Abstract
In plants, lateral roots originate from pericycle founder cells that are specified at regular intervals along the main root. Here, we show that Arabidopsis (Arabidopsis thaliana) SKP2B (for S-Phase Kinase-Associated Protein2B), an F-box protein, negatively regulates cell cycle and lateral root formation as it represses meristematic and founder cell divisions. According to its function, SKP2B is expressed in founder cells, lateral root primordia and the root apical meristem. We identified a novel motif in the SKP2B promoter that is required for its specific root expression and auxin-dependent induction in the pericycle cells. Next to a transcriptional control by auxin, SKP2B expression is regulated by histone H3.1/H3.3 deposition in a CAF-dependent manner. The SKP2B promoter and the 5′ end of the transcribed region are enriched in H3.3, which is associated with active chromatin states, over H3.1. Furthermore, the SKP2B promoter is also regulated by H3 acetylation in an auxin- and IAA14-dependent manner, reinforcing the idea that epigenetics represents an important regulatory mechanism during lateral root formation.
Plants have evolved different root architectures depending on the genotype and on the surrounding environment. Both the number and position of lateral roots (LR) are major determinants of the root system architecture. Together with root hairs, these lateral organs are responsible for maximizing the surface needed to acquire water and nutrients from the soil. Classical studies (Charlton, 1996) and also more recent work have shown that LR are continuously initiated at a predictable distance above the growing root tip and correlate with the periodic fluctuations in DR5 expression, a marker that labels the auxin response (De Smet et al., 2007; Moreno-Risueno et al., 2010). Lateral root formation follows an acropetal development, where the lateral root primordia (LRP) are found nearest to the root tip, whereas more mature LR are encountered closer to the root-shoot junction (Fahn, 1974). In Arabidopsis (Arabidopsis thaliana), LR originate from pericycle cells located in front of the xylem poles (Dolan et al., 1993). However, not all of these xylem pole pericycle cells show the same potential to divide, since only a few of them, called founder cells, acquire the potential to divide and to form LRP (Casimiro et al., 2003). How do these founder cells become specified and differentiated from their neighboring cells? Recent results indicated that the events that determine LR positioning take place in the upper region of the root apical meristem, between the meristem and the elongation zone, in an auxin-dependent manner and involve the Aux/IAA28-dependent auxin-response module (De Smet et al., 2007; De Rybel et al., 2010). This module regulates the expression of GATA23, a transcription factor involved in founder cell specification (De Rybel et al., 2010). Later, the IAA14/SLR module regulates the first founder anticlinal cell division as a previous step that triggers the formation of LRP. The slr-1 mutation generates a dominant nondegradable IAA14/SLR protein that blocks LR formation (Fukaki et al., 2002). Despite recent advances in identifying the molecular mechanisms that govern the LR position and number, this process is still an intriguing question.
Here, we report on the function of the F-box protein SKP2B (for S-Phase Kinase-Associated Protein2B) in LR development. SKP2B, which is homologous to the human (Homo sapiens) cell cycle S-Phase Kinase-Associated Protein2 (Skp2; del Pozo et al., 2002), regulates the stability of the cyclin-dependent kinase inhibitor KRP1 (Ren et al., 2008). In this work, we show that SKP2B regulates LR formation by repressing founder cell division. SKP2B is expressed during the entire LR development and in the root meristem. We have identified a novel motif needed for root-specific SKP2B expression in LRP and founder cells and auxin induction in the pericycle. Using yeast one-hybrid and chromatin immunoprecipitation (ChIP) analyses, we found that histone H3.3 binds to the SKP2B promoter. Defects in histone H3.1/H3.3 deposition alter SKP2B expression and LR development. Furthermore, we have found that CAF-1, a histone H4/H3.1 chaperone, regulates the expression of SKP2B in the founder cells and LRP. Finally, we demonstrate that acetylation of histone H3 in K9 and K14, two marks associated with active transcription, occurs in the SKP2B promoter and that such modifications are auxin and IAA14/SLR dependent.
RESULTS
SKP2B Is Expressed in Dividing Tissues and during Early Stages of Lateral Root Initiation
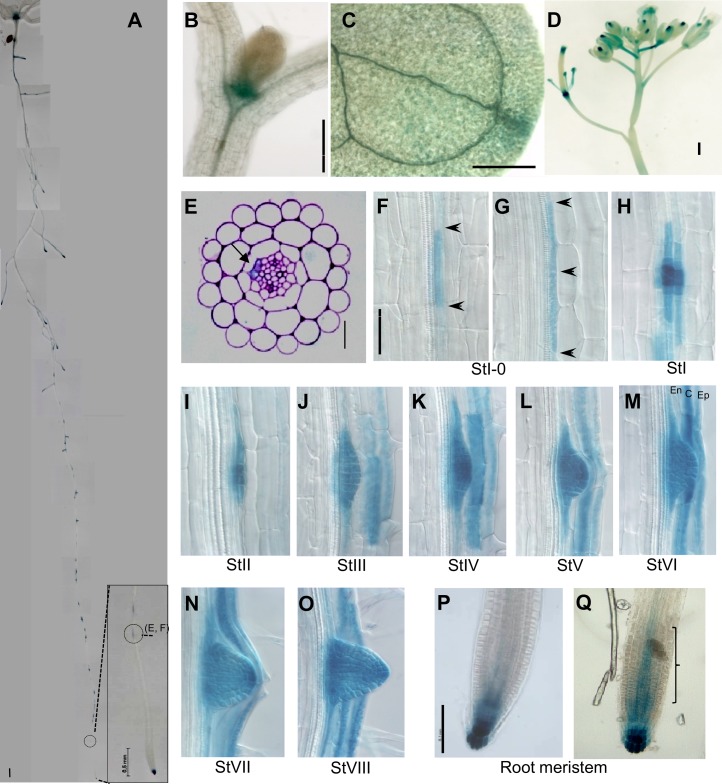
As SKP2B functions in the cell cycle, we studied its transcriptional regulation during the cell cycle. SKP2B showed two expression peaks that correlate with S and G2/M phases (Supplemental Fig. S1). To analyze its spatiotemporal expression pattern, we constructed a transgenic line expressing the GUS reporter under the control of the SKP2B promoter (named SKP2Bp:GUS). Histochemical GUS staining showed that SKP2B was expressed in dividing areas (shoot and root meristems), in the leaf vasculature, and in flowers (Fig. 1, A–D). In roots, SKP2B is expressed in the root apical meristem and in patches along the main root that correlate with LRP in all developmental stages, from stage 0 to VIII (Fig. 1, A and F–O). Microscopic analyses revealed that SKP2B was also expressed in undivided cells close to the root tip that was restricted to pericycle cells at the xylem pole (Fig. 1, E–G), likely corresponding to founder cells.
Figure 1.
SKP2B expression. A, SKP2Bp:GUS seedlings were grown for 12 d and then stained for GUS activity. At bottom right is a magnification showing the GUS-stained patches nearest to the root meristem. The dashed circles indicate the cross-section analyzed in E and F. B, SKP2B expression in the shoot meristem. C, Cotyledon and vascular tissue. D, Flower buds. E, Cross-section showing the GUS-stained patch nearest to the root meristem as shown in A and F. Arrowheads indicate pericycle cells stained for GUS activity. F to O, Representative images of LR formation at different developmental stages (St), from stage 0 to stage VIII, taken from a single root. P and Q, Images of two different and representative root meristems showing different GUS staining in the basal meristem (bracket). Bars = 0.5 mm (A–D), 20 µm (E), and 0.2 mm (F–Q). C, Cortex; En, endodermis; Ep, epidermis.
