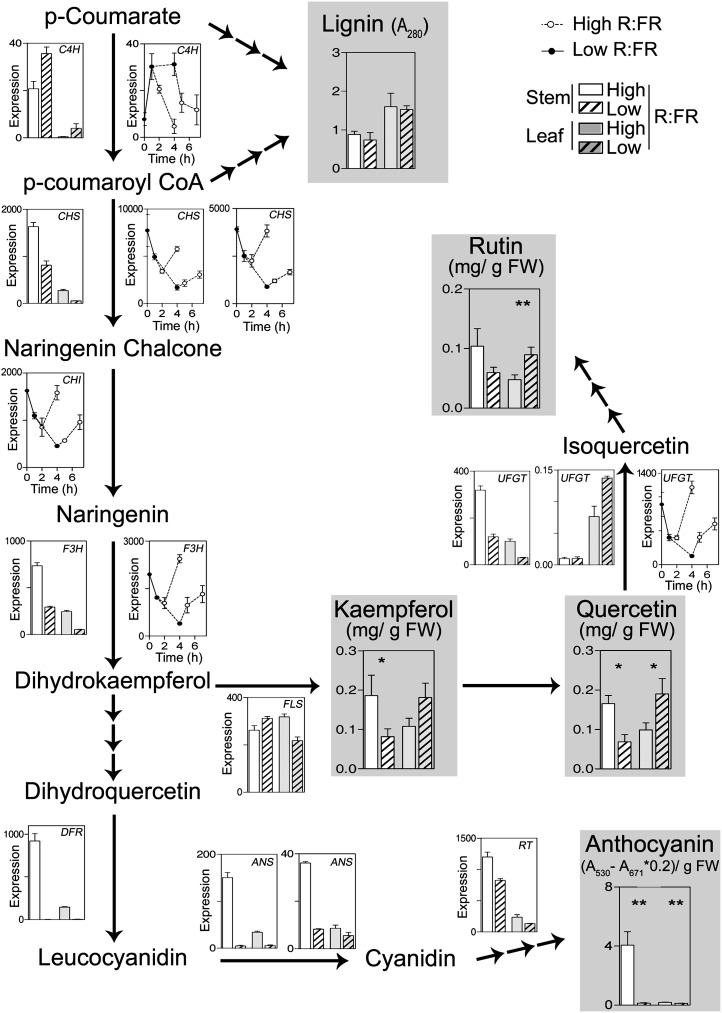
Figure 3 presents the expression patterns of all the genes of the flavonoid biosynthesis pathway (starting with p-coumaroyl-CoA and chalcone synthase) and the upstream phenylpropanoid pathway that showed significant responses to low R:FR in one or both microarray experiments. The genes involved in steps upstream of p-coumaroyl-CoA or in the branch toward lignin showed contrasting responses to R:FR, and lignin levels were unaffected by R:FR (Fig. 3). Conversely, the genes involved in the early steps of flavonoid biosynthesis, between p-coumaroyl-CoA and dihydrokaempferol, showed a rapid reduction in expression in the stem in response to low R:FR, which in some cases remained 4 d later (Fig. 3). Three genes involved in subsequent steps between dihydroquercetin and anthocyanin showed severe reductions of expression in the stem after 4 d of treatment. Consistent with the patterns of expression of upstream genes in the flavonoid pathway, anthocyanin levels were high only in the stem of high-R:FR controls and very low in the stem of low-R:FR-treated plants and the leaves of both groups (Fig. 3). Kaempferol and quercetin, which derive from dihydrokaempferol, also showed reduced levels in the stem of low-R:FR-treated plants.
Figure 3.
Reduced levels of flavonoids in the stem of low-R:FR-treated plants. The chart shows the steps of lignin and flavonoid (anthocyanin, flavonol) biosynthesis catalyzed by enzymes encoded by genes showing significant expression responses to R:FR. The expression in the stem and leaf of plants treated for 4 d with low R:FR compared with their high-R:FR controls and/or in the stem of seedlings exposed for 1 or 4 h to low R:FR and subsequently transferred back to high R:FR is shown when significant differences were observed (data are from Supplemental Tables S1 and S2). The levels of lignin, kaempferol, rutin, quercetin, and anthocyanin in plants treated for 4 d with low R:FR compared with their high-R:FR controls are indicated (data are means ± se of four to 12 plant replicates, and significant effects of low compared with high R:FR are indicated [t test, * P < 0.05, ** P < 0.01]). ANS, Anthocyanin synthase; C4H, transcinnamate 4-monooxygenase; CHI, chalcone isomerase-like protein; CHS, chalcone synthase; DFR, dihydroflavonol 4-reductase; F3H, flavanone 3β-hydroxylase; FLS, flavonol synthase; FW, fresh weight; IFR, isoflavone reductase; HQT, hydroxycinnamoyl-CoA quinate transferase; RT, rhamnosyltransferase; UFGT, flavonoid 3-glucosyltransferase; UGG, UDP-Glc:glucosyltransferase. The chart is based on the BRENDA database (http://www.brenda-enzymes.info; Scheer et al., 2011).
