Abstract
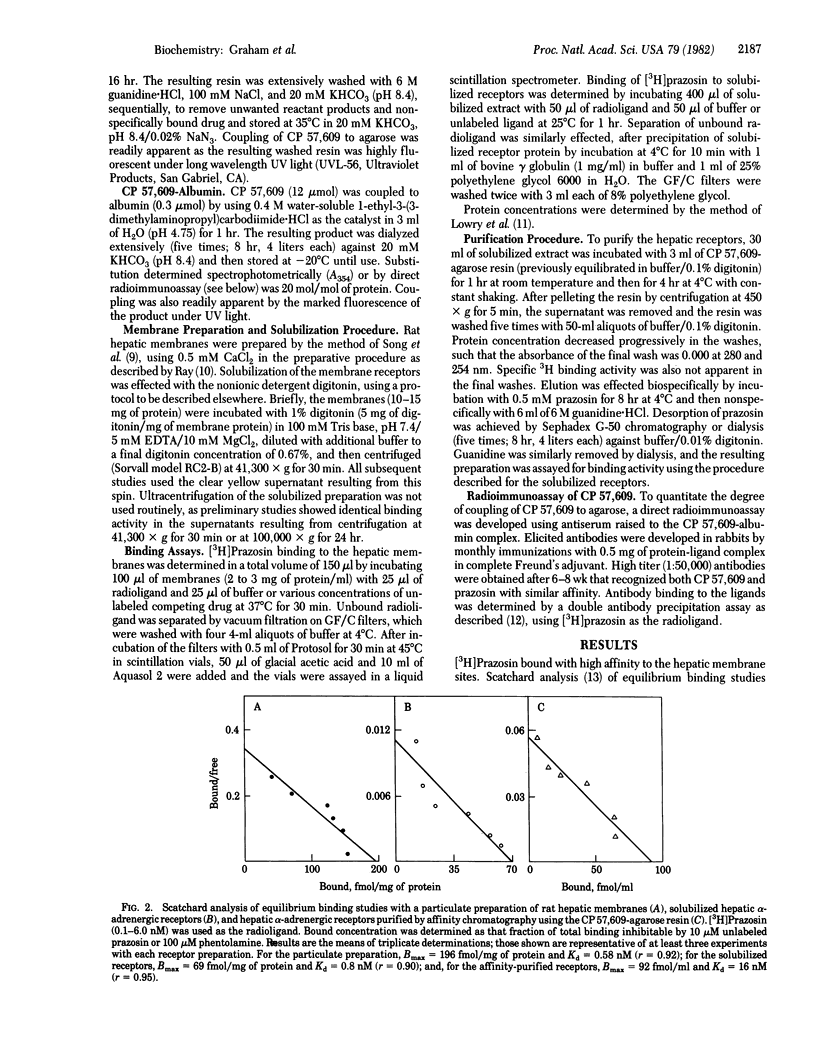
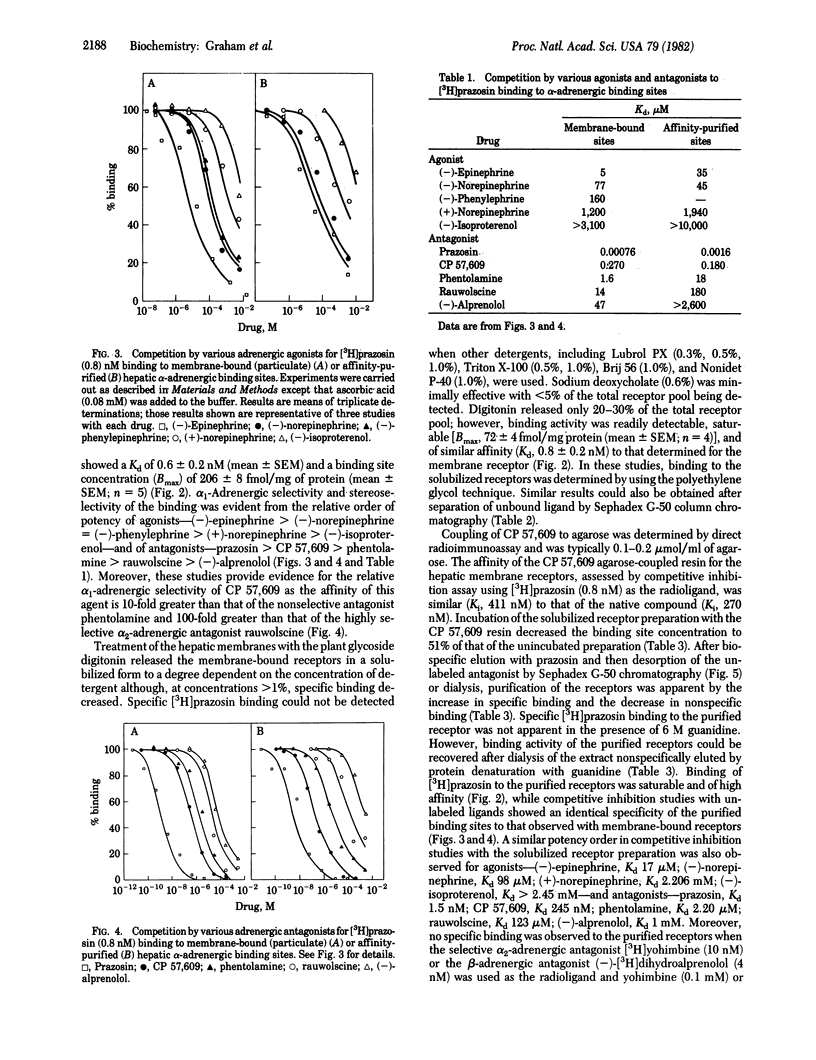
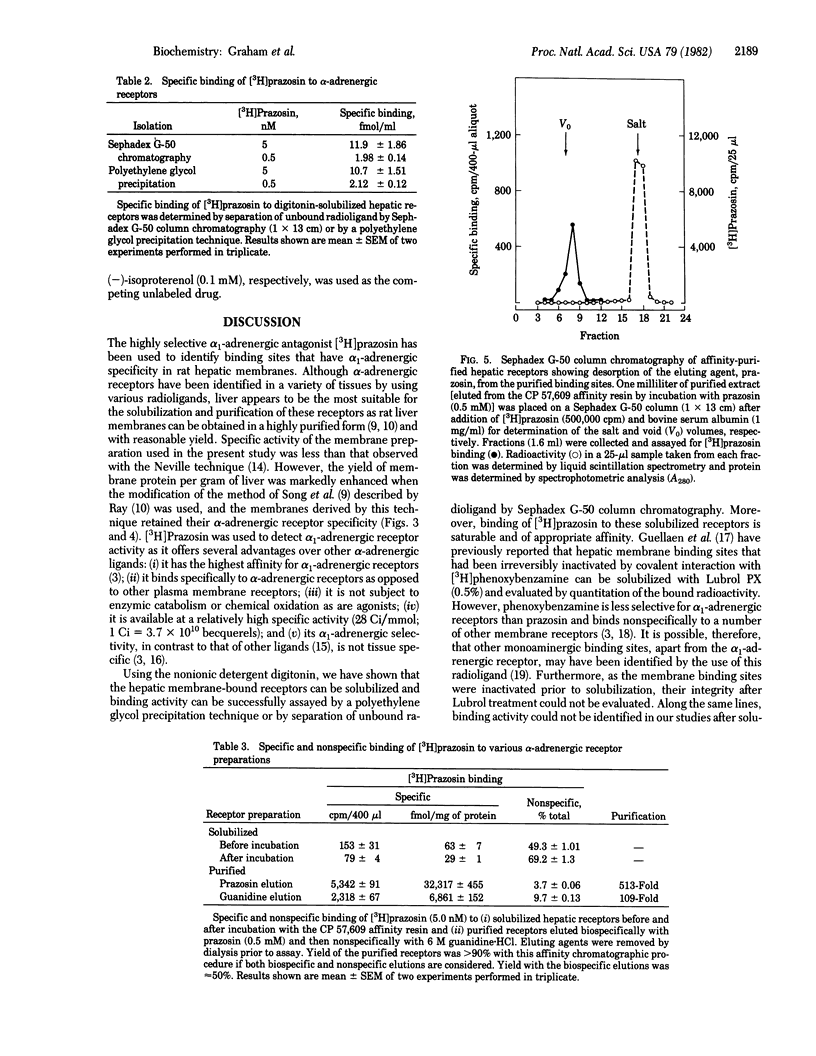
The highly selective alpha 1-adrenergic receptor antagonist prazosin was used to identify binding sites having alpha-adrenergic specificity in rat hepatic plasma membranes. Solubilization of the membrane-bound receptors was achieved by incubation with the nonionic detergent digitonin, and binding activity was assayed by using [3H]prazosin and a polyethylene glycol precipitation technique. Only 20-30% of the total receptor pool was released by the solubilization procedure. However, binding of [3H]prazosin was saturable [maximal value, 206 +/- 8 fmol/mg of protein (membrane) vs. 74 +/- 4 fmol/mg of protein (soluble)] and of high affinity [Kd, 0.6 +/- 0.2 nM (membrane) vs. 0.8 +/- 0.2 nM (soluble)]. To aid in purification of the receptors, an affinity resin was developed using an analog of prazosin, 2-(4-succinoylpiperazin-1-yl)-4-amino-6,7-dimethoxyquinazoline (CP 57,609; Kd 2.7 X 10(-7) M) immobilized via an amide linkage to agarose. The resulting resin demonstrated high affinity (Kd 3.2 X 10(-7) M) for the