In Arabidopsis, LR formation follows an acropetal sequence of development, with the earliest stages localized close to the root tip. The marker lines DR5p:GUS and GATA23 expression are considered to report the earliest events associated with LR initiation (Benková et al., 2003; Dubrovsky et al., 2008; De Rybel et al., 2010). Comparisons between DR5p:GUS and SKP2Bp:GUS expression revealed that SKP2B was expressed in all morphologically recognizable lateral primordia, including those located between two already developed LR far away from the root apical meristem (Supplemental Fig. S2A). However, about 20% to 25% of the morphologically detected LRP were not stained for the DR5:GUS (Supplemental Fig. S2B), indicating the occurrence of fully specified but developmentally arrested LRP having lost the auxin maximum required for further outgrowth.
SKP2B Negatively Regulates Lateral Root Formation
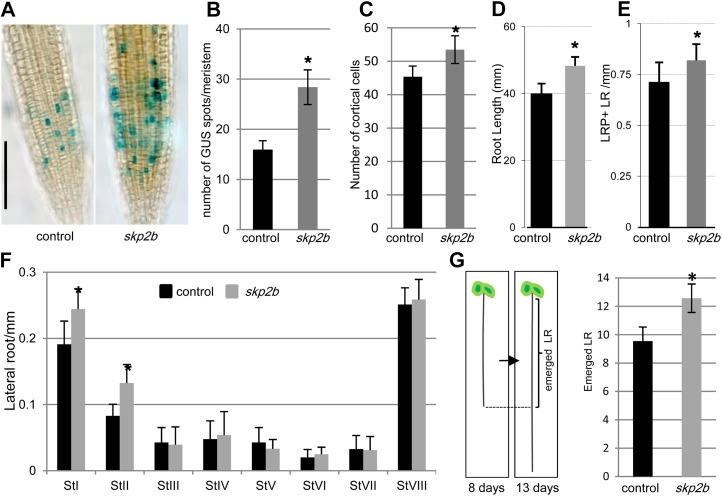
To analyze the role of SKP2B in cell division, we crossed the skp2b mutant (Ren et al., 2008) with a cell cycle marker, CYCB1-GUS (Colón-Carmona et al., 1999). We found that skp2b root meristems contain more dividing cells (represented as GUS-positive spots; Fig. 2, A and B) and bigger root meristem size than the wild type (Fig. 2C), indicating higher dividing activity in skp2b root meristems. In addition, we also found that the skp2b roots were longer than control roots (Fig. 2D). Next, we analyzed in detail LR formation in the skp2b mutant, finding that skp2b mutants developed more LR (primordia plus emerged LR) per millimeter than the control (Fig. 2E). When we analyzed the developmental stages of LRP (according to Malamy and Benfey [1997]), we found that 8-d-old skp2b roots contained significantly more LRP in stages I and II than the control, but we did not observe differences in the number of emerged LR (Fig. 2F). However, when we analyzed 13-d-old seedlings, the number of emerged LR was significantly higher in skp2b than in control plants (Fig. 2G). Taken together, these data indicate that SKP2B acts as a repressor of cell division and LR formation.
Figure 2.
SKP2B represses cell division. A, Representative images of GUS-stained root meristems of control (CYCB1;1p:CYCB1-GUS) and skp2b (skp2b/CYCB1;1p:CYCB1-GUS) seedlings grown for 8 d in MS medium on vertical plates. B, Quantification of CYCB1-GUS spots per meristem in control and skp2b mutant roots. *P < 0.0001 by two-sided t test (n = 30). C, Number of meristematic cortex cells in control and skp2b root meristems. *P < 0.0001 by two-sided t test (n ≥ 12). D, Root length of control and skp2b roots. E, Number of LRP plus emerged LR per millimeter. F, Number of LRP in different stages per millimeter of main root. *P < 0.0001 by two-sided t test (n = 12). G, Number of emerged LR in control and skp2b plants grown for 13 d in MS medium. The emerged LR were counted only in the portion of the root formed during the first 8 d (bracket). *P < 0.00001 by two-sided t test (n ≥ 35). In all cases, values represent means ± se. [See online article for color version of this figure.]
SKP2B Expression in the Root Is Regulated by Auxin
Auxin signaling plays a central role in the specification of founder cells (De Rybel et al., 2010) and during LRP development (for review, see Péret et al., 2009). Since SKP2B functions in LR formation, we decided to analyze whether auxin controls the expression of SKP2B in the root. After 3 h of auxin treatment, SKP2B was initially induced in the pericycle (Fig. 3A), but after 5 or 7 h, GUS staining was also localized in the surrounding cortex and epidermis, although staining was always stronger in the pericycle layer (Fig. 3A). These data are consistent with the finding that SKP2B expression increases in the pericycle cells after 2 and 6 h of auxin treatment (Parizot et al., 2010). In addition, treatment of SKP2Bp:GUS with 1-N-naphthylphthalamic acid (NPA), which inhibits auxin efflux and blocks LR development, eliminated SKP2B expression in the root, except from the root tip (Fig. 3B). It is possible that NPA impedes founder cell specification and LR formation and, consequently, SKP2B expression. To answer this, we grew Arabidopsis seedlings in medium containing 0 or 5 µm NPA for 7 d. Afterward, seedlings were transferred to fresh medium without NPA for an extra 3 d and LR were counted only in the root portions that were grown the first 7 d. As shown in Figure 3C, NPA severely compromised, but did not eliminate, the pericycle cell competence to further form LRP, suggesting that NPA does not completely block founder cell specification.
Figure 3.
Auxin regulates SKP2B expression. A, Histochemical GUS staining of 5-d-old SKP2Bp:GUS seedlings treated with 1 µm 2,4-D for 0, 3, 5, or 7 h. Bottom panels show higher magnifications of the root meristem and elongation zone. Bars = 0.5 mm (top panels) and 0.1 mm (bottom panels). B, Histochemical GUS staining of 5-d-old SKP2Bp:GUS seedlings grown with or without 10 µm NPA. Bar = 0.2 mm. Arrows point to GUS-stained LRP. C, Number of emerged LR in plants grown in medium with or without 5 µm NPA for 7 d and then an extra 3 d in MS medium. The emerged LR were counted only in the portion of the root formed during the first 7 d. *P < 0.00001 by two-sided t test (n ≥ 40). D, Histochemical GUS staining of root meristems and LRP of SKP2Bp:GUS(Ws) (where Ws indicates Wassilewskija ecotype) and iaa28/SKP2Bp:GUS(Ws). Bars = 0.2 mm. E, Total number of GUS-stained spots in SKP2Bp:GUS(Ws) and iaa28/SKP2Bp:GUS(Ws). F, Representative images of SKP2Bp:GUS(Ws) and iaa28/SKP2Bp:GUS(Ws) roots of seedlings grown 5 d in MS medium and 1 d in 1 µm 2,4-D in MS medium. Bars = 0.5 mm. G, Representative images of the more basal region of slr-1/SKP2Bp:GUS roots stained for GUS activity after treating them with 0 or 1 µm 2,4-D for 2 d. The arrows point to stained putative LRP. Bars = 0.2 mm. H, Higher magnification of the GUS-stained spot in slr-1/SKP2Bp:GUS treated with 2,4-D. Bars = 0.05 mm. [See online article for color version of this figure.]