The flavonoid synthesis genes that show reduced expression in the stem of tomato plants exposed to low R:FR did not respond to low R:FR in whole Arabidopsis seedlings (based on the analysis of publicly available data [Sessa et al., 2005]). In Arabidopsis, mutations at the PHYB or PHYD genes, involved in the perception of high R:FR, affect the accumulation of anthocyanin in the stem (Aukerman et al., 1997). Therefore, a putative response of anthocyanin synthesis genes in the stem of Arabidopsis could become diluted by the comparatively large proportion of biomass corresponding to cotyledons and expanding leaves.
Flavonol levels increased in the leaves (mainly quercetin and rutin) in response to low R:FR (Fig. 3). In the case of rutin, this leaf response could be due in part to the promotion of the most highly expressed UDP-Glc:flavonoid 3-O-glucosyltransferase genes in the leaves. Noteworthy, the At2g36790 gene, which encodes a UDP-Glc:flavonol-3-O-glycoside-7-O-glucosyltransferase, showed a 3-fold promotion of expression by low R:FR in the Arabidopsis leaf-rich samples (high R:FR, 32 ± 0; 4-d low R:FR, 117 ± 8; Sessa et al., 2005). In other words, this flavonol biosynthesis gene increases its expression in response to low R:FR in the leaves of tomato (Fig. 3) and in leaf-rich Arabidopsis samples.
Low R:FR Differentially Affects Photosynthesis and Respiration in the Leaf and Stem
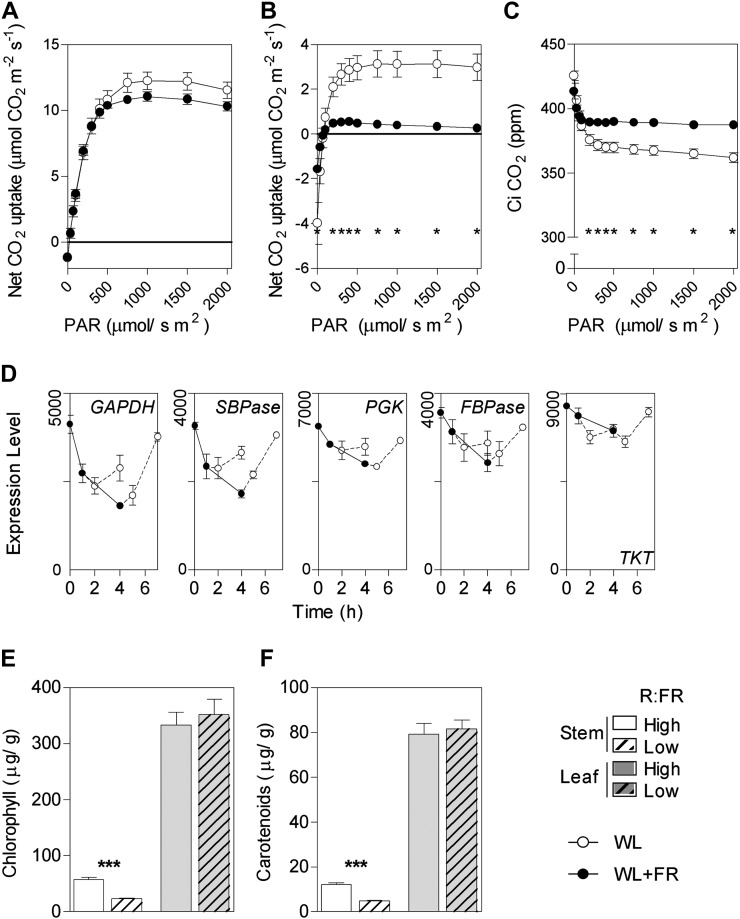
Photosynthesis-related genes are overrepresented in clusters S7 and S9, showing a rapid reduction in stem expression in response to low R:FR (Fig. 1C). These genes are linked mainly to dark reactions (Calvin cycle; Fig. 4D). In addition, genes related to isoprenoid biosynthesis and steroid metabolism, which represent steps upstream of chlorophyll and carotenoid biosynthesis, are overrepresented in clusters S8 and S9, respectively (Fig. 1C). These observations prompted us to measure the rates of net carbon dioxide exchange against irradiance in the stem and in the leaves of tomato plants exposed to low R:FR for 4 d. In the controls exposed to high R:FR, the stem showed a significant photosynthetic capacity, equivalent to approximately one-third of the leaf capacity at saturating irradiance (Fig. 4B). Noteworthy, in low-R:FR-treated stems, net carbon dioxide exchange reached the compensation point at 100 μmol m−2 s−1 and remained close to zero at higher irradiances (Fig. 4B). Furthermore, the rate of stem mitochondrial respiration (i.e. exchange rates at irradiance = 0) was approximately one-half in the plants treated with low R:FR. The internal concentration of carbon dioxide was lower in low-R:FR-treated stems, indicating that the limitation was nonstomatic (Fig. 4C). The levels of chlorophyll (Fig. 4E) and carotenoids (Fig. 4F) were also severely reduced in the stem of low-R:FR-treated plants, which is consistent with a strong downsize of the photosynthetic apparatus.
