Abstract
Auxin efflux carrier PIN-FORMED (PIN) proteins are thought to have central roles in regulating asymmetrical auxin translocation during tropic responses, including gravitropism and phototropism, in plants. Although PIN3 is known to be involved in phototropism in Arabidopsis (Arabidopsis thaliana), no severe defects of phototropism in any of the pin mutants have been reported. We show here that the pulse-induced, first positive phototropism is impaired partially in pin1, pin3, and pin7 single mutants, and severely in triple mutants. In contrast, such impairment was not observed in continuous-light-induced second positive phototropism. Analysis with an auxin-reporter gene demonstrated that PIN3-mediated auxin gradients participate in pulse-induced phototropism but not in continuous-light-induced phototropism. Similar functional separation was also applicable to PINOID, a regulator of PIN localization. Our results strongly suggest the existence of functionally distinct mechanisms i.e. a PIN-dependent mechanism in which transient stimulation is sufficient to induce phototropism, and a PIN-independent mechanism that requires continuous stimulation and does not operate in the former phototropism process. Although a previous study has proposed that blue-light photoreceptors, the phototropins, control PIN localization through the transcriptional down-regulation of PINOID, we could not detect this blue-light-dependent down-regulation event, suggesting that other as yet unknown mechanisms are involved in phototropin-mediated phototropic responses.
Plants have developed many adaptive responses to acclimate to their environments. Phototropism induced by unilateral blue-light irradiation is one such adaptive response, through which plants take advantage to capture light energy for photosynthesis. Classical physiological investigations have identified two types of phototropism, first and second positive phototropism (Iino, 2001; Whippo and Hangarter, 2006). First positive phototropism, which is induced by a pulse of blue light, shows a typical bell-shaped fluence response curve, and the magnitude of the responses depends on the total light fluence. Second positive phototropism is induced by a prolonged irradiation of blue light, and the magnitudes of phototropic responses are dependent on the duration of irradiation. Both types of phototropism are modulated by red-light treatment before phototropic stimulation through phytochromes, and in many cases, preirradiation with red light enhances the responses (Iino, 1988; Janoudi and Poff, 1991; Janoudi et al., 1997; Whippo and Hangarter, 2004). Several molecular mechanisms are proposed to be involved in the enhancement (Han et al., 2008; Tatematsu et al., 2004; Nagashima et al., 2008a; Kim et al., 2011).
Auxin asymmetry is one of the important mechanistic issues that has been studied extensively to further clarify the nature of the phototropic responses (Iino, 1990; Holland et al., 2009; Pedmale et al., 2010). According to the Cholodny-Went theory (Went and Thimann, 1937), unilateral light stimulation induces auxin gradients between the irradiated side and the shaded side, resulting in differential growth and a phototropic curvature. The hypothesis is generally accepted at present because supporting evidence has been provided in both dicots and monocots (Iino, 1991; Friml et al., 2002; Haga et al., 2005; Esmon et al., 2006; Haga and Iino, 2006; Ding et al., 2011). Molecular genetic studies have also indicated that the phototropin blue-light photoreceptors, phot1 (Huala et al., 1997) and phot2 (Sakai et al., 2001), play a fundamental role in the induction of the phototropic response with phototropin-binding proteins, such as NONPHOTOTROPIC HYPOCOTYL3 (Motchoulski and Liscum, 1999), ROOT PHOTOTROPISM2 (Sakai et al., 2000), and PHYTOCHROME KINASE SUBSTRATE (Lariguet et al., 2006). In addition, phototropin signaling appears to control the asymmetrical translocation of auxin. In fact, the NONPHOTOTROPIC HYPOCOTYL3 ortholog in rice (Oryza sativa), COLEOPTILE PHOTOTROPISM1, has been shown to be an essential mediator of auxin redistribution in coleoptiles during the phototropic response (Haga et al., 2005). It remains largely unknown, however, how auxin gradients are achieved in response to the activation of phototropin signaling (Iino and Haga, 2005; Holland et al., 2009).
