Abstract
Semidwarfism has been used extensively in row crops and horticulture to promote yield, reduce lodging, and improve harvest index, and it might have similar benefits for trees for short-rotation forestry or energy plantations, reclamation, phytoremediation, or other applications. We studied the effects of the dominant semidwarfism transgenes GA Insensitive (GAI) and Repressor of GAI-Like, which affect gibberellin (GA) action, and the GA catabolic gene, GA 2-oxidase, in nursery beds and in 2-year-old high-density stands of hybrid poplar (Populus tremula × Populus alba). Twenty-nine traits were analyzed, including measures of growth, morphology, and physiology. Endogenous GA levels were modified in most transgenic events; GA20 and GA8, in particular, had strong inverse associations with tree height. Nearly all measured traits varied significantly among genotypes, and several traits interacted with planting density, including aboveground biomass, root-shoot ratio, root fraction, branch angle, and crown depth. Semidwarfism promoted biomass allocation to roots over shoots and substantially increased rooting efficiency with most genes tested. The increased root proportion and increased leaf chlorophyll levels were associated with changes in leaf carbon isotope discrimination, indicating altered water use efficiency. Semidwarf trees had dramatically reduced growth when in direct competition with wild-type trees, supporting the hypothesis that semidwarfism genes could be effective tools to mitigate the spread of exotic, hybrid, and transgenic plants in wild and feral populations.
Semidwarfism is a valuable trait in many crop species and agricultural environments. In cereal crops, it can result in decreased lodging, increased yield, and improved harvest index (Dalrymple, 1985; Hedden, 2003). Therefore, it was a critical foundation of the “Green Revolution” that resulted in large improvements of yield in wheat (Triticum aestivum) and rice (Oryza sativa; Hargrove and Cabanilla, 1979; Perovic et al., 2008). Semidwarfism has had substantial benefits for fruit tree production, where it enables earlier fruit bearing, higher yields, and easier harvests in orchards (Battisini and Battisini, 2005). Semidwarf woody species are also extensively used in ornamental horticulture, where they allow more compact forms to be fit into small areas around homes and on streets and reduce the need for pruning to avoid interference with structures and transmission lines (Busov et al., 2003).
Although against the current orthodoxy of forest tree breeding, where height growth is emphasized, semidwarfism might also have benefits for wood and biomass production (Bradshaw and Strauss, 2001). Such trees could be useful if they were less prone to wind throw due to their shorter, stockier forms and expected greater allocation to roots. Reduced stature could also result in less bending and slanting of trunks in the face of wind and gravity on hillslopes and thus reduce the extent of reaction wood formation, which degrades the performance and value of solid wood and pulp products. Reduced height and increased allocation of growth to roots might enhance stress tolerance, soil nutrient uptake, bioremediation, and carbon sequestration.
Semidwarfism can be achieved by the modification of several types of genes and physiological mechanisms, but the most prevalent and advanced forms in agriculture affect GAs or their signaling (for review, see Busov et al., 2008). GAs are endogenous plant hormones that influence several aspects of plant growth and development, including seed germination, leaf expansion, shoot growth, cell division, flower induction, and fruit development (Sun and Gubler, 2004; Fleet and Sun, 2005; Swain and Singh, 2005). With respect to shoot growth, the most obvious effect of GA is its promotion of stem elongation by stimulating both cell elongation and division (Marth et al., 1956). GA modification also has significant effects on plant biochemistry, changing the amounts and distribution of a wide variety of metabolites in shoots and roots (Rossetto et al., 2003; Chen et al., 2004; Busov et al., 2006).
Little is known about how semidwarfism affects belowground growth. GA has been shown to play a controlling role in lateral root development (Gou et al., 2010), and GA and ethylene synergistically promote both the initiation and growth of adventitious roots (Osmont et al., 2007). In tomato (Solanum lycopersicum), isogenic GA-deficient mutants (gib) allocate more biomass to roots compared with shoots (Nagel et al., 2001). In poplar (Populus spp.), semidwarf transgenic plants grown in vitro had a lower shoot-to-root ratio, which was at least partly due to proliferation of lateral roots (Busov et al., 2006; Gou et al., 2010).
As a domestication trait, semidwarfism has been proposed as a means for mitigating the spread of transgenic plants within and outside of crop environments (Al-Ahmad et al., 2005). The genetic dominance of most semidwarfism transgenes would cause reduced height growth in transgene-containing progeny, reducing their ability to compete for light. Moreover, because of the close linkage of the semidwarfism genes to other genes that were cointroduced on the same plasmid, they would also powerfully retard their spread or introgression, even in cases where the linked transgene would, on their own, impart a selective advantage. However, there have been very few plant species where this concept has been explicitly tested (Al-Ahmad and Gressel, 2006; Gressel and Valverde, 2009), and we know of no examples in woody or perennial plants.
To study the effects of semidwarfism genes in a woody plant grown under field conditions, we inserted a number of dominant GA-modifying transgenes into hybrid poplar (Populus tremula × Populus alba), the widely recognized model woody plant for genomics and biotechnology (Herschbach and Kopriva, 2002; Brunner et al., 2004a; Tuskan et al., 2004). Most of the genes studied were overexpressed forms of GA 2-oxidase, GA-Insensitive (GAI), or Repressor of GAI-Like (RGL), all known to cause semidwarfism in other plant species. GA 2-oxidase is a major GA catabolic enzyme in plants, and GAI and RGL are negative regulators of the GA signal transduction pathway (Appleford et al., 2007; Busov et al., 2008). The transgenic trees were first analyzed in the greenhouse (Busov et al., 2006) and then assayed for their effect on height growth in a 2-year field trial (Zawaski et al., 2011), from which we selected 10 transgenic events that grew at approximately three-quarters the rate of wild-type trees. The goal was to select semidwarf trees whose phenotype was not so severe as to be irrelevant to possible crop uses but strong enough to give a clear phenotype in a field study. In this study, we analyzed changes in a number of morphological, physiological, and growth traits and investigated the prospect for semidwarfism to be used as a mitigation tool to reduce the frequency of spread of transgenic and exotic species.
RESULTS
Gene Expression Analysis
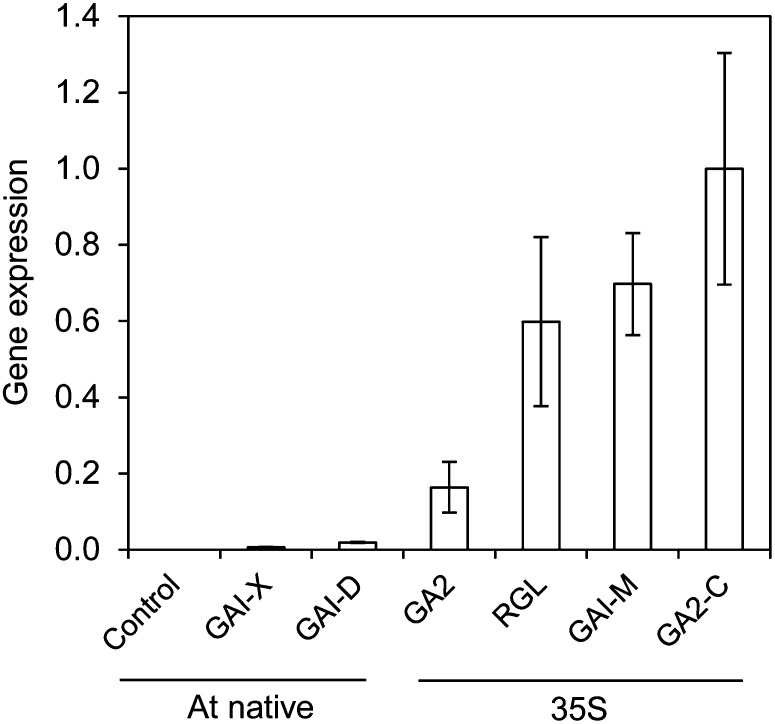
Quantitative real-time PCR (qPCR) analysis showed evidence of transgene expression for all six studied constructs, and the level varied widely among them (Supplemental Table S1). All of the constructs showing very high expression, including GA2, GAI-M, and RGL-1, were driven by the 35S promoter; in contrast, events driven by the native Arabidopsis (Arabidopsis thaliana) GAI promoter had much lower expression levels (Fig. 1). The strongest detected expression from the native GAI promoter was with the wild-type GAI gene (GAI-D); expression was considerably lower with the mutant gai gene (GAI-X).
Figure 1.
Variation in transgene expression among constructs, arranged in ascending order, and by promoter type (Arabidopsis native or 35S). Values are means ± se and were calculated from pooled biological replications and insertion events within construct types.