Figure 4.
Low R:FR severely reduces the stem photosynthetic capacity. A, Net carbon dioxide exchange in the leaves of plants treated for 4 d with low R:FR compared with their high-R:FR controls. B, The same in the stem of these plants. C, Internal carbon dioxide concentration for the stems shown in B. D, Low R:FR reduces the expression of Calvin cycle protein genes. E, Chlorophyll content in the stem and leaf of plants treated for 4 d with low R:FR compared with their high-R:FR controls. F, The same for carotenoid content. In A to C, E, and F, data are means ± se of six (A), three (B and C), or seven (E and F) plant replicates, and significant effects of low compared with high R:FR are indicated (t test, * P < 0.05, ** P < 0.01, *** P < 0.001). In A, maximum net carbon dioxide exchange is significantly different if the rates are corrected by using rates at 300 μmol m−2 s−1 as covariate. Data shown in D are from Supplemental Table S2. WL, White light.
The leaves also showed some reduction in maximum photosynthesis (rates of net carbon dioxide exchange at saturating irradiance; Fig. 4A), which is consistent with previous observations in the phyB mutant of Arabidopsis (Boccalandro et al., 2009). However, the magnitude of the leaf response is small when compared with that of the stem. Photosynthesis-related genes, in particular genes linked to light reactions, are overrepresented in cluster I6, showing reduced expression in the leaves in response to 4 d of low R:FR (Fig. 1B).
Low R:FR Differentially Affects Leaf and Stem Jasmonic Acid Levels
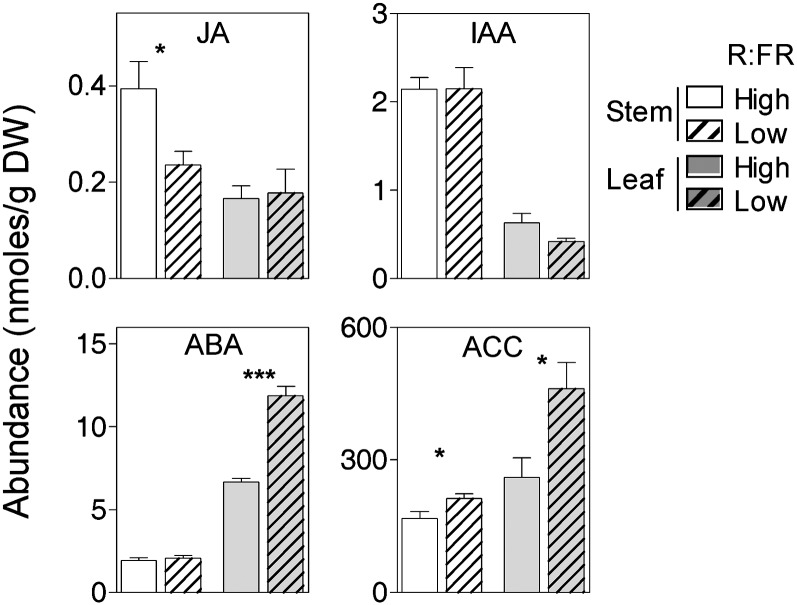
Jasmonic acid (JA), abscisic acid (ABA), ethylene (and its precursor 1-aminocyclopropane-1-carboxylic acid [ACC]), and auxin (indole-3-acetic acid [IAA]) have been reported to stimulate the synthesis of flavonoids (Feys et al., 1994; Jeong et al., 2004; Robson et al., 2010; Lewis et al., 2011). To investigate the signals potentially involved in the organ-specific patterns of expression of flavonoid biosynthesis genes and flavonoid compound levels, we measured the contents of these hormones in the stem and leaves of plants exposed for 4 d to low R:FR compared with the high R:FR controls.
The levels of JA were significantly reduced in the stem of low-R:FR-treated plants (Fig. 5), showing a pattern consistent with that of anthocyanin levels and the expression of anthocyanin synthesis genes. Noteworthy, the expression of the 12-oxophytodienoate-10,11-reductase gene involved in JA synthesis (Schaller et al., 1998; He et al., 2002) showed a rapid decrease in the stem of low-R:FR-treated plants (Supplemental Table S2). The levels of ABA increased in the leaves of low-R:FR-treated plants, those of ACC showed a similar promotion by low R:FR in the stem and leaves, and IAA contents were not significantly affected by R:FR (Fig. 5).
Figure 5.