Auxin efflux carrier PIN-FORMED (PIN) proteins are involved in many aspects of plant growth and development through the modulation of auxin flow and distribution (Petrášek and Friml, 2009). Arabidopsis (Arabidopsis thaliana) encodes eight PIN genes (Mravec et al., 2009). Among these, PIN3 is thought to play central roles in phototropism and gravitropism by regulating the lateral translocation of auxin (Friml et al., 2002; Ding et al., 2011; Rakusová et al., 2011). The pin3 mutants show impairment of phototropism and gravitropism (Friml et al., 2002; Nagashima et al., 2008b), and a reduction of asymmetrical auxin distribution during these tropisms (Ding et al., 2011; Rakusová et al., 2011). Ding et al. (2011) have reported that, under conditions of darkness, PIN3 proteins are expressed in the hypocotyl endodermis, which constitutes a barrier between the vasculature and the outer cell layers, and shows an apolar localization in endodermal cells. Under unilateral white-light irradiation, the PIN3 protein levels are greatly decreased in the outer lateral side of the endodermal cells on the irradiated hypocotyl side in a phot1-dependent manner. Thus, PIN3 proteins in the endodermal cells of the irradiated hypocotyl side appear to redirect auxin back into the vasculature and block its flow from the vasculature to the outer cell layers, most notably the epidermis. Moreover, the regulation of PIN3 subcellular localization appears to be an underlying mechanism of the asymmetrical translocation of auxin during phototropic responses.
The significance of PIN3 function in phototropism remains to be fully determined, however, as a substantial portion of the hypocotyl phototropism remains unaffected in pin3 mutants whereas auxin asymmetry is severely affected (Ding et al., 2011). In addition, Christie et al. (2011) have reported that hypocotyl phototropism is not severely impaired in dark-adapted pin3 single mutants, although pin3 mutation influences some aspects of phototropic responses. One possibility is that other PIN proteins have redundant functions with PIN3. For example, PIN1, PIN2, PIN4, and PIN7, which show subcellular localization on the plasma membrane, have similar protein structures to PIN3, harboring a long hydrophilic loop between the C-terminal and N-terminal transmembrane domains (for review, see Zažímalová et al., 2010). In this context, Ding et al. (2011) have examined the phototropic responses in etiolated seedlings of the pin3 pin7 double mutant and the pin2 pin3 pin7 triple mutant. Their findings indicate that the phototropic curvature of pin3 is slightly decreased by the additional mutation in pin7 but not in pin2. Christie et al. (2011) have also examined the phototropic responses in other mutants including pin1, pin2, pin4, pin7 and pin1 pin3. The dark-adapted pin1, pin2, pin4 and pin1 pin3 mutants show normal phototropic responses, but the pin7 mutant exhibits a lower phototropic curvature than the wild type. Although these results have suggested that PIN7 also plays some role in the phototropic responses, the significance of PIN functions in these systems remains unanswered at present.
ATP-BINDING CASSETTE subfamily B19 (ABCB19) is known as another type of auxin efflux carriers and as a regulatory component for PIN1 (Noh et al., 2001, 2003). The abcb19 mutants showed the enhancement of hypocotyl phototropism, indicating that the ABCB19 is a negative regulator for phototropism (Noh et al., 2003; Nagashima et al., 2008b). Recently, phot1 activated by blue light directly phosphorylates ABCB19, resulting in the inhibition of the function of ABCB19 (Christie et al., 2011), indicating that auxin is transported preferentially to the shaded side. Although they concluded that the ABCB19 is not directly involved in the lateral translocation of auxin, the investigation suggests that ABCB19 participates in the distribution of auxin during hypocotyl phototropism, and that auxin distribution is finely tuned in response to blue light by a complex mechanism.
In our current study, we distinguished the phototropic responses in three categories, pulse-induced phototropism (first positive phototropism), time-dependent second positive phototropism (dark treatment after a prolonged irradiation), and continuous-light-induced second positive phototropism. We investigated the phototropic responses of the Arabidopsis pin mutants under each of these light conditions. Our results clearly indicate that PIN1, PIN3, and PIN7 function redundantly in both pulse-induced phototropism and time-dependent phototropism. However, phototropism induced by continuous irradiation of blue light is not impaired in pin1 pin3 pin7 triple mutants, in addition to various pin single mutants. This indicates that PIN1, PIN3, and PIN7 at least are unnecessary for continuous-light-induced second positive phototropism. We propose that the phototropic responses are regulated by distinct molecular mechanisms; a PIN-dependent mechanism in which transient stimulation is enough to induce phototropism, and a PIN-independent mechanism in which continuous stimulation is necessary for phototropic responses. Furthermore, we have evaluated the phototropic responses in mutants of ABCB19, and a PIN-regulating protein kinase, PINOID (PID), and discuss the function and role of red-light preirradiation in hypocotyl phototropism.