Recently, it has been proposed that the auxin response IAA28 module regulates the specification of pericycle cells to become founder cells (De Rybel et al., 2010). The iaa28 mutant can still develop some LRP (Rogg and Bartel, 2001; De Rybel et al., 2010). Corroborating this observation, we found that iaa28/SKP2Bp:GUS plants developed LRP, although significantly fewer than control plants, and that all of these LRP expressed SKP2B (Fig. 3, D and E). Interestingly, SKP2B was weakly induced by auxin in the iaa28 roots compared with wild-type roots (Fig. 3F), suggesting a possible role of IAA28 in controlling SKP2B expression. Later, these specified founder cells undergo an anticlinal cell division to start the development of the LRP. These anticlinal divisions are also controlled by auxin signaling, involving the activity of IAA14/SLR. A gain-of-function mutation in IAA14 (slr-1) leads to plants without LR (Fukaki et al., 2002). Histochemical analyses of slr-1/SKP2Bp:GUS showed GUS staining only in the root meristem (Fig. 3G). Auxin treatment of slr-1 did not induce SKP2B expression (Fig. 3G), except for a reproducible expression in a few pericycle cells in the differentiation zone (Fig. 3H) that could represent specified founder cells.
Mutations affecting auxin signaling reduce the number of LR (for review, see Mockaitis and Estelle, 2008). We crossed SKP2Bp:GUS with auxin signaling mutants (tir1-1, axr1-12, and ibr5-1) reported to develop fewer LR than the wild type. We found that auxin-dependent SKP2B induction was impaired in the axr1-12, a strong auxin signaling mutant (Hobbie and Estelle, 1995; Fig. 4A), while mutations in TIR1 or IBR5 slightly reduced SKP2B induction. Next, we studied the number of LR specified in these mutants, finding that tir1-1 and axr1-12 had a fewer number of GUS-stained LRP (Fig. 4B), while ibr5-1 developed a similar number to control roots, suggesting that IBR5 activity is needed for the emergence of LR rather than for LR specification, likely due to the function of the IAA28 module not being affected in this ibr5-1 mutant (Strader et al., 2008).
Figure 4.
Auxin signaling is needed for SKP2B expression. A, Histochemical GUS staining of 5-d-old SKP2Bp:GUS, tir1-1/SKP2Bp:GUS, axr1-12/ SKP2Bp:GUS, or ibr5-1/SKP2Bp:GUS roots. Seedlings were grown for 5 d in MS medium and then transferred to fresh medium containing 0 or 1 µm 2,4-D for 7 h. Representative images of the more basal region of the roots were made. Bars = 0.5 mm. B, Quantification of the number of LRP stained for GUS activity in the different mutants described above grown for 6 d in MS medium. Values represent means ± se. *P < 0.00001 by two-sided t test (n ≥ 40). [See online article for color version of this figure.]
Identification of a Novel Root-Specific Expression Motif
In order to identify domains responsible for SKP2B expression in founder cells and LRP, we generated different constructs containing deleted versions of the SKP2B promoter. Their expression pattern is summarized in Figure 5A. A promoter deletion containing 1 kb upstream from the ATG (SKP2B[1Kb]p:GUS) showed a similar expression pattern than for SKP2Bp:GUS. However, when we analyzed expression in the SKP2B[0.5Kb]p:GUS seedlings, it was restricted to the founder cells and LRP (Fig. 5B), losing expression in the aerial part of the plant (data not shown) and in the root meristem (Fig. 5B). When we analyzed the SKP2B[0.34Kb]p:GUS plants, we did not observe any GUS staining, while SKP2B[0.41Kb]p:GUS plants showed a similar expression pattern to SKP2B[0.5Kb]p:GUS plants (Fig. 5A). After auxin treatment, SKP2B[0.5Kb]p:GUS and SKP2B[0.41Kb]p:GUS seedlings showed GUS staining in the pericycle but not in the surrounding cortex or the epidermis (Fig. 5C).
Figure 5.
Dissection of SKP2B root expression. A, Representation of the different promoter regions used to generate transgenic plants that show expression in the root meristem, in the LRP, or in both. The SKP2B[1Kb-mut C(-397)A] construct bears a mutation at position −397 that replaces the cytosine by an adenine. B, Representative images of GUS-stained roots of SKP2Bp:GUS and SKP2B[0.5Kb]p:GUS plants showing a LRP (top panels) or a root meristem (bottom panels). Bar = 0.1 mm. C, Representative images of GUS-stained roots of SKP2B[0.5Kb]p:GUS plants treated with auxin showing staining in the pericycle cells close to the root tip (left panel) or in the differentiation zone (right panel). Bar = 0.1 mm. D, Representative images of GUS-stained roots of SKP2B[1Kb]p:GUS and SKP2B[1Kb-mut]p:GUS plants showing a LRP (top panels) or a root meristem (bottom panels). Bars = 0.2 mm. E, Higher magnification of representative images of GUS-stained roots of SKP2B[1Kb]p:GUS and SKP2B[1Kb-mut]p:GUS plants showing a LRP. Bars = 0.05 mm. F, Representative images of GUS-stained roots of 5-d-old SKP2B[1Kb]p:GUS and SKP2B[1Kbmut]p:GUS plants treated with 1 µm 2,4-D for 12 h. Bar = 0.1 mm. [See online article for color version of this figure.]
In the process of generating the SKP2B[1Kb]p:GUS lines, we identified a transformation event that did not render GUS staining in the LRP while the root meristem was still stained. After analyzing the insertion by sequencing, we found that this particular SKP2B[1Kb]promoter carried a mutation that replaced the cytosine in position −397 by an adenine. Next, by directed mutagenesis, we generated de novo this mutant construct (SKP2B[1Kb-mut]p:GUS), replacing the same cytosine −397 by adenine. SKP2B[1Kb-mut]p:GUS roots showed GUS staining in the root meristem but not in LRP (Fig. 5, D and E), demonstrating the relevance of this residue for its expression in LRP. In addition, this mutation also compromised the SKP2B auxin-dependent induction (Fig. 5F).
With this information, we analyzed the DNA sequence surrounding this cytosine using the PLACE motif search program (http://www.dna.affrc.go.jp/PLACE/index.html) to look for cis-elements. We found a plant motif denominated as “root-specific motif” located between nucleotides −387 and −409 from the ATG (Supplemental Fig. S3). We conducted an in silico analysis to search for promoters that contain at least one copy of this root motif, allowing only one mismatch in the sequence. We have identified more than 500 genes that contain this motif (Supplemental Table S1). When comparing these genes with those found in the pericycle cells (De Smet et al., 2008; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE6349), we found that about 60% of these genes are expressed in the pericycle cells, while only 36.5% ± 3.8% was obtained with random sampling (three different random samples of 600 genes each). Moreover, 4% of the genes that contain this motif are induced in response to auxin in the pericycle (Supplemental Table S1), while random sampling only retrieved 0.15% ± 0.028%. These data indicate a positive correlation between the presence of this motif and pericycle expression.