Low R:FR alters the hormonal balance. JA, ABA, ACC, and IAA in the stem and leaves of plants treated for 4 d with low R:FR compared with their high-R:FR controls. Data are means ± se of five plant replicates, and significant effects of low compared with high R:FR are indicated (t test, * P < 0.05, *** P < 0.001). DW, Dry weight.
Light-Dependent Induction of Anthocyanin by JA
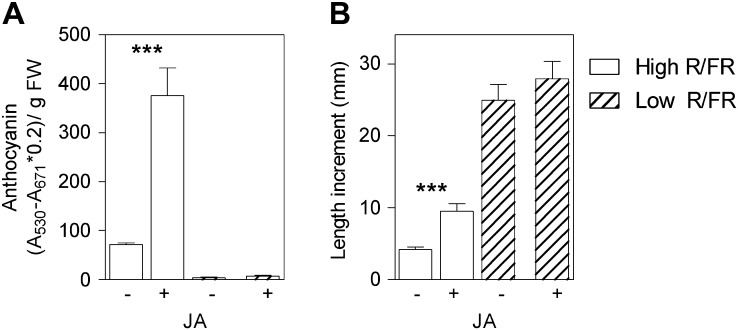
To investigate the physiological role of the stem-specific reduction in JA content, we selectively added JA to the stem of plants exposed to either high or low R:FR. Exogenous JA increased anthocyanin levels in the high-R:FR controls (Fig. 6A), suggesting that the higher levels of endogenous JA under this light condition (compared with low R:FR) would be a requisite for the observed high anthocyanin levels. Exogenous JA had no effects in low-R:FR-treated plants (Fig. 6B), suggesting that the reduction of anthocyanin contents by low R:FR would be the result of reduced sensitivity to JA.
Figure 6.
Exogenous JA applied to the stem promotes stem anthocyanin (A) and stem growth (B) in high- but not in low-R:FR-treated plants. The stem was painted with a brush soaked in a water solution containing 450 μm JA and 0.015% (v/v) Silwet, twice during the first day of treatment and daily during the subsequent 3 d of treatment. Controls were painted similarly but without JA. Data are means ± se of three (A) or seven (B) plant replicates, and significant effects of JA are indicated (t test, *** P < 0.001). FW, Fresh weight.
Previous results have provided genetic evidence for a negative role of JA signaling in shade avoidance (Robson et al., 2010). Addition of JA to the stem promoted the growth of this organ in tomato plants grown under high R:FR but did not promote stem growth at low R:FR (Fig. 6B). Therefore, JA also reduced shade avoidance in our system (note the reduced difference in length increment between high and low R:FR in JA-treated stems).
DISCUSSION
When plants are shaded by neighbors, the photoassimilates that fuel growth become limited by light availability. In response to the low R:FR signals of dense vegetation, the growth of the stem is promoted to place the leaves at a higher position, with better chances to capture light for photosynthesis (Smith, 1982; Casal and Smith, 1989; Ballaré, 1999; Morelli and Ruberti, 2002; Franklin and Whitelam, 2005; Casal, 2012). This shade-avoidance response involves enhanced growth when the available photoassimilates may already be limiting. Thanks to the high sensitivity to small reductions in R:FR caused by far-red light reflected from neighbors, in growing canopies the stem growth response can anticipate actual shade (Ballaré et al., 1987), but even so, shade-avoidance reactions continue after actual shade is established and photoassimilates become scant. The analysis of the tomato stem transcriptome and follow-up experiments reported here reveal mechanisms that reduce the energetic cost of shade-avoidance responses.
Genes involved in early steps of the flavonoid synthesis pathway and downstream genes leading to anthocyanin synthesis showed comparatively high levels of expression in the stem of plants exposed to high R:FR, a strong (and often rapid) reduction in response to low R:FR, and lower expression levels in the leaves (Fig. 3). A similar pattern was observed for the end product, as anthocyanin levels were high only in the stem of high R:FR controls. The biosynthesis of flavonols diverges from that of anthocyanin beyond dihydrokaempferol. The levels of the flavonols kaempferol and quercetin were also reduced in the stem in response to low R:FR. Flavonol levels were high in the leaves, where they were not reduced by low R:FR (Fig. 3), in accordance with previous results in Brassica napus (Gerhardt et al., 2008) and Betula pendula (Tegelberg et al., 2004). Actually, in tomato leaves, we observed increased quercetin and rutin levels in response to low R:FR, which correlated with the promotion of expression by low R:FR of some flavonol-related genes in these organs (Fig. 3). Low R:FR also reduced the expression of early genes in the isoprenoid and steroid pathways in the stem (Fig. 1C). Some of the downstream products of the latter genes, such as chlorophyll and carotenoids, were also severely reduced in the stem (Fig. 4, E and F), while ABA, which is another downstream product, was unaffected in this organ (Fig. 5), indicating the occurrence of additional control points.