RESULTS
Hypocotyl Phototropism in pin Mutants
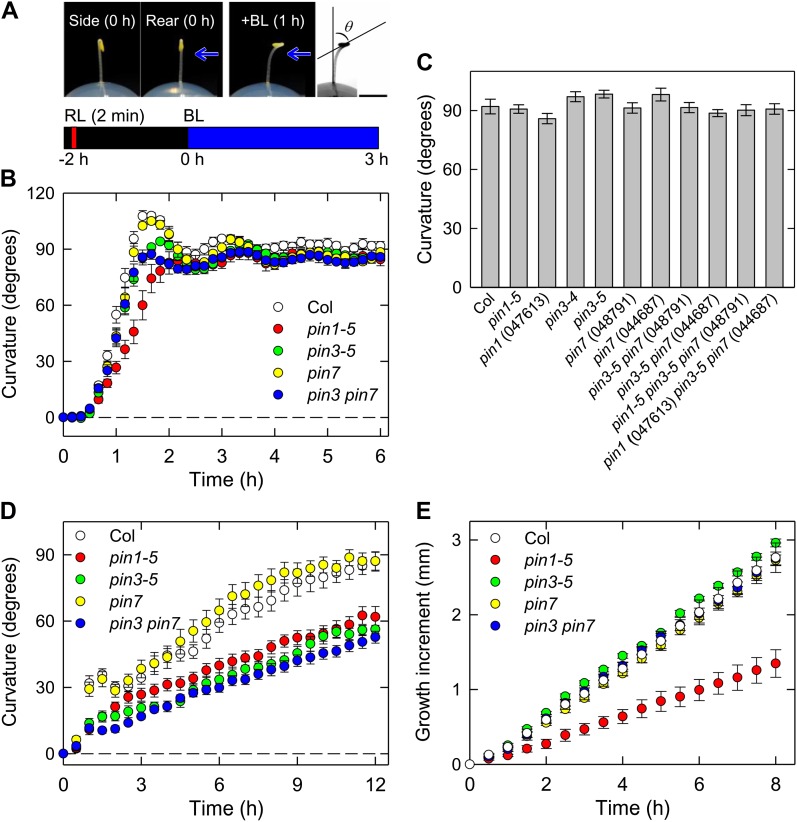
We investigated the phototropic mutants that have been previously described, including phot and root phototropism2, using seedlings grown along the surface of vertically oriented agar medium (Sakai et al., 2000, 2001). Under such conditions, a long-term exposure to blue-light irradiation (6–12 h) is required to induce clear phototropic curvatures. Similar experimental conditions are commonly used for the analysis of phototropism in Arabidopsis seedlings. Because the impairments of phototropic curvatures observed in the pin3 and pin7 mutants are marginal (Friml et al., 2002; Nagashima et al., 2008b; Christie et al., 2011; Ding et al., 2011), we have developed the following experimental conditions. As shown in Figure 1A (top), the etiolated seedlings were grown on the surface of the agar medium, and the hypocotyls, which did not contact the agar, were expected to show greater curvature responses. Furthermore, it is known that the relationship between the hook position and direction of phototropic stimulation influences hypocotyl phototropism in Arabidopsis (Khurana et al., 1989). Hence, the hypocotyls were irradiated with unilateral blue light perpendicular to the hook plane (Fig. 1A, top). In addition, the seedlings were irradiated with an overhead red light before phototropic stimulation to enhance phototropic curvatures (Fig. 1A, bottom). These conditions improved the development of phototropic curvatures and reproducibility of the phototropic responses (Fig. 1B). In wild-type seedlings, the phototropic curvature was initiated 30 min after the onset of the stimulation and reached maximal levels beyond the horizontal position at 90 min (Fig. 1B). After overshooting, the curvature gradually attenuated and reached the horizontal level with oscillation. We established, therefore, a highly sensitive method to induce hypocotyl phototropism in comparison with typical methods used previously, in which long-term irradiation is required. Wang et al. (2009) reported a similar kinetics using a high resolution method even when seedlings grown along the surface of agar medium were used. However, more than 3 h of light stimulation was still required to induce the maximum phototropic curvature. As a consequence, red-light pretreatment and a relationship between the hook position and direction of phototropic stimulation are critical points to establish a highly sensitive method presented here.