The SKP2B Promoter Is Enriched in Histone H3.3
Next, we wanted to get insight into the upstream molecular signaling that controls SKP2B expression in LRP. To do this, we conducted a yeast one-hybrid screen using the SKP2B[0.41Kb] promoter. We chose this promoter region because the root-specific motif identified, fused to a minimal 35S, was not sufficient to drive SKP2B expression in planta (data not shown). We isolated 10 clones that, after retesting in a medium containing 5, 10, 15, or 20 mm 3-amino-triazole (3-AT), only seven of them still activated the HIS3 marker (C. Manzano and J.C. del Pozo, unpublished data). Sequence analysis of these clones revealed that three of them corresponded to the AT5G10980 gene, which encodes for the histone variant H3.3 (Okada et al., 2006). Previously, one-hybrid screenings using other promoters have recovered the three H3 types (Ditzer and Bartels, 2006). However, we did not find other H3 variants in our screening. This fact led us to evaluate whether H3.1 could also bind the SKP2B[0.41Kb] promoter. We cloned HISTONE H3.1 and H3.3 in the pGAD424 vector and transformed them into the yeast strain containing the SKP2B[0.41KB]p construct. We observed that yeast transformed with the H3.1 clone were able to grow only in the presence of a low 3-AT level (5 mm), while those transformed with H3.3 grew up to 20 mm 3-AT (Supplemental Fig. S4), suggesting that H3.3 has a higher affinity for the SKP2B promoter than H3.1. This appealing result led us to investigate the H3 status across the SKP2B gene by mapping H3.1 and H3.3 occupancy in DNA extracted from roots. ChIP analyses using plants expressing Myc-tagged H3.1 or H3.3 (Stroud et al., 2012; see “Materials and Methods”) followed by PCR amplification of different regions (Fig. 6A) revealed that the SKP2B promoter contained mostly H3.3, while H3.1 was untraceable (Fig. 6B). When the coding region was analyzed, the H3.3 amount increased significantly, but now the H3.1 was detectable, consistent with active transcription of this gene (Fig. 6B). We found that root-expressed genes (see “Materials and Methods”; Supplemental Fig. S5) also showed H3.3/H3.1 enrichment, but in these cases the levels of H3.1 detected in the promoters were much higher than in the SKP2B promoter (Fig. 6B). Since H3.3 is associated with actively transcribed chromatin and SKP2B is highly transcribed in roots upon auxin treatment, we assessed the effect of this hormone on H3.3 deposition. We found that H3.3 deposition did not increase by auxin treatment (Supplemental Fig. S6), suggesting that H3.3 deposition might be more related to a specific cell type expression than to auxin response.
Figure 6.
The SKP2B promoter is regulated by histone H3 deposition. A, Schematic representation of At1g77000 (SKP2B) and localization of the primers used for ChIP-PCR. B, ChIP-PCR analysis of different SKP2B promoter fragments and root-expressed genes using chromatin extracted from roots of 7-d-old H3.3-MYC or H3.1-MYC Arabidopsis seedlings. C, Histochemical GUS staining of SKP2Bp:GUS, fas1/SKP2Bp:GUS, and fas1/SKP2B[0.5Kb]p:GUS roots. Seedlings were grown for 6 d in MS medium. Bar = 0.1 mm or 0.05 mm (right panels). RM, Root meristem. D, Histochemical GUS staining of SKP2Bp:GUS, fas1/SKP2Bp:GUS, and fas1/SKP2B[0.5Kb]p:GUS roots treated with auxin (1 µm 2,4-D) for 0, 3, 5, or 7 h. E, Quantification of the root length (mm), LRP (emerged plus nonemerged), and emerged LR per millimeter in 10-d-old fas1-4/SKP2Bp:GUS and SKP2Bp:GUS seedlings. Values represent means ± se. *P < 0.005 by two-sided t test (n ≥ 20). [See online article for color version of this figure.]
HIRA1 chaperone replaces H3.3 for H3.1 in differentiating cells after they exit the cell cycle (Lennox and Cohen, 1988). A HIRA1 homolog was identified in Arabidopsis (Phelps-Durr et al., 2005), but its function is still poorly known, and mutations in this gene result in an embryonic-lethal phenotype, complicating genetic studies. On the other hand, the CAF-1 complex is dedicated to the replication-coupled deposition of H3.1/H4 dimers (Polo and Almouzni, 2006), and viable mutants in Arabidopsis for CAF-1 complex subunits, fas1 and fas2, have been described (Serrano-Cartagena et al., 1999; Kaya et al., 2001). Thus, using these mutants, we decided to study whether alterations in H3.1 deposition influence SKP2B expression. Histochemical analyses of fas1-4/SKP2Bp:GUS or fas2-1/SKP2Bp:GUS eliminated SKP2B expression in LRP but not in the main root meristem or in the LRP surrounding cortex and epidermis (Fig. 6C; Supplemental Fig. S7). We also found that SKP2B auxin induction was compromised in the fas1-4 mutant (Fig. 6D), suggesting that the correct H3.1 incorporation is needed for both its cell-specific expression and auxin induction. Unexpectedly, when we analyzed in fas1-4 the expression of SKP2B[0.5Kb]p, a promoter region that specifically drives the expression in LRP, we found correct GUS staining in LRP (Fig. 6C) as well as auxin induction in the vascular tissue (Fig. 6D). These data indicate that the maintenance of SKP2B expression in founder cells and LRP relies on the SKP2B[0.5Kb]p region, but it is influenced by CAF-1 function in the proximal upstream region.
Next, we wondered whether the lack of SKP2B expression in the LRP in the fas1-4 mutant is a general effect on LRP-expressed genes or is locus specific. To study this, we generated fas1-4/GATA23p:GUS plants. GATA23 is only expressed in early LRP (De Rybel et al., 2010), and mutation in FAS1 did not affect its expression in early LRP (Supplemental Fig. S7), suggesting that the regulation of SKP2B expression by CAF-1 activity is locus specific and might represent a good example of how H3.1/H3.3 deposition regulates gene expression.
Finally, using the SKP2Bp:GUS reporter, we studied LR formation in the fas1-4 mutant. We found that fas1-4/SKP2Bp:GUS developed a lower number of LRP and emerged LR per root length than SKP2Bp:GUS plants (Fig. 6F), indicating that FAS1 is needed for LR specification and emergence.
The SKP2B Promoter Is Regulated by Auxin-Dependent Histone Acetylation
In addition to histone H3 exchange, H3 acetylation on promoters plays an important role in regulating gene transcription. We carried out ChIP analyses using an antibody that recognizes H3K9ac and H3K14ac as well as PCR amplification of different regions of the SKP2B promoter. We found that the SKP2B promoter was labeled by H3K9/K14ac (Fig. 7A). Next, we analyzed its H3 acetylation level in response to auxin in both wild-type and slr-1 roots. Interestingly, we found that auxin significantly promotes acetylation in the SKP2B promoter and to a lesser extent in the coding region, and such acetylation was significantly reduced in the slr-1 background. In this mutant, auxin treatment slightly increased the acetylation level in the SKP2B promoter, but never to the control level (Fig. 7B). Similarly, we detected that root-expressed promoters of CYCB1;1, GRP, PIN6, and ACTIN2 (ACT2) also contained acetylated H3 (Fig. 7C). The slr-1 dominant mutation also seems to reduce the H3 acetylation level in the root-expressed promoters, but the reduction was significantly lower than in SKP2Bp. The bigger changes were found in the CYCB1;1 promoter, which was expected, since the expression of this locus is induced by auxin in the root (Himanen et al., 2002), and in the PIN6 promoter, in which acetylation level was reduced by auxin treatment (Fig. 7C).
Figure 7.
Acetylation in the SKP2B promoter is regulated by auxin. A, ChIP assays using chromatin isolated from roots of 7-d-old wild-type plants. Three different regions in the promoter (a–c) and one in the coding region (d) were PCR amplified and separated on an agarose gel. As a control, the ChIP assays were carried out using anti-IgG. B, Relative acetylation levels on the SKP2B locus. ChIP assays of 7-d-old wild-type (WT) or slr-1 mutant Arabidopsis roots treated with or without auxin (aux) using antibodies specific for diacetylated H3. As a control, the ChIP assays were carried out using anti-IgG. Quantitative PCR was used for relative quantification. The data were normalized to the levels in the wild type. aP < 0.001, bP < 0.02, cP < 0.05 by two-sided t test (n = 6). Values represent means ± se. C, Relative acetylation levels on promoters of root-expressed genes. The data were normalized to the levels in the wild type. aP < 0.001, bP < 0.02, cP < 0.05 by two-sided t test (n = 6). Values represent means ± se. D and E, Representative images of the root meristem of 5-d-old SKP2Bp:GUS and DR5p:GUS treated with or without TSA (5 µm) during 12 h in liquid MS medium. Arrows indicate the first LRP labeled by GUS staining. Bars = 0.1 mm in D and 0.5 mm in E. [See online article for color version of this figure.]