solubilized receptors, as determined by competitive inhibition assay. The degree of substitution to the resin was determined by a direct radioimmunoassay using antibodies against albumin-complexed CP 57,609 and found to be 0.1 to 0.2 mumol/ml of agarose. Affinity chromatography using the resin resulted in 513-fold purification in a single step. Moreover, the specificity of the purified binding sites was similar to that of membrane-bound receptors. This novel affinity resin should thus provide a powerful tool for isolating the receptor protein in quantities sufficient for detailed biochemical characterization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Cambridge D., Davey M. J., Massingham R. Prazosin, a selective antagonist of post-synaptic alpha-adrenoceptors [proceedings]. Br J Pharmacol. 1977 Mar;59(3):514P–515P. [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Guellaen G., Aggerbeck M., Hanoune J. Characterization and solubilization of the alpha-adrenoreceptor of rat liver plasma membranes labeled with [3H]phenoxybenzamine. J Biol Chem. 1979 Nov 10;254(21):10761–10768. [PubMed] [Google Scholar]
- Hoffman B. B., Lefkowitz R. J. [3H]WB4101--caution about its role as an alpha-adrenergic subtype selective radioligand. Biochem Pharmacol. 1980 Jun 1;29(11):1537–1541. doi: 10.1016/0006-2952(80)90605-x. [DOI] [PubMed] [Google Scholar]
- Koch-Weser J., Graham R. M., Pettinger W. A. Drug therapy. Prazosin. N Engl J Med. 1979 Feb 1;300(5):232–236. doi: 10.1056/NEJM197902013000505. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]
- Rockson S. G., Homcy C. J., Haber E. Anti-alprenolol antibodies in the rabbit. A new probe for the study of beta-adrenergic receptor interaction. Circ Res. 1980 Jun;46(6):808–813. doi: 10.1161/01.res.46.6.808. [DOI] [PubMed] [Google Scholar]
- Song C. S., Rubin W., Rifkind A. B., Kappas A. Plasma membranes of the rat liver. Isolation and enzymatic characterization of a fraction rich in bile canaliculi. J Cell Biol. 1969 Apr;41(1):124–132. doi: 10.1083/jcb.41.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K. G., Liepmann P., Baldessarini R. J. Inhibition of dopamine-stimulated adenylate cyclase activity by phenoxybenzamine. Eur J Pharmacol. 1978 Nov 15;52(2):231–234. doi: 10.1016/0014-2999(78)90211-x. [DOI] [PubMed] [Google Scholar]