Figure 1.
Phototropism, gravitropism, and elongation growth in Arabidopsis hypocotyls. A, Illustration of the experimental procedures used to evaluate hypocotyl phototropism. Representative pictures of the shoot of a dark-grown Arabidopsis seedling are shown on the top. The left (side view) and middle (rear view) images are of a 2-d-old dark-grown seedling prior to phototropic induction. The seedling was pretreated with overhead red light (RL) at 20 μmol m−2 s−1 for 2 min. After 2 h, the hypocotyl was irradiated with blue light (BL) at 0.17 μmol m−2 s−1 perpendicularly to the hook plane to induce phototropic curvature. The representative picture shown on the right was taken 1 h after the onset of blue light. Black bar, 2 mm. The bottom shows the experimental scheme used to analyze phototropism. B, Time course analyses of hypocotyl phototropism. The etiolated seedlings were pretreated with overhead red light at 20 μmol m−2 s−1 for 2 min, and after 2 h, they were stimulated continuously with unilateral blue light at 0.17 μmol m−2 s−1. Phototropic curvatures were determined at 10-min intervals. The pin1-5, pin3-5, and pin7 (Salk_048791) single mutants, and the pin3 pin7 double mutant were used. The data shown are the means ± se from nine to 10 seedlings. C, Hypocotyl phototropism of pin mutants. The hypocotyls of pin single, double, and triple mutants were stimulated with unilateral blue light, and the hypocotyl curvatures were determined 3 h after the onset of blue light. The data shown are the means ± se from 10 to 19 seedlings. D, Time courses of hypocotyl gravitropism. The etiolated seedlings were displaced by 90°. Gravitropic curvatures were determined at 30-min intervals. The data shown are the means ± se from 10 to 22 seedlings. E, Time course analysis of the elongation growth of hypocotyls. The hypocotyl lengths were determined at 30 min intervals for 8 h. The data shown are the means ± se from eight to 10 seedlings.
Using our method, we investigated hypocotyl phototropism of the pin3 and pin7 single mutants and the pin3 pin7 double mutant (Fig. 1B). In the pin3 mutant, overshooting was slightly reduced, but the hypocotyl curvatures at the steady-state levels and the kinetics of the phototropic response were quite similar to the wild type. The pin7 mutant did not show any significant phototropic response changes compared with the wild type. The pin3 pin7 double mutant exhibited similar kinetics to the pin3 mutant, although impairments in overshooting were slightly greater than those observed in the pin3 mutant. Similar results were also observed even when one order lower of fluence rates were used (data not shown). Like the wild type, these mutants grew linearly for at least 8 h (time 0 corresponds to the onset of phototropic stimulation) when the seedlings were grown under complete darkness (Fig. 1E). Under the same growth conditions, displacement of the pin3 mutant to the horizontal position caused impaired hypocotyl gravitropism as reported previously (Friml et al., 200; Fig. 1D). Our analysis of the pin7 single mutant and the pin3 pin7 double mutant demonstrated that PIN7 is not involved in hypocotyl gravitropism (Fig. 1D). These results indicate that PIN3 and PIN7 are not required for hypocotyl phototropism under the experimental conditions used.
We also investigated the possible involvement of PIN1 in hypocotyl phototropism. Because pin1 null mutants are completely sterile (Okada et al., 1991), PIN1/pin1 heterozygous seedlings were used for the physiological experiments and pin1 homozygous mutants were identified by genotyping after the experiments. The hypocotyls of the pin1 homozygous mutant curved slowly, requiring a longer time to reach the maximum curvature (around 40 min later than the wild type), and overshooting was not clearly observed (Fig. 1B). However, the steady-state level of the curvature was similar to the wild type. Significant impairment of the gravitropic responses was also observed in the pin1 mutant (Fig. 1D), which has not been reported previously. Furthermore, in contrast to the pin3 and pin7 mutants, elongation growth was found to be impaired in the pin1 mutant, and the growth rate was found to be reduced by about 50%, although the pin1 hypocotyls grew in a linear fashion (Fig. 1E). The results suggest that the reduction in the phototropic curvature rate observed in the pin1 mutant (Fig. 1B) is not the result of auxin gradient defects mediated by PIN1-dependent lateral translocation, but from defects in elongation growth mediated by the PIN1-dependent basipetal polar transport of auxin.