GA Analysis
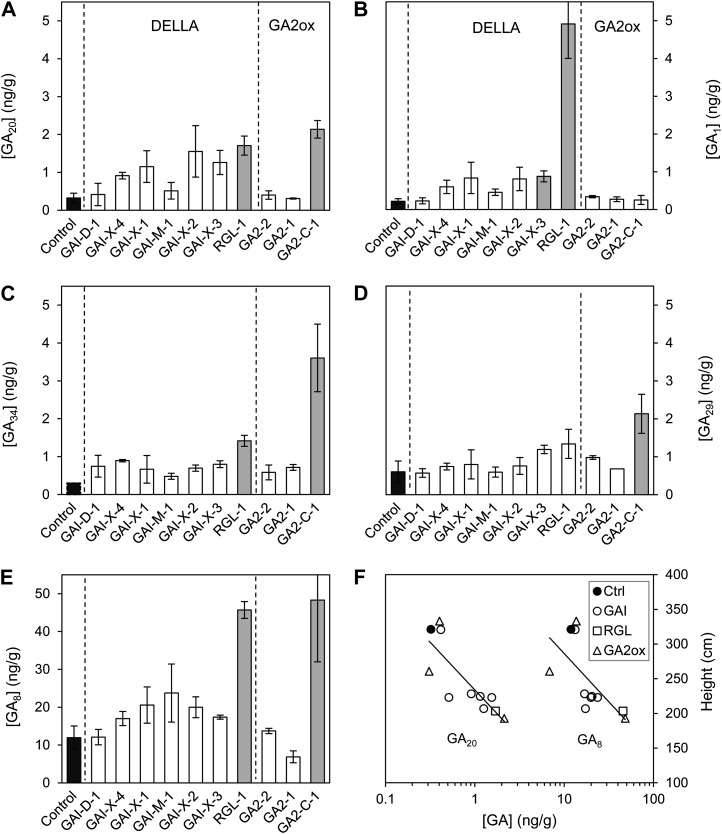
The endogenous GAs occurred in generally decreasing abundances as follows: GA8 > > GA20 > GA34 = GA29 > GA1 > GA4 (Fig. 2). ANOVA revealed that the GA quantities varied significantly across the different events for GA1, GA8, and GA34 (all P < 0.01) and for GA20 (P < 0.05). When log-transformed GA values for individual transgenic events were compared with the control, GA1 and GA8 were significantly greater in the RGL-1 event (P < 0.01) and GA20 was significantly greater in the GA2-C-1 event (P < 0.05). The GA1 precursor, GA20, was generally elevated across the semidwarfs, and GA34, the 2-hydroxylated catabolite from the alternative GA1 precursor, GA4, was somewhat elevated. The pattern for the bioeffector GA1 varied across the transgene types. It was elevated in some DELLA events and dramatically in the single RGL event, but it was unaltered in the GA2ox semidwarfs. The abundant GA1 catabolite, GA8, was elevated in the DELLA-type events, especially in RGL, and also in the most dwarfed GA2ox event, GA2-C-1.
Figure 2.
A to E, Levels of endogenous GAs (means ± se) for GA20 (A), GA1 (B), GA34 (C), GA29 (D), and GA8 (E). The lines are grouped by transgene type and then sequenced by declining height in the field study; shaded bars indicate significant (P < 0.05) difference from the control (regarding GA34, see “Materials and Methods”). F, Field study heights versus the levels of GA20 or GA8, with a logarithmic scale. For clarity, the GA8 position for GAI-D-1 was slightly offset. For the GA20 regression, r2 = 0.68, P = 0.002; for the GA8 regression, r2 = 0.46, P = 0.023. Supplemental Table S2 presents ratios of GA levels and heights compared with the control.
The ordering of events by height showed that GA levels were positively correlated with the extent of dwarfism and, thus, inversely correlated with plant height (Fig. 2). The strongest associations with height were observed for GA20 and GA8 (Fig. 2F), and a significant but moderate association was also observed for GA34 (r2 = 0.35; P < 0.05). A strong association between increased GA1 and decreased height was specifically displayed for the GA-insensitive DELLA events (r2 = 0.61; P < 0.01). Comparative values of GA levels and heights of transgenic events, versus those of the control, are provided in Supplemental Table S2.
Rooting Efficiency
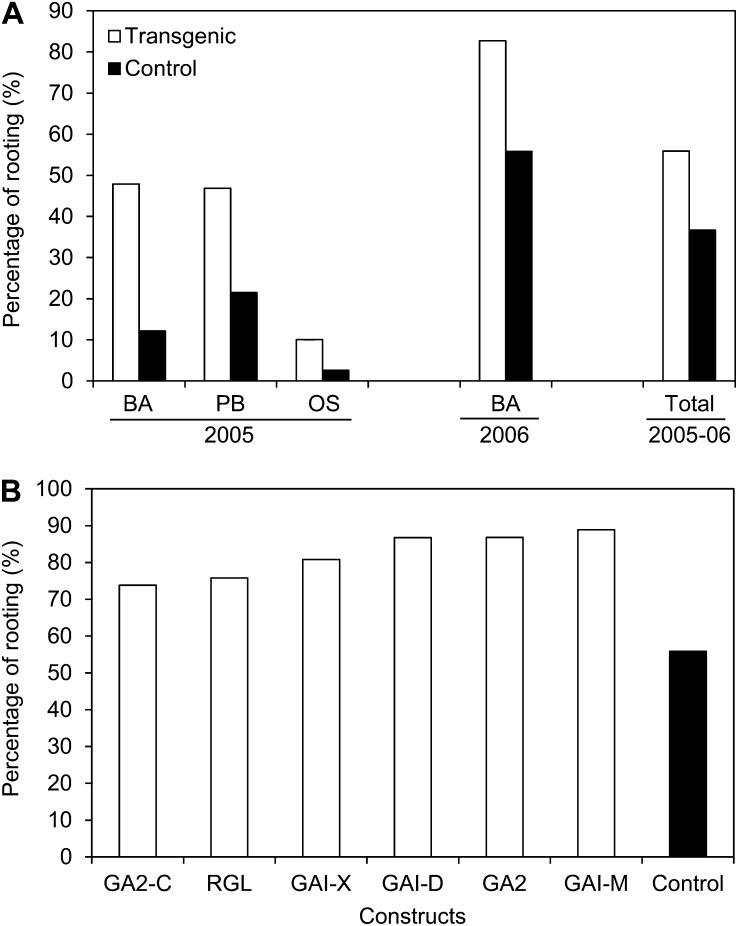
To propagate sufficient material for our field experiments, we used two commercial nurseries as well as our own greenhouse at Oregon State University (OSU) in 2005, and then a single commercial propagator in 2006. Rooting efficiency of transgenic events was higher in all locations (P < 0.001), and the trend was repeated in 2006 (Fig. 3). When constructs were considered individually, all of the transgenic events rooted with higher efficiency than controls (P < 0.001) based on results at the Broadacres Nursery in 2006. On a genotype basis, however, the plants that rooted most strongly tended to have GA profiles most similar to that of the control and little to modest dwarfism (GAI-D, GA2, and GAI-M), whereas the transgenic plants that rooted most poorly had the highest GA levels and among the strongest dwarfism (GA2-C and RGL-1; compare Figs. 4 and 5). However, all the transgenic plants tested were substantially superior to controls in rooting ability.
Figure 3.
Rooting efficiency was increased in all semidwarf transgenic events. A, Plants were sent to two commercial propagators (Broadacres Nursery [BA] and Premier Botanicals [PB]) or rooted in our greenhouse at OSU (OS) in 2005. B, Results from 2006 at Broadacres Nursery are shown by construct.
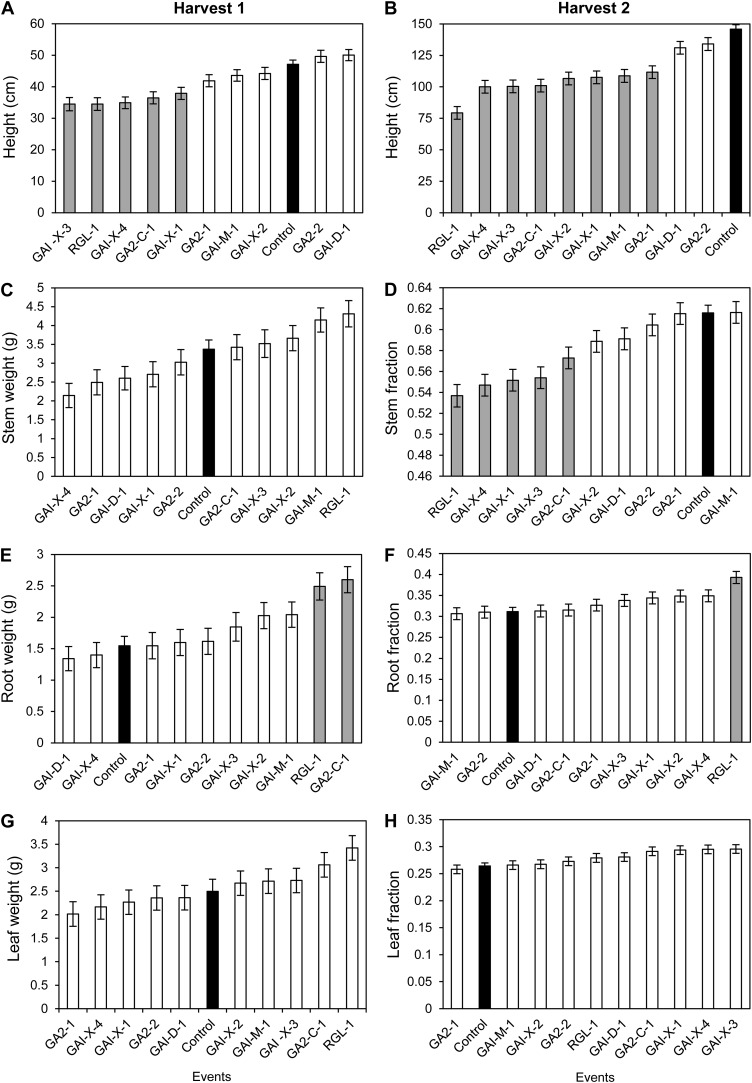
Figure 4.
Growth and allocation of biomass in the raised-bed study during early (harvest 1; A, C, E, and G) and later growth stages (harvest 2; B, D, F, and H); only results from statistically significant effects are shown. The bars represent means (least square) ± se. Supplemental Figure S1 shows dry weight and height allocation among roots, stems, and leaves. Black bars are controls; white bars are transgenic events; and gray bars are statistically significant (P < 0.05) events compared with controls.
Figure 5.