Next, we analyzed the effect of trichostatin A (TSA), a histone deacetylase inhibitor, on SKP2Bp:GUS and on the auxin signaling marker DR5p:GUS. Short TSA treatment (12 h) led to higher and delocalized GUS staining in the basal meristem and transition zone in SKP2Bp:GUS roots (Fig. 7D). TSA-treated DR5p:GUS seedlings also showed significantly increased GUS staining in the vasculature of the basal meristem and 2transition/differentiation zone (Fig. 7D), similar to what was found in auxin-treated seedlings. Conversely, we found lower levels of GUS staining in the most basal LRP in TSA-treated roots (Fig. 7E). Remarkably, when seedlings were grown for 3 d in the presence of TSA, instead of 12 h, the root growth was significantly delayed (Supplemental Fig. S8, A and B). Moreover, we found that a 3-d TSA treatment blocked the auxin-dependent induction of SKP2B:GUS and DR5:GUS reporters (Supplemental Fig. S8, C and D). It has been described that TSA treatment was able to promote LR formation in the slr-1 mutant (Fukaki et al., 2006). When slr-1 was treated with TSA for 3 d, we found SKP2B expression in specific cells in the pericycle (Supplemental Fig. S8E), likely corresponding to founder cells. Remarkably, all these SKP2B expression points appeared only on the root sections grown in the presence of TSA. Taken together, our data suggest that H3 acetylation regulates auxin responsiveness in the basal meristem and SKP2B expression in the root.
DISCUSSION
SKP2B Is a Negative Cell Cycle Regulator in the Root System
Both SKP2A and SKP2B were identified by their homology with the human Skp2, which is a key regulator of cell division (del Pozo et al., 2002). SKP2A is an auxin-binding F-box protein that functions as a positive regulator of cell division (Jurado et al., 2008, 2010). Despite the high homology of both F-box proteins, SKP2B functions as a negative regulator of cell division in the root meristem and in the founder cells.
Here, we show that the skp2b mutant develops a higher number of LRP in stages I and II than wild-type roots, suggesting that SKP2B participates in the first anticlinal division of founder cells, an idea that is supported by the SKP2B expression pattern. Based on this, we think that SKP2B might contribute to maintain founder cells undivided until the correct developmental time. However, although statistically significant, the increase in the number of LRP is not stunning. This could be explained by the redundant mechanisms that govern the cell division process and the fact that deprivation of SKP2B function is partially compensated by the functions of other proteins, attenuating the skp2b root phenotype. This partial compensation has been shown for other cell cycle proteins such as Cdt1 (Nishitani et al., 2001) and p27/Kip1 (Müller et al., 1997; Carrano et al., 1999; Amador et al., 2007). In addition, this idea is supported by the fact that the double mutant for SKP2B and RKP1 (a KPC1-related RING finger protein), another E3 ligase that collaborates in KRP1 proteolysis (Ren et al., 2008), develops more LRP in early stages than either single mutant or wild-type plants (Supplemental Fig. S9). In view of these results, it is reasonable to think that additional and redundant mechanisms govern founder cell division and that SKP2B function is just one of them.
This role as a negative cell division regulator might conflict with the proposed role of SKP2B in the degradation of the cell division repressor KRP1 (Ren et al., 2008). However, it is possible that SKP2B degrades other targets in addition to KRP1, as has been shown for many E3 ligases, including HsSkp2, which targets cell cycle repressors such as p27 (Kossatz et al., 2004) and cell cycle activators such as E2F1 (Marti et al., 1999) or cyclin E (Yeh et al., 2001). In addition, histological analyses of the KRP1p:GUS plants show that KRP1 is not expressed in roots in normal developmental conditions (G.-T. Kim, personal communication).
Identification of a Novel and Specific Root Motif
Until now, few root-specific motifs have been described. In this work, we have identified a promoter domain and a motif that are needed for specific root expression of SKP2B. An in silico search using this motif led us to identify more than 500 genes that contain it in their promoters (Supplemental Table S1). It is remarkable that more than 60% of these genes are expressed in the pericycle, suggesting that this motif might be needed for pericycle expression. A mutation in the cytosine at position −397 of this motif blocked SKP2B expression in LRP and almost blocked its auxin responsiveness. An in silico search revealed the existence of an Auxin Response Element (Aux-RE) downstream of this motif (Supplemental Fig. S3), suggesting that this cytosine might influence this Aux-RE. One possibility is that this cytosine is regulated by methylation. However, we do not think that methylation is important for SKP2B regulation, since SKP2Bp:GUS plants treated with 5-aza-2′-deoxycytidine, an inhibitor of DNA methylation, did not show differences in GUS staining (data not shown). Recently, the screening of a Ds-element enhancer trap line in Arabidopsis led to the identification of a root-specific promoter in the At1g73160 gene (Vijaybhaskar et al., 2008), which contains a copy of the LR-specific motif identified here. Based on these observations, we can conclude that the motif identified in this work is important to confer expression in LRP as well as to the auxin response.
Recent work indicated that the IAA28 auxin response module functions in founder cell specification while IAA14/SLR regulates LRP initiation (Vanneste et al., 2005; De Rybel et al., 2010). Confirming previous results, we found that the iaa28 mutant develops fewer LRP than the wild type, and all of the LRP detected expressed SKP2B. However, the auxin responsiveness of SKP2B was severely compromised in this mutant, suggesting that the IAA28 module regulates the expression of SKP2B in response to auxin. Interestingly, we found that the dominant slr-1 mutation completely blocks SKP2B expression in founder cells and LRP. It is possible that, just after founder cell specification, degradation of IAA14/SLR in founder cells is needed to maintain founder cell status and, later, to allow the first anticlinal division. These data suggest that in the LR formation program, SKP2B regulates cell division downstream of IAA28 and IAA14/SLR auxin response modules. Despite the fact that the SKP2B promoter contains two Aux-RE in the promoter, suggesting a direct regulation by IAA28 and/or IAA14, we do not know whether the regulation is directed or not. Further experiments involving ChIP analyses will answer this question.
Here, we have shown that SKP2Bp:GUS labeled all morphologically recognizable LRP. Conversely, GUS staining is not detected in all LRP in DR5p:GUS plants, a widely used marker to study LR development for being the earliest reporter associated with this process (Benková et al., 2003; Dubrovsky et al., 2008), suggesting that some of these LRP are arrested (Zolla et al., 2010; this work). Taken together, we think that SKP2Bp:GUS is an excellent and trustworthy maker to study LR development in different conditions or mutant backgrounds. For example, the use of this marker has easily shown that mutations in AXR1 affect LR specification while a mutation in IBR5 affects LR emergence more than specification (Fig. 4B).
Epigenetic Regulation of SKP2B
Here, we present evidence that SKP2B is regulated by novel mechanisms involving histone exchange and auxin-dependent acetylation.