In order to investigate hormonal changes potentially involved in the observed flavonoid responses, we measured the levels of IAA, ABA, JA, and ACC in the stem and leaves, as these hormones had been implicated in the induction of flavonoids. In young Arabidopsis seedlings, low R:FR increased the abundance of IAA (Tao et al., 2008; Hornitschek et al., 2012), but this was not the case in the petiole and lamina of Arabidopsis leaves (Kozuka et al., 2010) or in the stem and leaves of tomato (Fig. 5). We did observe the promotion of auxin-related genes (Fig. 2C) typically reported for Arabidopsis. One of the possibilities is that in Arabidopsis leaves and tomato organs, low R:FR cause IAA tissue redistribution rather than changes in organ IAA levels. The diversion of auxin toward outer stem cell layers has also been proposed for Arabidopsis hypocotyls (Keuskamp et al., 2010). Auxin transport could be favored by the reduced flavonoid levels observed under low R:FR (Brown et al., 2001). Low R:FR increased ACC levels in tomato as reported for sorghum (Sorghum bicolor; Finlayson et al., 1998, 1999) and tobacco (Nicotiana tabacum; Pierik et al., 2004) and increased ABA levels in the leaves as reported for the phyB mutant of Arabidopsis (González et al., 2012). However, the patterns of these hormone changes do not reflect the organ dependency of flavonoid responses. Only JA decreased in the stem and not in the leaves in parallel with the flavonoid patterns. Previous reports had also shown no JA changes in the leaves of Arabidopsis exposed to different R:FR (Moreno et al., 2009). Adding JA selectively to the stem increased anthocyanin levels in the stem of seedlings exposed to high R:FR but not in those exposed to low R:FR (Fig. 6). These results suggest that anthocyanin levels are high under high R:FR due to increased JA levels and increased sensitivity to JA.
In Arabidopsis, the coi1, jar1, jin1, and jai mutants show enhanced promotion of hypocotyl elongation by low R:FR, indicating that JA biosynthesis and signaling genes reduce shade-avoidance responses (Robson et al., 2010). Application of JA to the stem of tomato yielded a result consistent with the latter observation, as it reduced the response to low compared with high R:FR (Fig. 6A). In contrast to the case of Arabidopsis, this reduced response in tomato was caused by enhanced growth under high R:FR and not reduced growth under low R:FR. Therefore, the observed reduction of JA levels in the stem of tomato exposed to low R:FR could be part of a positive feedback loop of shade-avoidance responses in this organ.
In tomato, nonleaf green organs, including the stem, are potentially quite active photosynthetically (Hetherington et al., 1998). However, in some cases, the tomato stem shows no net carbon dioxide exchange, because its photosynthetic capacity can be enough to refix the respired carbon dioxide (Xu et al., 1997). Here, we show that in plants grown under high R:FR, the stem certainly has significant photosynthetic capacity, equivalent to approximately one-third that of the leaves. However, under low R:FR, this capacity is substantially reduced, and net rates of carbon dioxide exchange are close to zero at irradiances above the compensation point (Fig. 4B). There would be no benefit in maintaining the maximum potential of the photosynthetic apparatus when shade limits the expression of this potential. The reduction of photosynthesis has a nonstomatic origin (Fig. 4C) and could be caused by limitations in the Calvin cycle (Fig. 4D). In addition, the rates of mitochondrial respiration are also strongly reduced by low R:FR.
Taken together, these results indicate that in response to low R:FR, the stem photosynthetic apparatus is downsized. In the stem, photosynthetic and photoprotective pigment abundances decline, the photosynthetic capacity becomes minimized, and a positive relationship between R:FR and JA levels appears as an important component of the control of anthocyanin content. In turn, these changes reduce the energetic cost of producing and maintaining a longer stem, and this is manifested in lower respiration rates. The low R:FR-induced savings are very selective, as they mainly involve the stem and not the leaves, and within the stem, they involve processes linked to light (which is becoming scant) and not processes related to the stem physical support function, as lignin concentrations are not reduced.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of tomato (Solanum lycopersicum; La Germinadora) were sown on wet paper at 22°C under continuous white light (photosynthetically active radiation [PAR] = 51 µmol m−2 s−1). Seven-day-old seedlings were transplanted to 70-cm3 pots containing equal amounts of perlite (Perlome), peat moss (Finca Don Calvino), and vermiculite (Intersum) and watered as needed with a solution containing 1 g L−1 Hakaphos R (Compos). Plants were grown under continuous light at 130 µmol m−2 s−1 PAR (Li-188B sensor; LI-COR) provided by fluorescent tubes, and the temperature was 22°C. The R:FR (Skye meter SKR 100, remote probe SHR1100; Skye Instruments) was 4.6.