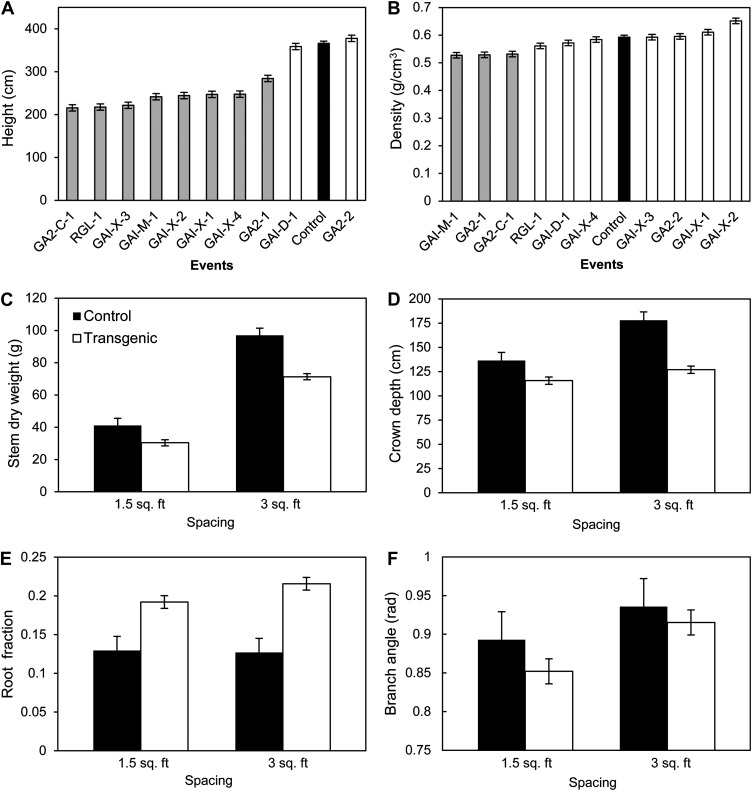
Growth and allocation of biomass in relation to density in the high-density field study. Spacing is given in feet as per the original study (where 1.5 feet = 0.45 m, 3.0 feet = 0.91 m). The bars represent means (least square) ± se. A and B represents height and stem density, respectively, for all events, where black bars are controls; white bars are transgenic events; and gray bars are statistically significant (P < 0.05) events compared with controls. C to F represent the transgenic pool versus control for shoot dry weight, crown depth, root fraction, and branch angle, respectively. Supplemental Figure S2 presents dry weight partitioning among roots and stems and height differences between high- and low-density plantings.
Raised-Bed Study
We employed raised beds to study the allocation of growth in small trees because nearly complete root biomass harvests were feasible (see “Materials and Methods”). We analyzed the results from two harvests in the raised beds; trait means are given in Figure 4 and Supplemental Table S3. Beds were not statistically significant (P < 0.05) as main effects or interactions for harvest 1 (data not shown), but they were for several traits in harvest 2 (Table I). Events were a significant source of variation for most size and mass traits in both harvests 1 and 2. Transgenics as a group were statistically different from controls only for height in harvest 1 but were differentiated also for height, diameter, stem weight, stem fraction, and leaf fraction in harvest 2. Events varied significantly for all traits except root weight and leaf weight in harvest 2. At final harvest, the transgenic events that showed the greatest degree of semidwarfism also tended to have the largest root weights, a higher proportion of root biomass, a lower proportion of stem biomass, and a higher proportion of leaf biomass (Fig. 4).
Table I. Summary of results from ANOVA based on the raised-bed study.
** P < 0.01; * P < 0.05; –, not significant.
| Variable | Harvest 1 |
Harvest 2 |
|||
|---|---|---|---|---|---|
| Event | Transgenic Versus Control | Event | Bed | Transgenic Versus Control | |
| Height (cm) | ** | ** | ** | ** | ** |
| Diameter (cm) | ** | – | ** | ** | ** |
| Volume (cm3) | ** | – | ** | ** | ** |
| Stem weight (g) | ** | – | ** | ** | ** |
| Root weight (g) | ** | – | – | ** | – |
| Leaf weight (g) | * | – | – | ** | – |
| Stem fraction | * | – | ** | – | ** |
| Root fraction | – | – | ** | ** | – |
| Leaf fraction | – | – | ** | ** | * |
High-Density Field Study
We evaluated tree physiognomy through a wide variety of measurements of plant stature, volume, biomass, and crown form taken during or at the conclusion of the second year of growth (Fig. 5; Table II; trait means and se values are given in Supplemental Tables S4 and S5). Unsurprisingly, most of the growth and yield types of traits were strongly affected by block in this agronomic field site as well as by plant spacing. The denser of the two plantings tended to give trees that were smaller, had shorter live crowns, and steeper branch angles. Events were significantly different for every trait measured, with the exception of root dry weight (Table II). For example, the most dwarfed events tended to have lower shoot weights, shorter live crowns, steeper branch angles, and substantially higher root fractions.
Table II. Summary of ANOVA from the high-density field study.
**P < 0.01; * P < 0.05; –, not significant; NA, not applicable.
| Variable | Effect |
Estimates, High Density (1.5 Feet) |
Estimates, Low Density (3 Feet) |
|||||
|---|---|---|---|---|---|---|---|---|
| Block | Event (E) | Spacing (S) | E × S | Transgenic | Control | Transgenic | Control | |
| Shoot height (cm) | ** | ** | ** | – | 241 | 321 | 290 | 412 |
| Shoot diameter (cm) | ** | ** | ** | – | 1.77 | 2.02 | 2.40 | 3.15 |
| Volume index (cm3) | ** | ** | ** | – | 802 | 1,345 | 1,571 | 4,149 |
| Crown depth (cm) | ** | ** | ** | ** | 116 | 136 | 115 | 178 |
| Crown volume (m3) | ** | ** | ** | – | 0.535 | 0.460 | 0.552 | 1.93 |
| Branch length (cm) | ** | ** | – | 46.3 | 41.9 | 58.2 | 72.8 | |
| Stem dry weight index (kg) | ** | ** | ** | ** | 0.03 | 0.04 | 0.07 | 0.10 |
| Root fraction | ** | ** | ** | ** | 0.192 | 0.130 | 0.216 | 0.127 |
| Root dry weight (g) | – | – | ** | – | 99.9 | 116 | 326 | 347 |
| Shoot dry weight (g) | ** | ** | ** | ** | 30.4 | 41.1 | 71.3 | 97.0 |
| Root-shoot dry weight ratio | ** | ** | ** | ** | 3.5 | 2.824 | 4.65 | 3.62 |
| Stem density (g/cm3) | ** | ** | ** | – | 0.574 | 0.594 | 0.591 | 0.592 |
| Branch angle (rad) | ** | ** | ** | ** | 48.8 | 51.2 | 52.4 | 53.6 |
| Midvein angle (rad) | – | ** | – | – | 81.6 | 85.1 | 82.5 | 85.3 |
| Petiole angle (rad) | – | ** | – | – | 52.1 | 51.1 | 53.1 | 49.6 |
| Petiole length (cm) | * | ** | NA | NA | 3.90 | 4.16 | NA | NA |
| Leaf blade area (cm2) | – | ** | NA | NA | 75.1 | 77.3 | NA | NA |
| Chlorophyll concentration (mg L−1) | – | ** | NA | NA | 10.5 | 8.99 | NA | NA |
| Δ13C | ** | ** | NA | NA | 17.6 | 17.9 | NA | NA |
| PC1 | ** | ** | NA | NA | 1.714 | 0.343 | NA | NA |
| PC2 | ** | ** | NA | NA | 0.105 | −0.021 | NA | NA |
| PC3 | ** | ** | NA | NA | 0.972 | −0.194 | NA | NA |
Events and spacing interacted significantly in determining stem and shoot weight, branch angle, crown depth, and root-shoot and root-total fractional biomass ratios (Table II; Fig. 5). The changes in shoot weight and crown depth between spacings were larger for the control plants than for the transgenic plants, showing a greater tolerance to variation in spacing by the semidwarf transgenic plants. In contrast, branch angle of the transgenic events became steeper in the higher planting density more so than did the control plants. Likewise, root fraction, which was nearly twice as large in the transgenic as in the control plants at high density, was reduced much more in the transgenic plants than in the control plants at low density, although it was still approximately one-third greater than for the nontransgenic controls.