Histone H3.1 is incorporated into nucleosomes in dividing cells during DNA synthesis, while H3.3, a replacement variant that can substitute for H3.1, is incorporated into nucleosomes during transcription, and it is generally associated with actively transcribed chromatin both in animals and plants. This exchange provides a fast and dynamic gene activation mechanism of loci that are normally repressed by histone modifications (Ahmad and Henikoff, 2002; McKittrick et al., 2004; Schwartz and Ahmad, 2005; Ingouff and Berger, 2010). CAF-1 activity is involved in depositing H3.1/H4 dimers during DNA replication. Our data clearly show that the SKP2B promoter and gene body are enriched in H3.3 over H3.1 and that the lack of FAS1 activity affects root development, reducing the LR density, which can be explained by defects in founder cell specification or by defects in the development of LRP. It is remarkable that mutations in FAS1 or FAS2 genes eliminate the expression of SKP2B in LRP but not in the root meristem or in the cortex/epidermis surrounding the LRP. It is possible that, in CAF-1 mutants, the impossibility of a correct deposition of H3.1 leads to an incorrect deposition of other histones and epigenetic marks, which might make difficult the correct exchange for H3.3 and proper transcriptional activation. In animal cells, the disruption of CAF-1-dependent H3.1 incorporation during replication activates an alternative salvage pathway in which HIRA deposits H3.3 at replication sites (Ray-Gallet et al., 2011). Unlike in mammals, where CAF-1 function is essential (Quivy et al., 2001), in Arabidopsis, mutants for CAF-1 subunits (FAS1 or FAS2) are fully viable, although they show defects in the root and shoot meristems (Serrano-Cartagena et al., 1999; Kaya et al., 2001). Our results indicate that in Arabidopsis the lack of CAF-1 activity eliminates SKP2B expression from LRP. At first glance, this result might be surprising, since higher H3.3 incorporation correlates with an increase in gene expression. However, in plants, it is unknown how or what type of H3 are deposited in CAF-1 mutants, but knockout mutants for CAF-1 subunits are fully viable, suggesting that plants have evolved alternative pathways to overcome this lack of H3.1 deposition. In addition, Arabidopsis has several isoforms of H3.1 and H3.3 that might have different functions in vivo or use different chaperones for deposition, offering higher versatility. However, additional analyses of how and what types of H3 are deposited in different chaperone mutants will be necessary to understand this, and they will be the subject of future work.
The fact that mutations in FAS1 do not impede the expression of SKP2B[0.5Kb]p:GUS in LRP suggests that this promoter region acts as an autonomous LRP expression module that is not directly regulated by CAF-1 function in founder cells and LRP. Conversely, the incorrect deposition of H3.1 in fas1 might lead to inaccurate H3.3 (or other histones) deposition and/or epigenetic marks, altering the activity of a regulatory element located upstream of the [0.5Kb]SKP2B promoter that influences SKP2B expression in LRP. However, we cannot discard the possibility that the fas1-4 mutation changes the levels of a gene that regulates SKP2B expression. At present, the SKP2B promoter seems to respond only to auxin signaling, which is mainly regulated by the activity of Aux/IAA and ARF transcription factors (Mockaitis and Estelle, 2008). Analyzing the transcriptome of the fas1 mutant (Schönrock et al., 2006), we did not find changes in any Aux/IAA or ARF, suggesting that that the lack of SKP2B expression in LRP in fas1 is likely due to an incorrect H3.1/H3.3 deposition in its promoter rather than to changes in the levels of SKP2B regulatory proteins. This SKP2B regulation by CAF-1 seems to be specific, since GATA23, an early expressed gene in LRP, is properly expressed in the fas1-4 mutant, making SKP2B expression a good example of how H3.1/H3.3 deposition might control gene expression in a specific cell type. Although speculative at this time, it is possible that H3.1/H3.3 exchange on specific loci would be one of the molecular mechanisms involved in founder cells specification in the basal meristem along with other mechanisms already proposed (De Rybel et al., 2010; Moreno-Risueno et al., 2010).
Acetylation on H3K9 and K14 significantly correlated to changes in locus expression, linking these marks to gene activation (Markowetz et al., 2010). The facts that the SKP2B promoter accumulates these marks in response to auxin and that TSA treatment alters SKP2B expression indicate that a correct H3 acetylation is needed for proper SKP2B expression, at least in the root. Recently, is has been shown that auxin regulates gene transcription by readjustments in chromatin epigenetics. Auxin regulates changes in the acetylation levels of several promoters through SAGA-like complexes (Anzola et al., 2010). TOPLESS (TPL), a transcriptional corepressor, influences the auxin-mediated repression through the function of histone deacetylase complexes (Szemenyei et al., 2008). It has been suggested that TPL or a TPL-like gene might interact with IAA14/SLR to repress, via deacetylase activities, ARF7/19-auxin signaling during LR development. Auxin treatment causes a significant increase of the H3K9/K14ac levels preferentially in the promoter of SKP2B, and this acetylation is diminished in the slr-1 mutant. Supporting this role of histone acetylation in auxin response and root development, it has been shown that treatment with TSA, a histone deacetylase inhibitor, partially rescues the LR formation defect in slr-1 (Fukaki et al., 2006). We have shown that TSA activated SKP2B expression in founder cells/LRP in the slr-1. Since SKP2B was initially expressed at the position of transference to TSA-containing medium and in the most apical part of the roots, we think that TSA is promoting founder cell specification and division of these cells in the slr-1 roots rather than activating the division of prespecified founder cells. In addition, we show that a short-time TSA treatment induces the auxin response marker (DR5p:GUS) and SKP2B expression in the founder cell specification zone. Conversely, long-term TSA treatment completely blocks the auxin response of both SKP2Bp:GUS and DR5:GUS, suggesting that acetylation/deacetylation balance is critical for auxin responsiveness. In light of these results, it is tempting to speculate that H3.3 deposition and H3 acetylation in K9/K14 in an auxin- and IAA14/SLR-dependent pathway activate SKP2B expression in roots and likely are needed for founder cell specification. However, to unravel the molecular mechanisms that regulate SKP2B expression and LR formation via IAA14 will require further experiments.
MATERIALS AND METHODS
Plant Material and Constructs
In this work, we have used the following Arabidopsis (Arabidopsis thaliana) plants: control or wild type (Columbia ecotype), tir1-1 (Ruegger et al., 1998), skp2b (Ren et al., 2008), axr1-12 (Hobbie and Estelle, 1995), slr-1 (Fukaki et al., 2002), iaa28 (Rogg and Bartel, 2001), ibr5-1 (Monroe-Augustus et al., 2003), fas1-4 (Ramirez-Parra and Gutierrez, 2007), and fas2-1 (Serrano-Cartagena et al., 1999). These plants were grown under sterile conditions on vertically oriented Murashige and Skoog (MS; one-half MS salts, 1% Suc, and 1% plant agar [Duchefa]) plates at 22°C with 16 h of light and 8 h of dark. For auxin treatment, plants were grown on vertical MS plates for 5 d and then transferred to MS liquid medium with 1 µm 2,4-dichlorophenoxyacetic acid (2,4-D) for the indicated times. For TSA treatments, seedlings were grown for 5 d on solid vertical MS plates and then treated with 10 µm TSA (Sigma) in liquid MS medium for 12 h. These seedlings were them subjected of GUS staining.