Light Treatments
Twenty-four-day-old plants were separated into two groups. The control group continued to grow under the high-R:FR (4.6) conditions described above. The low-R:FR (0.05) group received continuous supplementary far-red light (7.8 µmol m−2 s−1) provided from both sides by incandescent lamps in combination with a water filter (10 cm width), a red filter (no. 026; Lee Filters), and three blue acrylic filters (2.5 mm thick; Paolini 2031).
Stem and Leaf Growth
The length of the first internodes (the stem between the cotyledonary node and the first true leaf node) was measured with a ruler to the nearest millimeter. Dry weight was determined after drying at 70°C during 1 d. Leaf area was measured with a LI-3100m (LI-COR). For detailed kinetics, the plants were photographed with a Canon Power Shot A520 camera, and the images of the different time points were aligned using Photoshop 7.0 to record internode length increments.
Microarray Experiments
In the first experiment, the first pair of leaves and the first internode of both low-R:FR and control plants (three biological replicates) were harvested in liquid nitrogen, and total RNA was extracted with the RNAEasy Plant Mini Kit (Qiagen). In the second experiment, only the first internode was harvested at different times after transfer from high to low and from low to high R:FR (two biological replicates per time point). Copy DNA and copy RNA synthesis and hybridization to Affymetrix Tomato Gene Chips were performed following Affymetrix instructions. Expression data were normalized to the total sum of expression values of each microarray (Clarke and Zhu, 2006), restricted by presence criteria (presence flags for all the replicates of at least one condition), and used either for factorial ANOVA with R:FR and organ as factors or for one-way ANOVA (seven treatments). P values provided by ANOVA were used to calculate q values (Storey and Tibshirani, 2003). The cutoff values for the effects of treatments, R:FR, organ, and interaction in the factorial ANOVA of the first experiment and for the effects of treatments in the second experiment were set at P < 0.05 or q < 0.10, which ever was reached first. These values are provided in Supplemental Tables S1 and S2. To identify the clusters, we used the standard setting of the dChip software (Li and Wong, 2003).
Lignin
Relative lignin levels were determined using the thioglycollic acid method (Bruce and West, 1989). Samples containing 200 mg of plant material were stored at −20°C and subsequently homogenized. Absolute methanol (25 mL) was added to the samples, which were vacuum filtered and rinsed with further methanol on filter paper. The solid material was dried at 60°C for at least 24 h, combined with 5 mL of 2 m HCl and 0.5 mL of 80% (v/v) thioglycollic acid (Riedel-deHean), and placed in boiling water for 4 h. After centrifugation (30,000g, 10 min, 4°C), the pellets were washed with distilled water, resuspended in 5 mL of 0.5 m NaOH, and incubated at 25°C for 18 h. After further centrifugation, 1 mL of concentrated HCl was added to the supernatants and incubated for 24 h at 4°C. Finally, the samples were centrifuged (1,000g, 10 min, approximately 25°C) and the pellet was resuspended in 3 mL of 0.5 m NaOH, where absorbance was determined spectrophotometrically at 280 nm.
Flavonol Levels
Samples were ground with liquid nitrogen, and flavonoids were extracted in methanol:hexane (1:0.5, v/v, per 0.1 g fresh weight) and stirred overnight at 25°C with rotation at 200 rpm, followed by centrifugation at 13,000 rpm for 10 min. The methanol fraction was dried in a rotary evaporator, and the sample was resuspended in 400 µL of methanol (protocol based on Torres et al. [2005]). Resuspended extracts (5 μL) were injected into an Agilent 1100 HPLC system fitted with an Eclipse XDB-C18 column. Gradient elution was conducted at a flow of 1 mL min−1 at 40°C. Mobile phase solvents (HPLC grade) were water:acetic acid (99.90:0.10, v/v; A) and acetonitrile:acetic acid (99.90:0.10, v/v; B). The initial concentration was 15% B, and linear gradient elution was carried out to 36.5% B at 30 min. Detection was achieved with the Agilent 1100N G1365 MWD module at 270, 280, 254, and 220 nm.
Chlorophyll, Carotenoid, and Anthocyanin Levels
The samples were harvested in 1 mL of acetone (pure solvent) and incubated in darkness at −20°C for at least 3 d. Absorbance was measured at 661.6, 644.8, and 470 nm to calculate chlorophyll and carotenoid levels (Lichtenthaler and Buschmann, 2001). For anthocyanin levels, plant material was extracted in 1 mL of 1% (w/v) HCl methanol. Measurements of A530 were corrected for chlorophyll absorption (657 nm; Mancinelli et al., 1991).
Leaf Photosynthesis
A portable gas-exchange system (LI-COR 6400; LI-COR) was used to obtain curves of CO2 exchange against PAR provided by the red and blue diode gas-exchange system (6400-02B light-emitting diode light source; LI-COR) in fully expanded first leaves and first internode stems. The area included in the 6-cm2 chamber was recorded for each sample. Measurements started at 2,000 µmol m−2 s−1 PAR in the references chamber and decreased stepwise to 1,500, 1,000, 750, 500, 400, 300, 200, 100, 70, 35, and 0 µmol m−2 s−1.