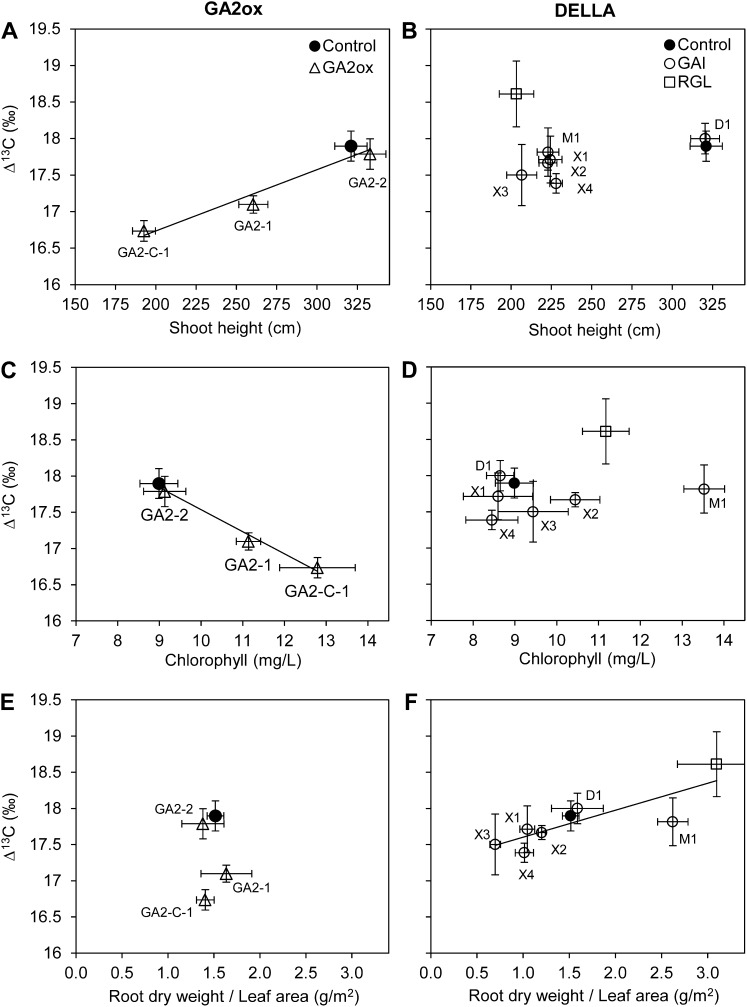
The transgenic events varied widely in shoot morphology and physiology, as shown by significant differences in chlorophyll, branch angle, petiole angle, leaf blade area, and carbon isotope discrimination in leaf tissue (Δ13C; Table II). Although based on a small number of data points, for GA2ox there was a very strong association between Δ13C and height (Fig. 6A) and chlorophyll (Fig. 6B). Chlorophyll and height were also strongly associated (r2 = 0.98; P = 0.008 [data not shown]). Thus, the more severely dwarfed events had darker green leaves with increased chlorophyll and reduced Δ13C. In contrast, for the DELLA events, there was no consistent association between Δ13C and height or chlorophyll (Fig. 6, B and D). For those GA-insensitive dwarfs, there was increased Δ13C accompanying the increase in the ratio of root dry weight to leaf area, which could provide a measure of the potential balance between water uptake and transpirational water loss.
Figure 6.
Leaf carbon isotope discrimination values for transgenic, semidwarf poplars versus height (A and B), leaf chlorophyll concentration (C and D), and root dry weight-leaf area, an index of potential transpiration, based on ratios (E and F). The left panels display the GA2ox transgenics, and the right panels show the DELLA-type transgenics. For the GAI semidwarfs, the specific genotypes are abbreviated (i.e. X3 = GAI-X-3; right panels). Only the statistically significant linear regression lines are plotted: A, r2 = 0.95, P = 0.028; C, r2 = 0.96, P = 0.008; F, r2 = 0.68, P = 0.012.
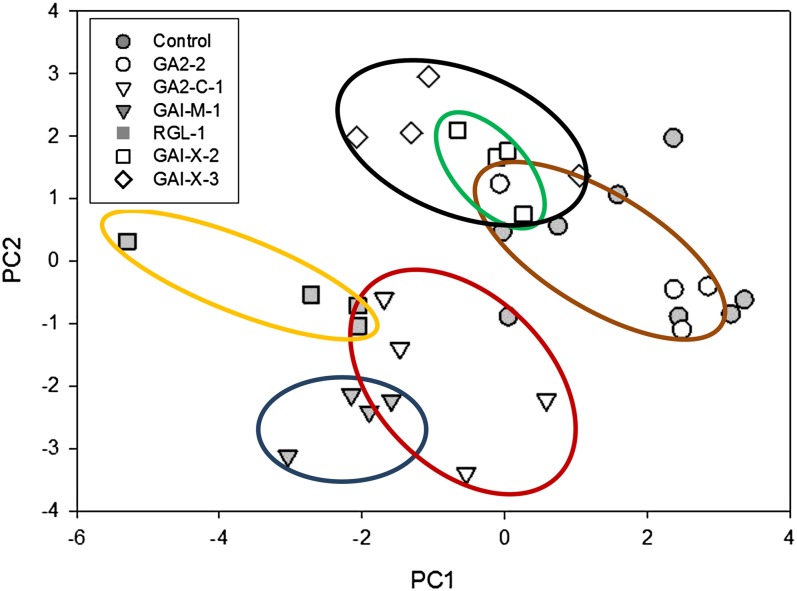
To summarize and integrate the extensive variation among transgenic events, we conducted a principal components analysis (PCA; Table III). The first PCA vector (PC1) accounted for approximately one-quarter of the variance and was strongly related to aboveground growth traits such as shoot dry weight, volume, and crown depth, but it also reflected variation in leaf and shoot morphology, but to a lesser extent. PC2 accounted for approximately one-fifth of the total variance and was strongly related to stem density, chlorophyll concentration, and leaf blade area. PC2 also reflected leaf and branch morphological traits such as midvein angle, petiole angle, petiole length, and branch length. PC3 accounted for 15% of the variance and reflected Δ13C strongly. PC3 was positively related to growth rate and mass traits and negatively related to some aspects of leaf and branch morphological traits such as midvein angle, leaf blade area, and branch angle. The distinct combinations of characteristics from the different transgenic events were evident in the relationship of Δ13C to other traits (Fig. 6). RGL-1 was often distinctive and had the greatest increase in the root-leaf ratio, a slight change in chlorophyll, and increased Δ13C. The GA2ox mutants GA2-1 and especially GA2-C-1 had elevated chlorophyll, little change in the root-leaf ratio, and decreased Δ13C. The mutant GAI-M-1 had the highest chlorophyll and also substantially elevated root-leaf ratio, and Δ13C was similar to that of the control. When PC1 versus PC2 values were graphed, the unique characteristics of each construct compared with the control were clearly visible (Fig. 7). RGL was again among the most distinctive, and the two GAI and the two GA2 constructs were clearly differentiated from each other.
Table III. Eigenvectors (multiplied by 100) from PCA, based on the high-density field study.
| Variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| Stem dry weight | 40.90 | −16.12 | 33.23 |
| Root dry weight | 27.32 | −4.76 | 35.65 |
| Aboveground volume index | 41.67 | −13.14 | 33.68 |
| Stem density | −1.60 | 43.66 | 7.97 |
| Midvein angle | 25.40 | −24.90 | −34.21 |
| Petiole angle | 24.73 | −32.09 | −19.88 |
| Branch angle | 25.98 | 3.34 | −30.97 |
| Branch length | 16.12 | 24.36 | 4.10 |
| Crown depth | 41.82 | −5.60 | −5.89 |
| Chlorophyll concentration | −19.03 | −43.40 | −5.95 |
| Petiole length | 31.52 | 32.85 | −5.79 |
| Leaf blade area | 18.77 | 47.44 | −29.98 |
| Δ13C | −13.91 | 11.82 | 53.70 |
| Percentage variance | 27.23% | 18.05% | 14.60% |
Figure 7.
Morphological and growth variation among constructs based on PCA. Ovals show the main concentrations of points for five constructs and the nontransgenic control. [See online article for color version of this figure.]
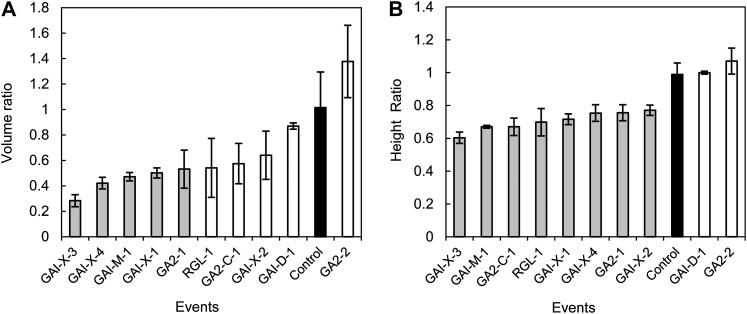
When the transgenic semidwarf trees were interplanted with nontransgenic trees, they were ineffective competitors (Fig. 8). All but two of the events had significantly reduced height, approximately 60% to 70% that of the control. This difference was increased further, to 25% to nearly 60% of controls among significantly different transgenic events, when stem volume was analyzed. This indicates that stem volume was more attenuated than height growth as a result of competition with taller trees.
Figure 8.
Growth as a ratio to nontransgenic controls when transgenic events were grown in competition with the wild type. A and B represent volume and height ratios, respectively. The bars represent means (least square) ± se. Black bars are controls; white bars are transgenic events; and gray bars are statistically significant (P < 0.05) events compared with controls.
DISCUSSION
The main goal of this study was to determine how transgene-imparted semidwarfism affected growth, morphology, aspects of physiology, and competitiveness of a tree under intensive cultivation. Although semidwarfism is widely used to increase yields in rice and wheat (David and Otsuka, 1994; Perkins, 1997), it has not been productively employed in maize (Zea mays) or many other cereals. Yield potential has mainly resulted from improved harvest index associated with dwarfing in rice and wheat, whereas in maize, tolerance to closer planting, not dwarfing, has been the major driver (Evans and Fischer, 1999). Thus, although reduced stature of fruit, ornamental, and street trees has clear value, and it is possible that semidwarf trees could also have specialized uses such as for bioremediation, stress tolerance, or carbon sequestration (Ragauskas et al., 2006) as a result of their increased allocation to root growth (Busov et al., 2006; Gou et al., 2010), it is unclear whether semidwarfism could be beneficial in promoting wood yield in dense plantings. It was also unclear whether semidwarfism observed in open grown trees would also obtain in mixed plantings, as trees compensate strongly to favor height over stem growth under competition for light. For semidwarfism to be accepted as a mitigation trait against transgene spread (Gressel, 1999), it was essential to demonstrate its effects under strong competition in the field.