Because we did not observe any significant contribution of PIN1, PIN3, or PIN7 to phototropic responses except overshooting under our experimental conditions, we generated a pin1 pin3 pin7 triple mutant to further investigate hypocotyl phototropism (Fig. 1C). Unexpectedly, we found no significant defects in continuous-light-induced phototropism even in the pin1 pin3 pin7 triple mutants, indicating a very limited contribution of these PINs to this process. It has been reported that PIN2, mainly expressed in the root, does not function in Arabidopsis hypocotyls (Luschnig et al., 1998). It has also been shown that pin1 pin3 pin4 pin7 quadruple mutants show severe developmental defects (Benková et al., 2003; Blilou et al., 2005), thus preventing us from using this mutant in our current analysis.
Pulse-Induced Phototropism in pin Mutants
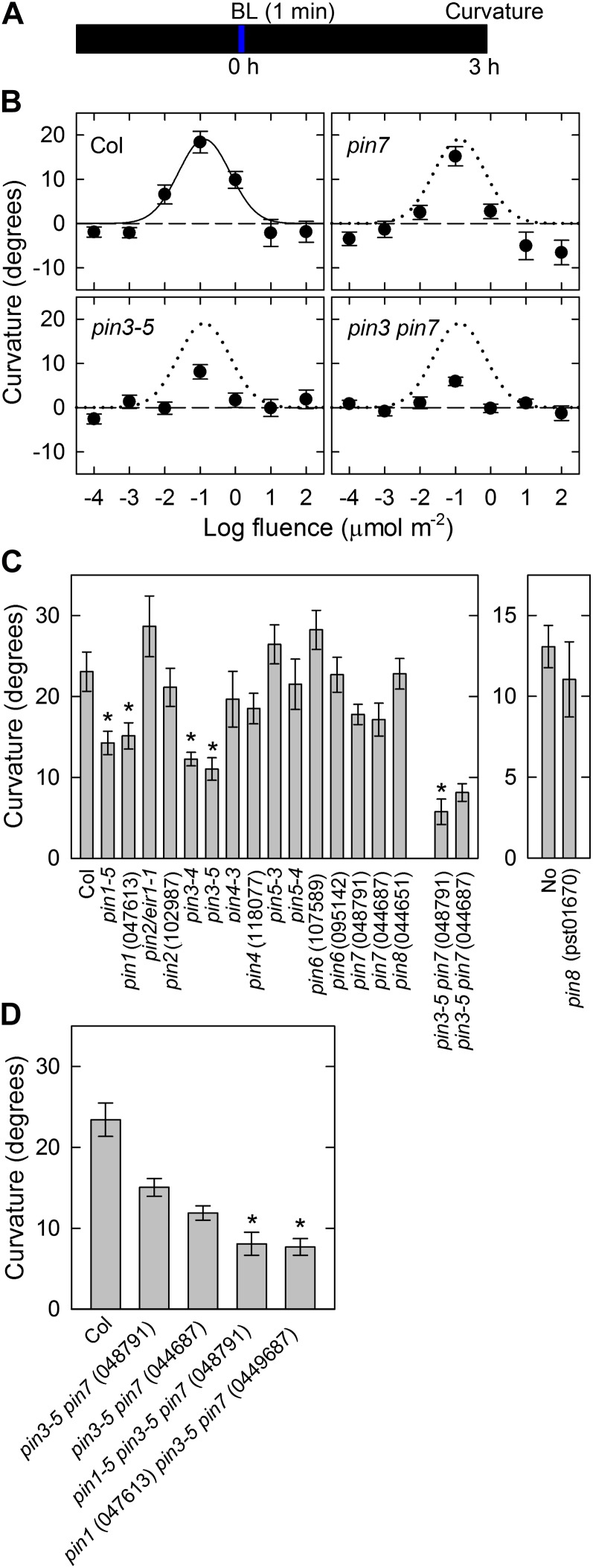
The continuous-light-induced second positive phototropism was found not to be impaired even in the pin1 pin3 pin7 triple mutants, indicating that the contribution of PINs to phototropism remained unclear. Because the roles of the PINs in pulse-induced phototropism have not been reported previously, we investigated using pin mutants whether these proteins have any critical functions in pulse-induced phototropism. Dark-grown hypocotyls were irradiated with unilateral blue light for 1 min at various fluence rates to obtain several fluences, and the hypocotyl curvature was determined 3 h after the onset of blue light (Fig. 2A). In wild-type seedlings, a pulse treatment induced a positive phototropic curvature (Fig. 2B), and the fluence-response curve was a typical bell shape with a peak at total fluence of 0.1 μmol m−2. The hypocotyls had reached a steady-state level 3 h after the onset of the stimulation at the optimal fluence (Supplemental Fig. S1A). Pulse-induced positive phototropism was also induced in the pin3, pin7, and pin3 pin7 mutants with typical bell-shaped curves. However, the curvature responses were reduced significantly in the pin3 mutant and slightly in the pin7 mutant, respectively. Furthermore, the pin3 pin7 double mutant showed that impairment of the phototropic curvature was greater than that observed in the pin3 mutant. These results indicated that PIN3 and PIN7 function additively, and that PIN3 has the major role in pulse-induced phototropism whereas PIN7 functions in a lesser capacity in this process.