Analysis of Hormone Abundances
Freeze-dried stem and leaf tissue was finely chopped with a razor blade, and the masses were measured (2.74–3.2 mg dry weight). A mixture of stable isotope-labeled hormones including 0.76 ng of [13C2]JA, 1 ng of [13C6]IAA, 10 ng of [2H6]ABA, and 10 ng of [2H4]ACC was added to each sample replicate in a 1.7-mL centrifuge tube. A total of 500 μL of methanol warmed to 55°C was added, and the tissue was ground with a micropestle and reextracted with 500 μL of methanol and then with 500 μL of 80% ethanol. The extractions were centrifuged, and the cleared supernatants were pooled after each extraction. The pooled extracts were dried, and the residue was resuspended in 800 μL of chloroform and partitioned against 1 mL of water adjusted to pH 9.0 with NH4OH. The aqueous fraction was recovered, adjusted to pH 5.0 with acetic acid, and partitioned against 1 mL of ethyl acetate. The organic fraction (JA, IAA, ABA) was dried and then methylated with ethereal diazomethane. The aqueous fraction containing ACC was dried, redissolved in 100 μL of 0.1 n acetic acid, and applied to a 100-μL Dowex 50 column prewashed with 500 μL of 0.1 n acetic acid. The column was washed with 500 μL of 0.1 n acetic acid. A total of 80 μL of 2 n NH4OH was applied to the column and eluted to waste, and the free ACC was collected in the next 180 μL of 2 n NH4OH and then dried. The ACC was derivatized with 80 μL of 3 mm phthalic anhydride at 90°C for 40 min, dried, and then methylated with ethereal diazomethane. Samples were analyzed on an Agilent 7890A/5975C XL gas chromatography-mass spectrometry apparatus equipped with a 0.25-mm × 30-μm DB-5MS column (0.25-μm film) using pulsed splitless injection. Helium was used as the carrier gas at 0.75 mL min−1. The inlet was maintained at 250°C, and the oven was ramped from 45°C (2.25-min initial hold) to 250°C at 40°C per minute, held at 250°C for 3 min, and then ramped to 290°C at 40°C per minute. The ion source temperature was maintained at 230°C, and the quadrupole was heated to 150°C. The ion source was operated in electron-impact mode, and both scan and selected ion data were acquired. Two ion pairs were monitored for each hormone, and the larger fragments were used for quantification (JA, 193, 195, 224, 226 mass-to-charge ratio [m/z]; IAA, 130, 136, 189, 195 m/z; ABA, 162, 166, 190, 194 m/z; ACC, 213, 216, 245, 249 m/z).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Stem and leaf transcriptome responses to R:FR.
Supplemental Table S2. Rapid changes in stem transcriptome in response to R:FR.
Glossary
- R:FR
red/far-red ratio
- GO
Gene Ontology
- JA
jasmonic acid
- ABA
abscisic acid
- ACC
1-aminocyclopropane-1-carboxylic acid
- IAA
indole-3-acetic acid
- PAR
photosynthetically active radiation
- m/z
mass-to-charge ratio
References
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. (1997) A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9: 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL. (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4: 97–102 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Sánchez RA, Scopel AL, Casal JJ, Ghersa CM. (1987) Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ 10: 551–557 [Google Scholar]
- Boccalandro HE, Rugnone ML, Moreno JE, Ploschuk EL, Serna L, Yanovsky MJ, Casal JJ. (2009) Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol 150: 1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK. (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RJ, West CA. (1989) Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol 91: 889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (2012) Shade avoidance. The Arabidopsis Book 10: e0157, doi/0110.1199/tab.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA, Boylan M, Vierstra RD, Quail PH. (1995) Is the far-red-absorbing form of Avena phytochrome A that is present at the end of the day able to sustain stem-growth inhibition during the night in transgenic tobacco and tomato seedlings? Planta 197: 225–232 [Google Scholar]
- Casal JJ, Smith H. (1988) Persistent effects of changes in phytochrome status on internode growth in light-grown mustard: occurrence, kinetics and locus of perception. Planta 175: 214–220 [DOI] [PubMed] [Google Scholar]
- Casal JJ, Smith H. (1989) The function, action and adaptive significance of phytochrome in light-grown plants. Plant Cell Environ 12: 855–862 [Google Scholar]
- Clarke JD, Zhu T. (2006) Microarray analysis of the transcriptome as a stepping stone towards understanding biological systems: practical considerations and perspectives. Plant J 45: 630–650 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Yanovsky MJ, Kay SA. (2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA, Lee IJ, Morgan PW. (1998) Phytochrome B and the regulation of circadian ethylene production in sorghum. Plant Physiol 116: 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA, Lee IJ, Mullet JE, Morgan PW. (1999) The mechanism of rhythmic ethylene production in sorghum: the role of phytochrome B and simulated shading. Plant Physiol 119: 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC. (2005) Phytochromes and shade-avoidance responses in plants. Ann Bot (Lond) 96: 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt KE, Lampi MA, Greenberg BM. (2008) The effects of far-red light on plant growth and flavonoid accumulation in Brassica napus in the presence of ultraviolet B radiation. Photochem Photobiol 84: 1445–1454 [DOI] [PubMed] [Google Scholar]