As expected, the majority of the selected semidwarf events had reduced shoot growth (stem volume and biomass) compared with controls. This was expected due to the effects of GA inhibition, which is likely to impact not only height growth but also cambial proliferation and cell development (Eriksson et al., 2000; Björklund et al., 2007; Mauriat and Moritz, 2009; Mauriat et al., 2011). It was also expected due to the short-term nature of the study, where even at high density trees had nearly full sunlight and moisture (due to irrigation most of the first growing season), such that faster growing trees could produce a larger photosynthetically active canopy and thus produce more stem mass. The cumulative growth benefit from the first year of nearly open growth is unlikely to be diluted away by only a single additional year of competition for light and moisture. The significant interaction between shoot dry weight and planting density (Fig. 5C), where the growth superiority of the control trees was much reduced at high compared with low planting density, supports the contention that had the trees been grown at higher density or for a longer time period (as might characterize a commercial stand), the growth advantages could have been much smaller. The approximately 50% higher root fraction in the transgenics compared with the controls (Fig. 5E) also suggests that the growth superiority of the controls at high density might be nullified if total biomass harvests or total carbon addition to the stand, rather than shoot harvests, were considered. Finally, for commercial purposes, it is also possible that a less severe degree of semidwarfism would be desirable. We had selected genotypes with approximately 75% of wild-type growth based on a previous study to make it likely that substantial effects were detectable. A more mild degree of semidwarfism might be commercially appropriate, depending on stand density, harvest cycles, the canopy structure of particular genotypes, and the specific application.
In addition to their semidwarfism, the disadvantage in shoot growth for the semidwarf transgenics, especially at low density, is also likely to have been a result of their distinctive morphology. They had higher chlorophyll content and steeper angles of their branches and leaf petioles, which might result in increased photosynthesis and more efficiency at intercepting light, but only under intense competition. Higher photosynthetic activity in older leaves of GA-deficient transgenics was previously reported and associated with their typical dark green foliage (Biemelt et al., 2004), which should reflect elevated nitrogen as well as the increased chlorophyll (Evans, 1989). The depth of their crowns was much less sensitive to increased competition than it was for the control trees (Fig. 5D), suggesting less ability to take advantage of the lower planting density. Finally, their proportionally larger root system might have been more efficient in reaching and extracting nutrients from the soil, but it would be a benefit primarily under high root competition. This trait, combined with their potentially higher water use efficiency (discussed below), could also be important for many forest and woody energy plantations on marginal soils with little or no fertilization and irrigation, regardless of the degree of competition.
One of the major outcomes of this study, consistent with our previous work under in vitro conditions and early greenhouse growth (Gou et al., 2010, 2011), is that root biomass fraction was enhanced in semidwarf trees. Both in the raised-bed environment, where we could most fully harvest and thus more accurately measure woody root biomass, and in the field site, the transgenics showed a higher fraction of root biomass. Even under the high-density field planting, where allocation to root growth was reduced relative to shoot growth among the transgenics (Fig. 5E), the transgenic trees showed approximately one-third higher root biomass fraction than control plants. Thus, GA alteration or insensitivity clearly and consistently leads to increased partitioning to root biomass growth, with potential consequences for the use of trees for carbon sequestration, bioremediation, erosion control, and moisture or nutritional stress tolerance. Finally, during propagation, we found that the semidwarf transgenic plants had consistently higher rates of adventitious rooting. The mechanisms by which GA affects lateral rooting in poplar have been described elsewhere (Gou et al., 2010); our results show that a similar mechanism appears to operate under field conditions and thus may facilitate genotype amplification during breeding and vegetative propagation.
There was significant genetic variation among transgenic events in leaf Δ13C (Table II), which provides an integrative measure of water use efficiency (the ratio of photosynthetic carbon uptake to transpirational water loss). There are a number of mechanisms by which modification of GA physiology could have given rise to alterations in Δ13C, including through elevated nitrogen and foliar enzymes associated with the observed dark green leaves as well as through variation in stomatal properties. Stomatal conductance is a key factor controlling leaf Δ13C (Farquhar et al., 1989), and while there is limited evidence for direct effects of GA on stomatal response, GA inhibitors have been shown to decrease stomatal conductance in trees (Guak et al., 2001). GA is typically an antagonist to abscisic acid, and abscisic acid can directly influence stomatal function (Acharya and Assmann, 2009).
The trait modifications that were correlated with Δ13C suggest different potential applications for the different transgenic constructs studied. Particularly for the GA2ox events, the more severely dwarfed events had dark green leaves with increased chlorophyll content that should be associated with increased photosynthetic capacity (Fig. 6). This was tightly associated with decreased leaf Δ13C, indicating increased water use efficiency. Thus, these semidwarfs might be better suited to drought-stressed environments and could have more efficient water use in irrigated plantations. In contrast to the GA2ox semidwarfs, the more severe DELLA semidwarfs, and particularly GAI-M-1 and RGL-1, had an increased ratio of root mass to leaf area and thus potentially could sustain higher transpiration rates. This was associated with increased leaf Δ13C, as would be predicted from such a change. This would indicate decreased water use efficiency and suggests that these semidwarfs (with their proportionally larger root systems compared with the wild type) might be well suited for applications such as phytoremediation, in which increased water uptake could be desirable.
Our analyses of gene expression and GA levels were consistent with our previous work with transgenic semidwarf poplars, known mechanisms of action of the transgenes, and the tree phenotypes observed. Transgenes driven by the 35S promoter showed much stronger expression levels than did those driven by the GAI promoter; however, the transgenes driven by 35S, such as the bean (Phaseolus coccineum) GA2ox and the native GAI coding region, were generally the ones that we previously found to have weaker dwarfing effects (Zawaski et al., 2011). Therefore, much higher expression was needed for these transgenes to achieve the same level of dwarfing as for the mutant gai transgene (DELLA-less version, thus much less susceptible to degradation compared with GAI). Indeed, several orders of magnitude weaker expression of gai was able to achieve the same or a stronger level of semidwarfism than that of native GAI-containing transgenes.
Analysis of GA levels showed that GA1, GA8, and GA20 were negatively correlated with height, especially in the trees with DELLA domain-containing transgenes. This is a logical outcome because of the feedback regulation that DELLA domain proteins typically elicit on GA biosynthesis when overexpressed, resulting in higher bioactive GA concentrations (Peng et al., 1997; Cowling et al., 1998; Fu et al., 2001). This outcome is consistent with our prior GA analyses with other DELLA-type semidwarf hybrid poplars (Busov et al., 2006).
The low number of GA2ox-overexpressing transgenics (only three in this study) limited the statistical analysis of GA levels and height reduction with this transgene type. Previously, using a much larger number of independent events with the same transgene, we showed a highly significant, although nonlinear, correlation of transgene expression with height (Zawaski et al., 2011). Interestingly, despite the increased GA8 levels in the most dwarfed of the GA2ox-overexpressing transgenics, the bioactive GA1 was not significantly decreased, possibly due to a feedback mechanism that results in increased levels of the precursor GA20. This interpretation is also supported by the elevated GA29. Thus, the relatively mild phenotype of some of the GA2ox transgenics could be explained by feedback regulation compensating for increased catabolism.
Our study indicates that the different semidwarf types are not phenocopies of one another but instead display unique combinations of phenotypic traits. While all are characterized by depressed shoot elongation, alterations to other aspects of growth and development vary, probably reflecting the distinctive combinations of particular GAs and GA signaling, along with other interactions such as those between GAs and other phytohormones (Pearce et al., 2004; Gou et al., 2010; Zawaski et al., 2011). For example, as discussed above, we observed opposing responses in leaf Δ13C in the catabolic GA2ox semidwarfs versus the GA-insensitive DELLA dwarfs, despite similar changes in height. Nonetheless, all of the transgenic events were reasonably well adapted, surviving and showing generally normal bud set, cold hardiness, and bud flushing in spring. It is likely that by studying an even larger range of constructs and events, even more distinctive morphological and physiological diversity could be produced and potentially utilized in hybrid poplar breeding.
Reduced height growth confers a very significant disadvantage in the competition for light (Schwinning and Weiner, 1998), especially in shade-intolerant trees such as poplars. Thus, dwarfism has been proposed as a means for mitigating the risk of spread for fitness-promoting transgenes in annual crops (Gressel, 1999) and also in trees (Bradshaw and Strauss, 2001; Strauss et al., 2004). Dominant genes for semidwarfism that are tightly linked, and preferably flanking, other transgenes could impart a very strong selective disadvantage to all volunteers or progeny resulting from mating with wild relatives that contain the transgenes. Because of tight linkage, the rate of segregation of the dwarfism genes from the transgenes by recombination should be extremely low. Here, we tested the competitiveness, and thus the prospect for continued transgene spread in the environment, by interplanting the transgenic lines with control plants. We found that, as predicted, semidwarf transgenics were very poor competitors when intermixed with control plants. Most of the semidwarf genotypes grew only 30% to 60% in stem volume compared with that of the control trees in this short-term study. This suggests that even if stand yields of clones of semidwarf trees were similar to those of taller trees at full rotations, as surmised above, semidwarf trees would still be unlikely to survive in a mixed stand. The fitness disadvantage should be far greater in the wild, where poplar trees are established in dense patches of seedlings and almost all of these die during their first growing season (Stettler, 2009). Thus, our study supports the prospect that semidwarfism could be used as a mitigating strategy to greatly reduce the risk and significance of transgene dispersal.
CONCLUSION
Our studies suggest that for trees grown for specialty purposes such as for biofuels, carbon sequestration, bioremediation, and under highly stressful environments, or when they present risks of species or transgene invasiveness, semidwarfism genes may be of value. This results from their increased morphological and physiological diversity, and their high yield and allocation to root growth, particularly in high-density plantings.