To generate the transgenic lines that harbor the different constructs containing the full promoter or deletions of the SKP2B promoter fused to GUS, the promoter regions were amplified by PCR and cloned into pDONOR221 by recombination using the GATEWAY BP Clonase enzyme mix (Invitrogen). Then, these promoter regions were mobilized to pGWB3 (Nakagawa et al., 2007) by recombination using the GATEWAY LR Clonase enzyme mix (Invitrogen). The full-length promoter containing 1,750 bp upstream of ATG (SKP2Bp:GUS) was used to generate transgenic plants in three different Arabidopsis ecotypes, Columbia (SKP2Bp:GUS), Landsberg erecta [SKP2Bp:GUS(Ler)], and Wassilewskija [SKP2Bp:GUS(Ws)], using the floral dip method (Clough and Bent, 1998). Several independent transgenic lines were analyzed for GUS staining, all of them showing the same expression pattern. Three tandem repetitions of the root-specific motif (from −393 to −409) were fused to the −50 35S minimum promoter (Tucker et al., 2002) and transgenic plants were generated. To generate the point position mutant promoter, we used the Quick Change Multi Site Directed Mutagenesis kit (Stratagene) using pDONOR221-SKP2B[1Kb] as a template. We changed the cytosine at position −397 to adenine to generate pDONOR221-SKP2B[1Kb-mut C(-397)A]. After mutagenesis, the whole DNA was sequenced to discard undesired mutations and then transferred to pGWB3 vector by LR recombination to generate SKP2B[1Kb-mut C(-397)A]p:GUS.
GUS Assays
Histochemical GUS staining was performed as described by del Pozo et al. (2006). Photographs were taken using a Leica MZ9.5 stereomicroscope with a DCF280 camera or a Leica MD2000 microscope with a DCF300 camera.
Yeast One-Hybrid Analysis
The promoter region of SKP2B containing 410 bp upstream from the ATG was cloned into the Gateway-adapted pHISi-1 vector to prepare reporter yeast harboring HIS3. The construct was linearized with XhoI, and 1 µg was used for yeast transformation using the strain Y187. The transformation was carried out as described in the Matchmaker protocol (Clontech; http://www.clontech.com/ES/Products/Protein_Interactions_and_Profiling/Yeast_Two-Hybrid/ibcGetAttachment.jsp?cItemId=17583&fileId=5877836&sitex=10023:22372:US), using a complementary DNA (cDNA) library generated from mRNA isolated from auxin-treated 5-d-old Arabidopsis seedlings (kindly provided by W. Gray). The screening was carried out in a dropout base medium without Leu and His and containing 5 mm 3-AT. Approximately 1.2 million yeast transformants were screened, and 10 positive clones were isolated, which were regrown in a minimum medium containing 5, 10, and 20 mm 3-AT. The DNAs of these positive clones were PCR amplified and sequenced. The full-length cDNAs of HISTONE H3.1 and H3.3 were cloned in pGAD42 to transform into the yeast strain containing the SKP2B[0.41]p version to test the activation potential of both proteins.
ChIP Assays
To determine the H3.3 and H3.1 enrichment on the promoter, we used Arabidopsis transgenic plants expressing promoter and coding sequences of H3.1 (HTR13; At5g10390) or H3.3 (HTR5; At4g40040) fused in frame to a Myc tag and expressed under the control of their own promoters (Stroud et al., 2012). For the ChIP assays, we used chromatin-isolated roots of 7-d-old plants grown on MS agar plates in 16 h of light and 8 h of dark at 22°C. The ChIP experiment was carried out as described by Stroud et al. (2012). Chromatin was immunoprecipitated with 10 µg of anti-Myc antibody, clone 4A6 (Millipore), or anti-IgG (Abcam ab6703) used as a negative control. The different promoter fragments were amplified by PCR and resolved on agarose gels. We also amplified promoter regions of root-expressed genes. We used a near-localized SKP2B gene (At1g77100; PIN6), the cell cycle and auxin up-regulated gene CYCB1;1 (At4g37490), an auxin down-regulated gene (GRP; At4g30450), and ACT2 (At3g18780). The H3K9K14 ChIP assays and data analysis were carried out basically as described previously (Ramirez-Parra and Gutierrez, 2007) using chromatin isolated from root cells of 7-d-old plants. We fixed the roots in the presence of 3 mm sodium butyrate (Sigma), and immunoprecipitation was carried out with anti-H3ac antibody (Upstate-Millipore no. 06-599). FastStart DNA Master SYBR Green I (Roche) was used for quantitative real-time PCR. Data correspond to averages of two independent biological experiments and three independent quantitative PCR analyses per experiment. Primer sequences and conditions are available upon request.
Root Growth Assays and Microscopic Analysis
Primary root length was determined as described previously (Lucas et al., 2011). All data are mean values of at least 50 plants, and these experiments were repeated twice, obtaining similar values in each experiment. Data values were statistically analyzed using Student’s t function. Total numbers and stages of LRP were counted according to methods used previously (Malamy and Benfey, 1997), and root meristem size was calculated based on the number of meristematic cortex cells (Casamitjana-Martínez et al., 2003).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers BT024747.1 and AEE35924.1, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. SKP2B is cell cycle regulated.
Supplemental Figure S2. DR5p:GUS is not expressed in all LRP.
Supplemental Figure S3. Identification of a root-specific motif.
Supplemental Figure S4. H3.3 activates the SKP2B[0.5Kb] promoter in yeast one-hybrid analysis.
Supplemental Figure S5. Sequence of the promoter used in the ChIP analyses.
Supplemental Figure S6. H3.3 deposition in auxin-treated roots.
Supplemental Figure S7. SKP2Bp:GUS expression in fas1-4 and fas2-1.
Supplemental Figure S8. TSA inhibits auxin induction of SKP2B and DR5.
Supplemental Figure S9. LRP density in the rkp1 mutant.
Supplemental Table S1. In silico identification of genes containing the root-specific motif.
Acknowledgments
We thank Sara Navarro for her technical assistance. We also are in debt to W. Gray for providing the cDNA library. We thank O. Navarro for reading and typing the manuscript. We are also in debt to M. Estelle for providing the axr1-12 and tir1-1 mutants; B. Bartel for the ibr5-1 and iaa28 mutants; and M. Tasaka for the slr-1 mutant.