Figure 2.
Pulse-induced hypocotyl phototropism in dark-grown seedlings. A, Experimental scheme for phototropism. Hypocotyls were stimulated with unilateral blue light for 60 s, and curvatures were determined 3 h after the onset of stimulation. B, Fluence-response curves for pulse-induced phototropism. Hypocotyls were stimulated with unilateral blue light at various fluences. The data shown are the means ± se from 16 seedlings. The fitted curve obtained from Columbia (Col) data (solid line) was reproduced as dotted lines for comparison. C, Pulse-induced phototropism in pin mutants. The hypocotyls were stimulated with unilateral blue light at the optimal fluence (0.1 μmol m−2). The data shown are the means ± se from 15 to 22 seedlings. The asterisk shows a statistically significant difference between the wild type and pin mutants (Student’s t test, P < 0.05). In the case of pin3 pin7 double mutants, statistical analysis was conducted between pin3-5 and the double mutants. D, Pulse-induced phototropism in pin1 pin3 pin7 triple mutants. The hypocotyls were stimulated with unilateral blue light at the optimal fluence. The phototropic curvatures of segregated pin3 pin7 double mutants were also analyzed. The data shown are the means ± se from 14 to 17 seedlings. The asterisk shows a statistically significant difference between the segregated pin3 pin7 double mutants and the pin1 pin3 pin7 triple mutants (Student’s t test, P < 0.01).
To investigate whether the other PINs participate in pulse-induced phototropism, the hypocotyls of dark-grown pin mutants were stimulated with unilateral blue light at the optimum total fluence (i.e. 0.1 μmol m−2), and curvatures were determined 3 h after the onset of this stimulation (Fig. 2C). Pulse-induced phototropism defects were confirmed in the pin3, pin7, and pin3 pin7 allelic mutants. The phototropic curvature was found to be clearly impaired in the pin1 mutants. However, none of the other pin mutants, including pin2, pin4, pin5, pin6, and pin8, were greatly affected in terms of phototropism. These results suggest that in addition to PIN3 and PIN7, PIN1 plays a role in pulse-induced phototropism.
Because PIN1, PIN3, and PIN7 contributed to pulse-induced phototropism, the pin1 pin3 pin7 triple mutants were investigated next (Fig. 2D). Although a marginal degree of pulse-induced phototropism remained in the triple mutants, their phototropic curvature was reduced to one-third of the wild-type level. This suggested that PIN1, PIN3, and PIN7 have critical roles in pulse-induced phototropism. Furthermore, the impairment of phototropism in the triple mutants was greater than that observed in the pin3 pin7 double mutants suggesting that these PINs function additively in the pulse-induced phototropism.