MATERIALS AND METHODS
Overview of Experiments
We started our experiments in 2003 with a preliminary 2-year field study in Corvallis, Oregon, with seven constructs, 10 to 30 events per construct, and four trees per event (Zawaski et al., 2011). A very wide range of effects were seen, from extreme dwarfism to wild-type growth. From these trees, we vegetatively propagated a number of events with approximately 75% of the growth of wild-type trees for a raised-bed nursery study that was conducted in 2006 with 11 events derived from six constructs. Based partly on results from these studies, we selected 10 events from six constructs (Table IV) and used a commercial propagator to produce a large number of cloned copies of these events for the two high-density field studies that spanned two growing seasons, 2006 through 2007.
Table IV. Constructs used for transformation.
Code for event is given as “construct code-number of event.”
| Construct | Gene | Promoter | Terminator | Event | Code | Source of Gene |
|---|---|---|---|---|---|---|
| pLARS124 | GA 2-oxidase | 35S | NOS | 96-1, 232 | GA2-1, GA2-2 | Phaseolus coccineum |
| pNV17rgl | Atrgl-1 | 35S | NOS | 175 | RGL-1 | Arabidopsis |
| MpG3Ktg62 | AtGAI | 35S | 35S | 562-1 | GAI-M-1 | Arabidopsis |
| pA27c17-1 | PtaGA2- ox | 35S | OCS | 74 | GA2-C-1 | Hybrid poplar |
| pG3KD1 | AtGAI | Native | Native | 135 | GAI-d-1 | Arabidopsis |
| pG3Kλg | Atgai | Native | Native | 10-2, 102-2, 115, 117 | GAI-X-1, GAI-X-2, GAI-X-3, GAI-X-4 | Arabidopsis |
| Control | 717-1 | Control |
Transformation
We used a single female genotype, 717-IB4 (Populus tremula × Populus alba; provided by INRA-France), for all transformations. Transformation was performed using Agrobacterium tumefaciens strain C58/pMP90 (GV3101) essentially as described by Filichkin et al. (2007). After regeneration in selection medium, all transgenic events were verified for presence using PCR as described previously (Busov et al., 2003, 2006).
Gene Expression Analysis
We used qPCR to confirm the expression of the transgenes. RNA was extracted from young leaf tissues from two ramets of each transgenic event growing in a greenhouse in Corvallis, Oregon, using a modified Qiagen RNA extraction protocol (TURBO DNA-free kit; Ambion). All RNA samples were treated by DNase I to avoid genomic DNA contamination. First-strand synthesis of complementary DNA was carried out on 1 µg of total RNA using the SuperScript III First-Strand Synthesis System for qPCR (Invitrogen). The reverse transcription reactions were divided into aliquots and diluted three times, and 2 µL was used as a template for the PCR. Samples were run in triplicate with Platinum SYBR Green QPCR Super Mix-UDG (Invitrogen) on an Mx3000p real-time PCR system (Stratagene). The volume of the reaction was 20 µL, and the final primer concentration was 0.4 µm (primers are given in Supplemental Table S6). The best performing UBQ gene was used for normalization as described previously (Brunner et al., 2004b). The conditions for PCR were as follows: 50°C for 2 min, 95°C for 2 min, followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s. PCR efficiencies for all primers were checked by sequential dilution (1:5) of corresponding linearized vectors and were found to be 97% to 98%. The comparative method, where all data were presented relative to the UBQ internal control gene, was used for comparing the expression of transgenes between different constructs and events.
Rooting Efficiency
The rooting efficiency of transgenic events was tested at two commercial nurseries in the Willamette Valley of Oregon (Broadacres Nursery and Premier Botanicals) and at OSU in Corvallis in 2005. Rooting was performed in greenhouses using 8- to 10-cm-long stem cuttings. At Broadacres Nursery, the cuttings were placed in a 125-cm2 square pot with bottom heat at a temperature of 43°C. An intermittent mist/sprinkler water system was used for irrigating the cuttings from above, and gaseous CO2 was also pumped into the greenhouse. While planting, one to three buds were left above the soil for cuttings, and an approximately 50:50 perlite:pumice mixture was used as rooting medium. At Premier Botanicals, similar greenhouse and root heating was used, and the cuttings were also treated using high-phosphorous Peter’s fertilizer mix (J.R. Peters) and Homox Rooting Powder No. 8 (AgRx) before planting. A mixture of sand with general plant fertilizer (containing phosphorous) was used as rooting medium. At OSU, cuttings were first dipped into Rootone (rooting hormone with fungicide) and then potted in soil (Sunshine Professional Blend with 70%–80% peat moss and perlite) in 270-cm3 rose pots with heating from below. An intermittent mist system was used for irrigation. The plants were fertilized with 400 μg mL−1 20:10:20 (nitrogen:phosphorus:potassium) weekly after the cuttings were rooted.
Raised-Bed Study
We conducted a raised-bed study to allow the collection of intact root systems during early growth. The beds, approximately 0.6 m above the ground and 1.58 m wide and lined with plastic sheets below, could be dismantled and the soil washed away from seedlings to collect roots during each harvest (Fig. 9, A and B). The soil was 50% sand mixed with clay loam topsoil and had a drainage pipe (15-cm-diameter polyvinyl chloride with 1-cm-diameter holes) placed in the bottom center of each bed. The plants were irrigated to near saturation with a sprinkler system twice per day.
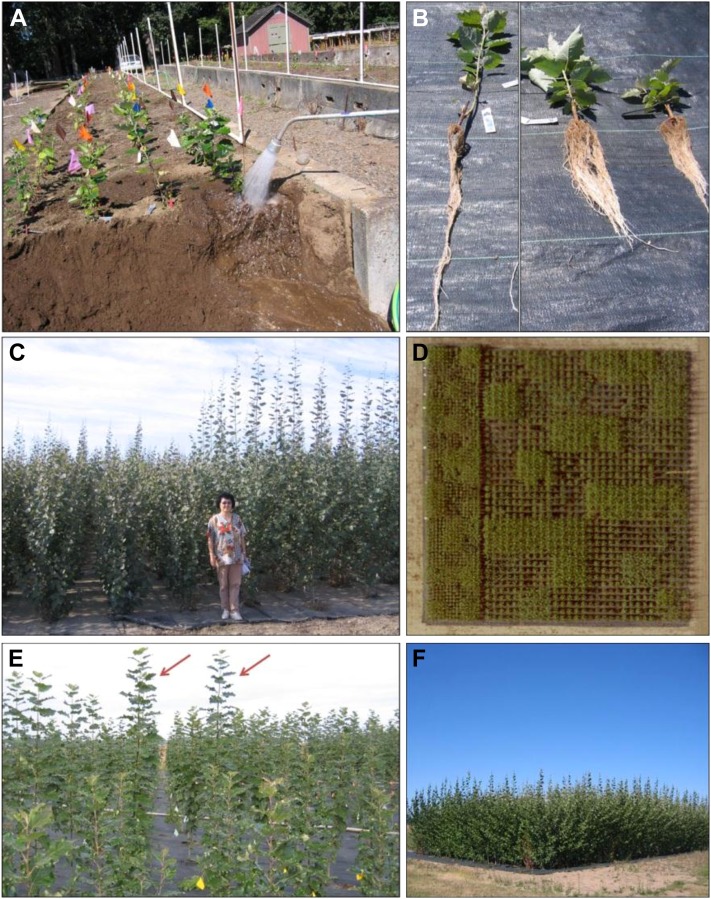
Figure 9.
Views of the field trials. A, Washing roots from plants harvested in the raised-bed study. B, View of plants harvested from raised beds; the left-most plant is a nontransgenic control, and the two plants on the right are from construct GAI-X-1. Note the more stocky and rooty morphology of the transgenics. C, Views of plants from the field study during second growing season (August), GA2-2 on the right and GAI-X-1 on the left. D, Aerial view of the field study showing the two densities and randomized 25-tree plots with differential growth. E and F, View of trees in the competition study during the first year of growth (E; arrows identify control trees approximately 2 m in height, surrounded by semidwarf transgenic trees) and plantation view during the second year of growth (F), where the tallest trees are approximately 5 m in height. [See online article for color version of this figure.]
We took 20- to 30-cm dormant cuttings from stems or branches of trees growing in the field, stored them at 4°C for about 2 months, and then sent them to commercial nurseries for rooting and propagation in February 2006. The rooted plants were returned to OSU, where they were acclimatized in a shadehouse (approximately 50% shade) for about 20 d before they were planted in the raised beds in June 2006 at a spacing of 32 × 45 cm. A randomized complete block design with three blocks was employed, so that each block could be harvested without disturbing the other blocks. Three beds with dimensions 11 × 22 × 0.6 m, and two beds with dimensions 1.6 × 17 × 0.9 m, were used for planting. Plants from each block were harvested at an approximately 40-d interval (mid July and then late August). Plants were separated into roots, stems, and leaves and then dried in an oven overnight at 60°C. From the dry weights, the tissue mass fractions (ratio of the weight of specific plant parts to total weight) were calculated.
High-Density Field Study
To determine how the effects of semidwarfism on yield and morphology were influenced by variation in stand density, we planted trees of single genotypes at two high-density spacings (referred to as the “yield study”; Fig. 9, C and D). In addition, to evaluate the extent to which intergenotypic competition influenced growth, we also planted a mixture of transgenic and wild-type trees at high density (referred to as the “competition study”; Fig. 9, E and F). The yield study was composed of trees planted at high (0.5 m × 0.5 m) and low (0.9 m × 0.9 m) spacing, with two blocks at each density where the trees were roughly grouped according to size at planting (smaller versus larger trees). Individual transgenic genotypes were grown in two randomly located 25-tree square plots within each block, and the nontransgenic controls were grown similarly but in four randomly replicated plots within each block. Only the central three or nine trees in each plot were measured, depending on the trait. For the competition trial, a similar structure was used but only a single density was employed (0.9 m × 0.9 m), and there were five transgenic trees placed on the diagonals in the center of each plot that were surrounded by nontransgenic trees on all sides, including a nontransgenic border row. These plots were replicated at random four times. The trees were planted at the Hyslop Field Station (Oregon State University College of Agricultural Sciences) in Corvallis (44.626°N, 123.214°W) on a Woodburn silt loam soil. The trees were grown for 2 years and irrigated during their first year.