Glossary
- LR
lateral roots
- LRP
lateral root primordia
- ChIP
chromatin immunoprecipitation
- NPA
1-N-naphthylphthalamic acid
- 3-AT
3-amino-triazole
- TSA
trichostatin A
- MS
Murashige and Skoog
- 2,4-D
2,4-dichlorophenoxyacetic acid
- cDNA
complementary DNA
References
- Ahmad K, Henikoff S. (2002) The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell 9: 1191–1200 [DOI] [PubMed] [Google Scholar]
- Amador V, Ge S, Santamaría PG, Guardavaccaro D, Pagano M. (2007) APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell 27: 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzola JM, Sieberer T, Ortbauer M, Butt H, Korbei B, Weinhofer I, Müllner AE, Luschnig C. (2010) Putative Arabidopsis transcriptional adaptor protein (PROPORZ1) is required to modulate histone acetylation in response to auxin. Proc Natl Acad Sci USA 107: 10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1: 193–199 [DOI] [PubMed] [Google Scholar]
- Casamitjana-Martínez E, Hofhuis HF, Xu J, Liu CM, Heidstra R, Scheres B. (2003) Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem maintenance. Curr Biol 13: 1435–1441 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ. (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Charlton WA. (1996) Lateral root initiation. In Y Waisel, A Eshel, U Kafkafi, eds, Plant Roots: The Hidden Half. Marcel Dekker, New York, pp 149–173
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P. (1999) Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20: 503–508 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Boniotti MB, Gutierrez C. (2002) Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell 14: 3057–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C. (2006) The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18: 2224–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, et al. (2010) A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20: 1697–1706 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frey NF, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al. (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- De Smet I, Vassileva V, De Rybel B, Levesque MP, Grunewald W, Van Damme D, Van Noorden G, Naudts M, Van Isterdael G, De Clercq R, et al. (2008) Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594–597 [DOI] [PubMed] [Google Scholar]
- Ditzer A, Bartels D. (2006) Identification of a dehydration and ABA-responsive promoter regulon and isolation of corresponding DNA binding proteins for the group 4 LEA gene CpC2 from C. plantagineum. Plant Mol Biol 61: 643–663 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E. (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn A. (1974) Plant Anatomy, Ed 2. Pergamon Press, New York, pp 49–64
- Fukaki H, Tameda S, Masuda H, Tasaka M. (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Taniguchi N, Tasaka M. (2006) PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation. Plant J 48: 380–389 [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7: 211–220 [DOI] [PubMed] [Google Scholar]
- Ingouff M, Berger F. (2010) Histone3 variants in plants. Chromosoma 119: 27–33 [DOI] [PubMed] [Google Scholar]
- Jurado S, Abraham Z, Manzano C, López-Torrejón G, Pacios LF, del Pozo JC. (2010) The Arabidopsis cell cycle F-box protein SKP2A binds to auxin. Plant Cell 22: 3891–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Diaz-Trivino S, Abraham Z, Manzano C, Gutierrez C, del Pozo JC. (2008) SKP2A, an F-box protein that regulates cell division, is degraded via the ubiquitin pathway. Plant J 53: 828–841 [DOI] [PubMed] [Google Scholar]
- Kaya H, Shibahara KI, Taoka KI, Iwabuchi M, Stillman B, Araki T. (2001) FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104: 131–142 [DOI] [PubMed] [Google Scholar]
- Kossatz U, Dietrich N, Zender L, Buer J, Manns MP, Malek NP. (2004) Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes Dev 18: 2602–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox RW, Cohen LH. (1988) The production of tissue-specific histone complements during development. Biochem Cell Biol 66: 636–649 [DOI] [PubMed] [Google Scholar]
- Lucas M, Swarup R, Paponov IA, Swarup K, Casimiro I, Lake D, Peret B, Zappala S, Mairhofer S, Whitworth M, et al. (2011) Short-Root regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol 155: 384–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Markowetz F, Mulder KW, Airoldi EM, Lemischka IR, Troyanskaya OG. (2010) Mapping dynamic histone acetylation patterns to gene expression in nanog-depleted murine embryonic stem cells. PLoS Comput Biol 6: e1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti A, Wirbelauer C, Scheffner M, Krek W. (1999) Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat Cell Biol 1: 14–19 [DOI] [PubMed] [Google Scholar]
- McKittrick E, Gafken PR, Ahmad K, Henikoff S. (2004) Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc Natl Acad Sci USA 101: 1525–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M. (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24: 55–80 [DOI] [PubMed] [Google Scholar]
- Monroe-Augustus M, Zolman BK, Bartel B. (2003) IBR5, a dual-specificity phosphatase-like protein modulating auxin and abscisic acid responsiveness in Arabidopsis. Plant Cell 15: 2979–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN. (2010) Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329: 1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Bouchard C, Rudolph B, Steiner P, Stuckmann I, Saffrich R, Ansorge W, Huttner W, Eilers M. (1997) Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/cdk2 complexes. Oncogene 15: 2561–2576 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. (2007) Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Taraviras S, Lygerou Z, Nishimoto T. (2001) The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J Biol Chem 276: 44905–44911 [DOI] [PubMed] [Google Scholar]
- Okada T, Singh MB, Bhalla PL. (2006) Histone H3 variants in male gametic cells of lily and H3 methylation in mature pollen. Plant Mol Biol 62: 503–512 [DOI] [PubMed] [Google Scholar]
- Parizot B, De Rybel B, Beeckman T. (2010) VisuaLRTC: a new view on lateral root initiation by combining specific transcriptome data sets. Plant Physiol 153: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Phelps-Durr TL, Thomas J, Vahab P, Timmermans MC. (2005) Maize rough sheath2 and its Arabidopsis orthologue ASYMMETRIC LEAVES1 interact with HIRA, a predicted histone chaperone, to maintain knox gene silencing and determinacy during organogenesis. Plant Cell 17: 2886–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo SE, Almouzni G. (2006) Chromatin assembly: a basic recipe with various flavours. Curr Opin Genet Dev 16: 104–111 [DOI] [PubMed] [Google Scholar]
- Quivy JP, Grandi P, Almouzni G. (2001) Dimerization of the largest subunit of chromatin assembly factor 1: importance in vitro and during Xenopus early development. EMBO J 20: 2015–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Parra E, Gutierrez C. (2007) E2F regulates FASCIATA1, a chromatin assembly gene whose loss switches on the endocycle and activates gene expression by changing the epigenetic status. Plant Physiol 144: 105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray-Gallet D, Woolfe A, Vassias I, Pellentz C, Lacoste N, Puri A, Schultz DC, Pchelintsev NA, Adams PD, Jansen LE, et al. (2011) Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol Cell 44: 928–941 [DOI] [PubMed] [Google Scholar]
- Ren H, Santner A, del Pozo JC, Murray JA, Estelle M. (2008) Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. Plant J 53: 705–716 [DOI] [PubMed] [Google Scholar]
- Rogg LE, Bartel B. (2001) Auxin signaling: derepression through regulated proteolysis. Dev Cell 1: 595–604 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev 12: 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönrock N, Exner V, Probst A, Gruissem W, Hennig L. (2006) Functional genomic analysis of CAF-1 mutants in Arabidopsis thaliana. J Biol Chem 281: 9560–9568 [DOI] [PubMed] [Google Scholar]
- Schwartz BE, Ahmad K. (2005) Transcriptional activation triggers deposition and removal of the histone variant H3.3. Genes Dev 19: 804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Cartagena J, Robles P, Ponce MR, Micol JL. (1999) Genetic analysis of leaf form mutants from the Arabidopsis Information Service collection. Mol Gen Genet 261: 725–739 [DOI] [PubMed] [Google Scholar]
- Strader LC, Monroe-Augustus M, Bartel B. (2008) The IBR5 phosphatase promotes Arabidopsis auxin responses through a novel mechanism distinct from TIR1-mediated repressor degradation. BMC Plant Biol 8: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud H, Otero S, Desvoyes B, Ramírez-Parra E, Jacobsen SE, Gutierrez C. (2012) Genome-wide analysis of histone H3.1 and H3.3 variants in Arabidopsis thaliana. Proc Natl Acad Sci USA 109: 5370–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA. (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386 [DOI] [PubMed] [Google Scholar]
- Tucker ML, Whitelaw CA, Lyssenko NN, Nath P. (2002) Functional analysis of regulatory elements in the gene promoter for an abscission-specific cellulase from bean and isolation, expression, and binding affinity of three TGA-type basic leucine zipper transcription factors. Plant Physiol 130: 1487–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, et al. (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17: 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaybhaskar V, Subbiah V, Kaur J, Vijayakumari P, Siddiqi I. (2008) Identification of a root-specific glycosyltransferase from Arabidopsis and characterization of its promoter. J Biosci 33: 185–193 [DOI] [PubMed] [Google Scholar]
- Yeh KH, Kondo T, Zheng J, Tsvetkov LM, Blair J, Zhang H. (2001) The F-box protein SKP2 binds to the phosphorylated threonine 380 in cyclin E and regulates ubiquitin-dependent degradation of cyclin E. Biochem Biophys Res Commun 281: 884–890 [DOI] [PubMed] [Google Scholar]
- Zolla G, Heimer YM, Barak S. (2010) Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot 61: 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]