- González CV, Ibarra SE, Piccoli PN, Botto JF, Boccalandro HE. (2012) Phytochrome B increases drought tolerance by enhancing ABA sensitivity in Arabidopsis thaliana. Plant Cell Environ (in press) [DOI] [PubMed] [Google Scholar]
- He Y, Fukushige H, Hildebrand DF, Gan S. (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128: 876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington SE, Smillie RM, Davies WJ. (1998) Photosynthetic activities of vegetative and fruiting tissues of tomato. J Exp Bot 49: 1173–1181 [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, et al. (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Jeong ST, Goto-Yamamoto N, Kobayashi S, Esaka M. (2004) Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci 167: 247–252 [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka T, Kobayashi J, Horiguchi G, Demura T, Sakakibara H, Tsukaya H, Nagatani A. (2010) Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol 153: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BSJ, Muday GK. (2011) Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol 156: 144–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wong WH. (2003) DNA-chip analyzer (dChip). In G Parmigiani, ES Garrett, R Irizarry, S Zeger, eds, The Analysis of Gene Expression Data: Methods and Software. Springer, New York, pp 120–141
- Li Y, Swaminathan K, Hudson ME. (2011) Rapid, organ-specific transcriptional responses to light regulate photomorphogenic development in dicot seedlings. Plant Physiol 156: 2124–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Buschmann C. (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In R Wrolstad, ed, Current Protocols in Food Analytical Chemistry. John Wiley & Sons, New York, pp F4.3.1–F4.3.8
- López-Juez E, Dillon E, Magyar Z, Khan S, Hazeldine S, de Jager SM, Murray JAH, Beemster GTS, Bögre L, Shanahan H. (2008) Distinct light-initiated gene expression and cell cycle programs in the shoot apex and cotyledons of Arabidopsis. Plant Cell 20: 947–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Sun N, Liu X, Jiao Y, Zhao H, Deng XW. (2005) Organ-specific expression of Arabidopsis genome during development. Plant Physiol 138: 80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli AL, Rossi F, Moroni A. (1991) Cryptochrome, phytochrome, and anthocyanin production. Plant Physiol 96: 1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli G, Ruberti I. (2002) Light and shade in the photocontrol of Arabidopsis growth. Trends Plant Sci 7: 399–404 [DOI] [PubMed] [Google Scholar]
- Moreno JE, Tao Y, Chory J, Ballaré CL. (2009) Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA 106: 4935–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Cuppens ML, Voesenek LA, Visser EJ. (2004) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136: 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson F, Okamoto H, Patrick E, Harris SR, Wasternack C, Brearley C, Turner JG. (2010) Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 22: 1143–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan R, Chinnappa CC, Staal M, Elzenga JTM, Yokoyama R, Nishitani K, Voesenek LACJ, Pierik R. (2010) Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol 154: 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Hennig P, Weiler EW. (1998) 12-Oxophytodienoate-10,11-reductase: occurrence of two isoenzymes of different specificity against stereoisomers of 12-oxophytodienoic acid. Plant Physiol 118: 1345–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer M, Grote A, Chang A, Schomburg I, Munaretto C, Rother M, Söhngen C, Stelzer M, Thiele J, Schomburg D. (2011) BRENDA, the enzyme information system in 2011. Nucleic Acids Res 39: D670–D676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G, Carabelli M, Sassi M, Ciolfi A, Possenti M, Mittempergher F, Becker J, Morelli G, Ruberti I. (2005) A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev 19: 2811–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (1982) Light quality, photoperception and plant strategy. Annu Rev Plant Physiol 33: 481–518 [Google Scholar]
- Storey JD, Tibshirani R. (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelberg R, Julkunen-Tiitto R, Aphalo PJ. (2004) Red:far-red light ratio and UV-B radiation: their effects on leaf phenolics and growth of silver birch seedlings. Plant Cell Environ 27: 1005–1013 [Google Scholar]
- Torres CA, Davies NM, Yañez JA, Andrews PK. (2005) Disposition of selected flavonoids in fruit tissues of various tomato (Lycopersicon esculentum mill.) genotypes. J Agric Food Chem 53: 9536–9543 [DOI] [PubMed] [Google Scholar]
- Xu H-L, Gauthier L, Desjardins Y, Gosselin A. (1997) Photosynthesis in leaves, fruits, stem and petioles of greenhouse-grown tomato plants. Photosynthetica 33: 113–123 [Google Scholar]