Growth Measurements
We measured tree height and crown depth using a height pole and stem diameter using a caliper. Branch and leaf angles were measured using a ruler with a weighted string and protractor. The branches measured were the second and third south-most facing branches, assessed from the apex of the tree. These branches were also measured for length using a meter stick. On the same two branches, we measured the size of the first two fully opened leaves below the tip of the branch, midvein angle, and petiole angle. Petiole length itself was measured on the same leaves, but only in the high-density blocks. Calculated variables included stem volume index (height × diameter squared) and crown volume (calculated using crown radius [r] = branch length × sine of branch angle; crown volume = πr2h, where h is the crown depth). For the competition trial, only height and diameter were measured, and stem volume index was calculated as a measure of vegetative fitness.
Postharvesting Measurements
Trees were harvested at the end of the second growing season, and we sampled leaves, roots, and stem sections to assess leaf area, chlorophyll, carbon isotope composition, wood density, and root dry weight. Two fully opened leaves were collected from the middle section of each tree in the high-density plantation, and their leaf area was determined using a leaf area meter (LI-3100 area meter; LI-COR). Chlorophyll was extracted with N,N-dimethylformamide (Inskeep and Bloom, 1985) from five randomly taken leaf discs from each of these leaves. These discs were kept in 5 mL of N,N-dimethylformamide in 10-mL tubes wrapped in aluminum foil and stored at 4°C for 7 d. After this time period, a Beckman DU-40 spectrophotometer was used to measure absorbance values at wavelengths ranging from 618 to 665 nm. For all trees, 0.3-m sections of the stem near the base were taken from the nine inner trees from each plot in early November 2007, dried in an oven at 38°C for 3 d, weighed, and stem volume was estimated by displacement of water in a graduated cylinder. From these measurements, stem density was calculated. All trees were uprooted in late November 2007 after the soils had saturated with fall rains. We used an excavator machine that allowed recovery of the woody roots (a modified fork-lift that went into the soil below the roots for each tree and lifted them out). The roots were then dried and weighed similar to the stem sections. Stem dry weight index was estimated from the product of stem volume index and wood density. For comparing allocation among genotypes, we calculated the root mass fraction (root dry weight-total dry weight) and root-shoot dry weight ratios. δ13C (a measure of the ratio of stable isotopes 12C and 13C) was measured on leaf samples taken from the high-density plantation using the stable isotope facility at the University of Wyoming (Finnigan Δ Plus XP online, with Costech EA 1108 Element Analyzer) and expressed relative to the Vienna Pee Dee Belemnite standard. Carbon isotope discrimination (Δ) was calculated from leaf sample δ13C, where Δ = (δair – δplant)/(1 + δplant), assuming an atmospheric source δ13C of −8‰ relative to Pee Dee Belemnite (Farquhar et al., 1989).
GA Level Determinations
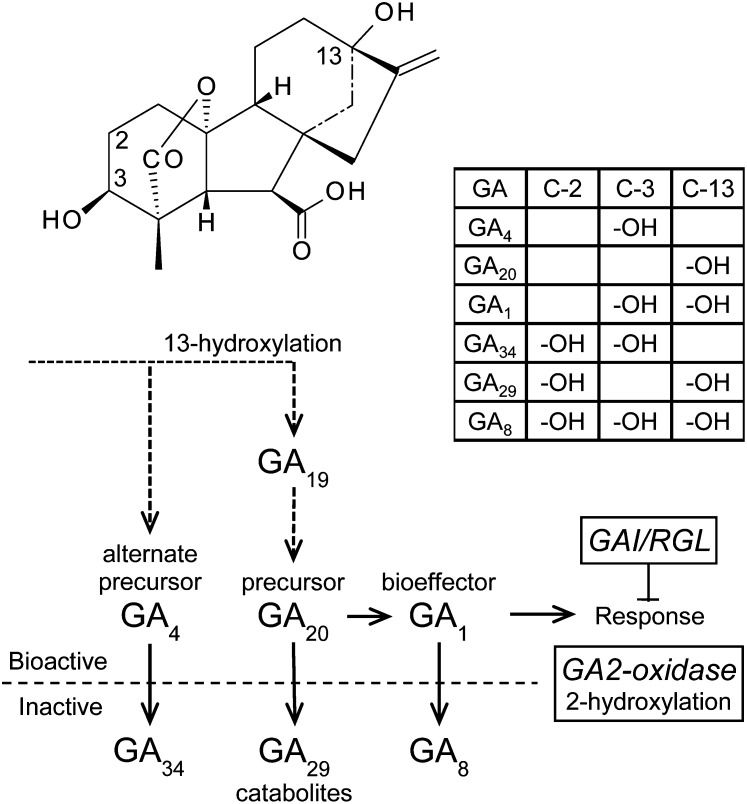
We analyzed endogenous GAs in the latter part of the metabolic pathway around GA1, the bioactive GA for shoot growth in angiosperms (Fig. 10). These seven GAs have been characterized from Populus spp. and include 2-hydroxylated GAs that are abundant in shoots and altered by GA2ox (Pearce et al., 2002; Busov et al., 2003, 2006). GA levels were analyzed from two ramets per event from the high-density field studies; for each tree, dormant shoots were taken and rooted in a greenhouse, then after plants were approximately 0.5 m in height and growing well, two to five young leaves were collected just below the shoot tip. These were freeze dried, stored, and then ground and extracted in 80% methanol with 2H2-labeled internal standards of GA1, GA4, GA8, GA19, GA20, GA29, and GA34 (from L.N. Mander, Australian National University). The GAs were purified and measured essentially as described by Busov et al. (2006) except that the methylated samples were trimethylsilylated and analyzed by gas chromatography with selected ion monitoring after NH2–Solid Phase Extraction, without intervening HPLC. This permitted simultaneous gas chromatography with selected ion monitoring analysis of the GAs and was successful except for GA19, which was not consistently or reliably quantified and thus was excluded from analyses. Also, GA4 occurred at only trace levels that were insufficient for confident comparison across the events, but the consistent detection of the [2H2]GA4 demonstrated the GA4 scarcity relative to the other GAs.
Figure 10.
The chemical structure of GA1, with indication of the C-2, C-3, and C-13 positions (top), and a table displaying the associated hydroxylations for the different GAs analyzed. The metabolic positions of the GAs are indicated, along with the alterations by the two transgene types (bottom).
Statistical Analysis
A fixed-effect one-way ANOVA was used for the raised-bed study, where the effect of interest was genotype in replicated blocks. The linear model was:
where yjkl is the response of the lth plant in the kth block of the jth event; μ is the overall mean; βj is the jth event effect; δk is the kth block effect; and εjkl is the experimental error, εjkl ∼ N(0, σ2). For the high-density field study, we analyzed data as a two-factor factorial in a randomized complete block with two factors, event and planting density, randomized in each of two blocks. The linear model was:
where yijkl is the response of the lth plot in the kth block in the jth event in the ith density; τi is the ith density effect; (τβ)ij is the interaction effect between event and planting density; and εijkl is the experimental error, εijkl ∼ N(0, σ2). Interactions between block and treatment factors were assumed to be negligible based on preliminary statistical analysis (data not shown) and thus are included in the experimental error term.
For the completely randomized competition study, we used a fixed-effect ANOVA. The linear model was:
where yjl represents the response of the lth plant in the jth treatment; and εjl represents experimental error εil ∼ N(0, σ2). All response variables were first tested for assumptions of homogeneity of variance and normality by plotting residuals, and all were found to follow the assumptions. We used PCA to examine associations among traits and to reduce the dimensionality of the data, using plot means within the high-density treatment to derive PCA loadings. The Proc GLM procedure and the Proc Princomp procedure in SAS/STAT version 9.2 (SAS Institute) were used for performing ANOVA and estimating least-square means, se values, multiple comparisons using Bonferroni tests, and PCA loadings. Least-square mean estimates and se values for each variable are given in Supplemental Tables S3 to S5.
Because endogenous GAs vary exponentially in plant tissues, values were log transformed (base 10) prior to statistical analysis with one-way ANOVAs. These were undertaken for each GA and followed by posthoc Dunnett t tests, comparing each transgenic event with the control.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Shoot heights from both harvest 1 and harvest 2; root, stem, and leaf biomass from harvest 1 and root, stem, and leaf biomass from harvest 2.
Supplemental Figure S2. Shoot height and shoot-root ratios in the high-density field study.
Supplemental Table S1. Variation in relative transgene expression among transgenic constructs.
Supplemental Table S2. Levels of GAs and height ratio for events relative to the control.
Supplemental Table S3. Raised-bed study trait means.
Supplemental Table S4. Field study trait means and se
Supplemental Table S5. Field study trait means and se (variables measured only in higher density blocks).
Supplemental Table S6. Primers used for qPCR; adaptor sequences are underlined.
Acknowledgments
We thank Ray and Sandra Ethel of Broadacres Nursery (Hubbard, OR) and Don Roberts of Premier Botanicals (Independence, OR) for propagation services and information on rooting frequency. We also thank Giles Pilate and Lise Jouanin (Institut National de la Recherche Agronomique-France) for access to the 717-1B4 poplar clone, and H. Toby Bradshaw (University of Washington) and Jerry Tuskan (Oak Ridge National Laboratory) for early discussions that helped to establish the conceptual framework for this research.
Glossary
- qPCR
quantitative real-time PCR
- OSU
Oregon State University
- Δ13C
carbon isotope discrimination in leaf tissue
- PCA
principal components analysis
References
- Acharya BR, Assmann SM. (2009) Hormone interactions in stomatal function. Plant Mol Biol 69: 451–462 [DOI] [PubMed] [Google Scholar]
- Al-Ahmad H, Galili S, Gressel J. (2005) Poor competitive fitness of transgenically mitigated tobacco in competition with the wild type in a replacement series. Planta 222: 372–385 [DOI] [PubMed] [Google Scholar]
- Al-Ahmad H, Gressel J. (2006) Mitigation using a tandem construct containing a selectively unfit gene precludes establishment of Brassica napus transgenes in hybrids and backcrosses with weedy Brassica rapa. Plant Biotechnol J 4: 23–33 [DOI] [PubMed] [Google Scholar]
- Appleford NEJ, Wilkinson MD, Ma Q, Evans DJ, Stone MC, Pearce SP, Powers SJ, Thomas SG, Jones HD, Phillips AL, et al. (2007) Decreased shoot stature and grain α-amylase activity following ectopic expression of a gibberellin 2-oxidase gene in transgenic wheat. J Exp Bot 58: 3213–3226 [DOI] [PubMed] [Google Scholar]
- Battisini A, Battisini G. (2005) Victor (R): a semi-dwarfing cherry rootstock for dry conditions. In GA Lang, ed, Proceedings of the IVth International Cherry Symposium. International Society Horticultural Science, Leuven, Belgium, pp 189–190
- Biemelt S, Tschiersch H, Sonnewald U. (2004) Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants. Plant Physiol 135: 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund S, Antti H, Uddestrand I, Moritz T, Sundberg B. (2007) Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J 52: 499–511 [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Strauss SH. (2001) Breeding strategies for the 21st century: domestication of poplar. In D Dickman, J Isebrands, JE Eckenwalder, J Richardson, eds, Poplar Culture in North America, Part B. NRC Research Press, Ottawa, Canada, pp 383–394
- Brunner AM, Busov VB, Strauss SH. (2004a) Poplar genome sequence: functional genomics in an ecologically dominant plant species. Trends Plant Sci 9: 49–56 [DOI] [PubMed] [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH. (2004b) Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol 4: 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busov V, Meilan R, Pearce DW, Rood SB, Ma C, Tschaplinski TJ, Strauss SH. (2006) Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta 224: 288–299 [DOI] [PubMed] [Google Scholar]
- Busov VB, Brunner AM, Strauss SH. (2008) Genes for control of plant stature and form. New Phytol 177: 589–607 [DOI] [PubMed] [Google Scholar]
- Busov VB, Meilan R, Pearce DW, Ma C, Rood SB, Strauss SH. (2003) Activation tagging of a dominant gibberellin catabolism gene (GA 2-oxidase) from poplar that regulates tree stature. Plant Physiol 132: 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang M, Jin S, Liu C, Chou M. (2004) Influence of exogenous GA3 and 6-BA on the growth and alkaloid content in Dendrobium nobile. J Plant Resour Environ 13: 7–11 [Google Scholar]
- Cowling RJ, Kamiya Y, Seto H, Harberd NP. (1998) Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol 117: 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple DG. (1985) The development and adoption of high-yielding varieties of wheat and rice in developing countries. Am J Agric Econ 67: 1067–1073 [Google Scholar]
- David CC, Otsuka K. (1994) Modern Rice Technology and Income Distribution in Asia. Lynne Rienner Publishers, Boulder, CO
- Eriksson ME, Israelsson M, Olsson O, Moritz T. (2000) Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat Biotechnol 18: 784–788 [DOI] [PubMed] [Google Scholar]
- Evans JR. (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19 [DOI] [PubMed] [Google Scholar]
- Evans LT, Fischer RA. (1999) Yield potential: its definition, measurement, and significance. Crop Sci 39: 1544–1551 [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol 40: 503–537 [Google Scholar]
- Filichkin SA, Difazio SP, Brunner AM, Davis JM, Yang ZK, Kalluri UC, Arias RS, Etherington E, Tuskan GA, Strauss SH. (2007) Efficiency of gene silencing in Arabidopsis: direct inverted repeats vs. transitive RNAi vectors. Plant Biotechnol J 5: 615–626 [DOI] [PubMed] [Google Scholar]
- Fleet CM, Sun TP. (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8: 77–85 [DOI] [PubMed] [Google Scholar]
- Fu X, Sudhakar D, Peng J, Richards DE, Christou P, Harberd NP. (2001) Expression of Arabidopsis GAI in transgenic rice represses multiple gibberellin responses. Plant Cell 13: 1791–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou J, Ma C, Kadmiel M, Gai Y, Strauss S, Jiang X, Busov V. (2011) Tissue-specific expression of Populus C19 GA 2-oxidases differentially regulate above- and below-ground biomass growth through control of bioactive GA concentrations. New Phytol 192: 626–639 [DOI] [PubMed] [Google Scholar]
- Gou J, Strauss SH, Tsai CJ, Fang K, Chen Y, Jiang X, Busov VB. (2010) Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 22: 623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressel J. (1999) Tandem constructs: preventing the rise of superweeds. Trends Biotechnol 17: 361–366 [DOI] [PubMed] [Google Scholar]
- Gressel J, Valverde BE. (2009) A strategy to provide long-term control of weedy rice while mitigating herbicide resistance transgene flow, and its potential use for other crops with related weeds. Pest Manag Sci 65: 723–731 [DOI] [PubMed] [Google Scholar]
- Guak S, Neilsen D, Looney NE. (2001) Growth, allocation of N and carbohydrates, and stomatal conductance of greenhouse grown apple treated with prohexadione-Ca and gibberellins. J Hortic Sci Biotechnol 76: 746–752 [Google Scholar]
- Hargrove TR, Cabanilla V. (1979) The impact of semidwarf varieties on Asian rice-breeding programs. Bioscience 29: 731–735 [Google Scholar]
- Hedden P. (2003) The genes of the green revolution. Trends Genet 19: 5–9 [DOI] [PubMed] [Google Scholar]
- Herschbach C, Kopriva S. (2002) Transgenic trees as tools in tree and plant physiology. Trees Struct Funct 16: 250–261 [Google Scholar]
- Inskeep WP, Bloom PR. (1985) Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol 77: 483–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth PC, Audia WV, Mitchell JW. (1956) Effect of gibberellic acid on growth and development of various species of plants. Bot Gaz 118: 106–111 [Google Scholar]
- Mauriat M, Moritz T. (2009) Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J 58: 989–1003 [DOI] [PubMed] [Google Scholar]
- Mauriat M, Sandberg LG, Moritz T. (2011) Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J 67: 805–816 [DOI] [PubMed] [Google Scholar]
- Nagel OW, Konings H, Lambers H. (2001) Growth rate and biomass partitioning of wildtype and low-gibberellin tomato (Solanum lycopersicum) plants growing at a high and low nitrogen supply. Physiol Plant 111: 33–39 [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. (2007) Hidden branches: developments in root system architecture. Annu Rev Plant Biol 58: 93–113 [DOI] [PubMed] [Google Scholar]
- Pearce DW, Hutt OE, Rood SB, Mander LN. (2002) Gibberellins in shoots and developing capsules of Populus species. Phytochemistry 59: 679–687 [DOI] [PubMed] [Google Scholar]
- Pearce DW, Rood SB, Wu R. (2004) Phytohormones and shoot growth in a three-generation hybrid poplar family. Tree Physiol 24: 217–224 [DOI] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins JH. (1997) Geopolitics and the Green Revolution: Wheat, Genes, and the Cold War. Oxford University Press, New York
- Perovic D, Foerster J, Welz G, Kopahnke D, Lein V, Loeschenberger F, Buerstmayr H, Ordon F. (2008) Marker-assisted wheat improvement: creating semi-dwarf phenotypes with superior Fusarium head blight resistance. Cereal Res Commun 36: 153–155 [Google Scholar]
- Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Jr, Hallett JP, Leak DJ, Liotta CL, et al. (2006) The path forward for biofuels and biomaterials. Science 311: 484–489 [DOI] [PubMed] [Google Scholar]
- Rossetto MRM, Purgatto E, Oliveira do Nascimento JR, Lajolo FM, Cordenunsi BR. (2003) Effects of gibberellic acid on sucrose accumulation and sucrose biosynthesizing enzymes activity during banana ripening. Plant Growth Regul 41: 207–214 [Google Scholar]
- Schwinning S, Weiner J. (1998) Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 113: 447–455 [DOI] [PubMed] [Google Scholar]
- Stettler RF. (2009) Cottonwood and the River of Time: On Trees, Evolution, and Society. University of Washington Press, Seattle
- Strauss SH, Busov VB, Ma C, Meilan R. (2004) Ten lessons from 15 years of transgenic Populus research. Forestry 77: 455–465 [Google Scholar]
- Sun TP, Gubler F. (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55: 197–223 [DOI] [PubMed] [Google Scholar]
- Swain SM, Singh DP. (2005) Tall tales from sly dwarves: novel functions of gibberellins in plant development. Trends Plant Sci 10: 123–129 [DOI] [PubMed] [Google Scholar]
- Tuskan GA, DiFazio SP, Teichmann T. (2004) Poplar genomics is getting popular: the impact of the poplar genome project on tree research. Plant Biol (Stuttg) 6: 2–4 [DOI] [PubMed] [Google Scholar]
- Zawaski C, Kadmiel M, Pickens J, Ma C, Strauss SH, Busov V. (2011) Repression of gibberellin biosynthesis or signaling produces striking alterations in poplar growth, morphology, and flowering. Planta 234: 1285–1298 [DOI] [PubMed] [Google Scholar]