Effects of Red-Light Pretreatment on Pulse-Induced Phototropism
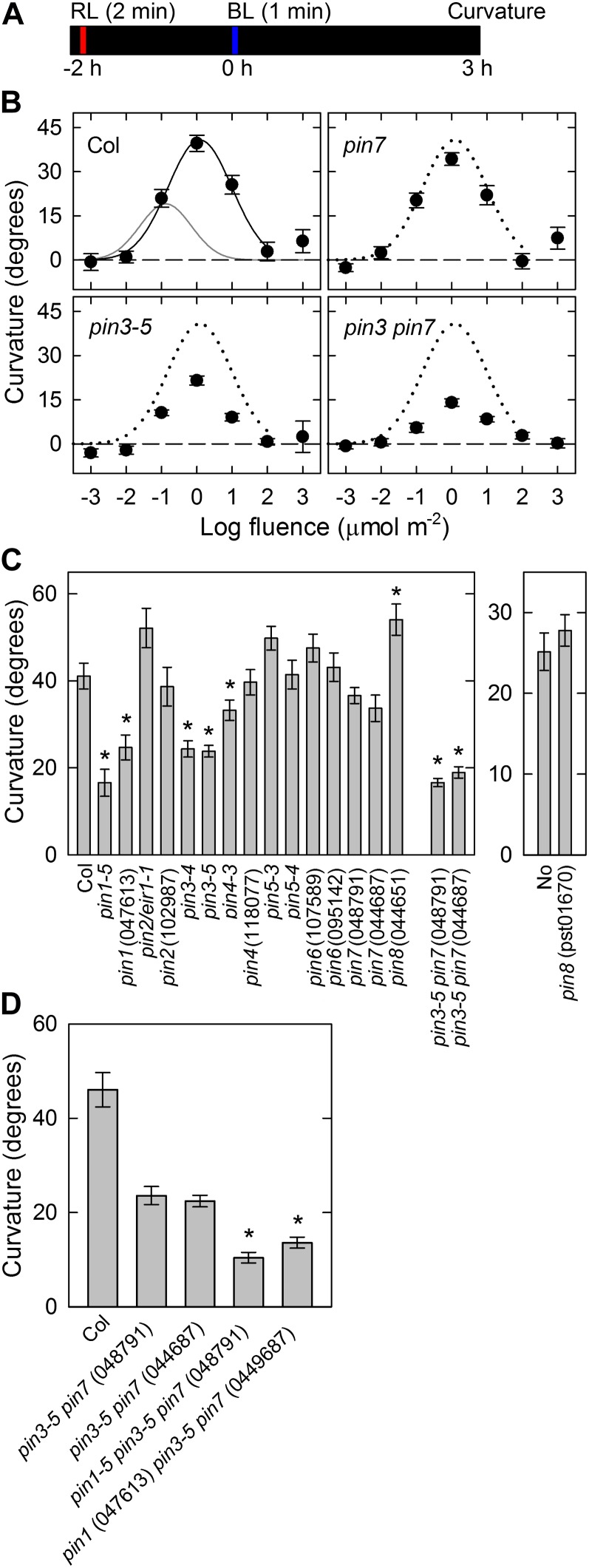
We next investigated effects of red light pretreatment on the pulse-induced phototropism in pin mutants because it is known that the phototropic curvatures are enhanced by this (Janoudi and Poff, 1991; Janoudi et al., 1997). Dark-grown seedlings were irradiated with overhead red light at 20 μmol m−2 s−1 for 2 min and incubated for 2 h under complete darkness (Fig. 3A). The hypocotyls were then stimulated with unilateral blue light for 1 min at various fluence rates, and the curvatures were determined 3 h after the onset of the stimulation, by which time the phototropic curvatures reached the steady-state levels (Supplemental Fig. S1B).
Figure 3.
Effects of red-light pretreatment on pulse-induced hypocotyl phototropism. A, Experimental scheme for phototropism. The seedlings were pretreated with overhead red light at 20 μmol m−2 s−1 for 2 min. Following 2 h, they were stimulated with unilateral blue light for 60 s, and curvatures were determined 3 h after the onset of stimulation. B, Fluence-response curves of pulse-induced phototropism. Hypocotyls were stimulated with unilateral blue light at various fluences. The data shown are the means ± se from 16 seedlings. The fitted curve obtained from Columbia (Col) data (solid line) was reproduced as dotted lines, and the gray line reproduces the curve of Figure 1B for comparison. C, Pulse-induced phototropism in pin mutants. Hypocotyls were stimulated with unilateral blue light at the optimal fluence (1 μmol m−2). The data shown are the means ± se from 16 to 23 seedlings. The asterisk indicates a statistically significant difference between the wild type and pin mutants (Student’s t test, P < 0.05). In the case of pin3 pin7 double mutants, the statistical analysis was conducted between pin3-5 and the double mutants. D, Pulse-induced phototropism in pin1 pin3 pin7 triple mutants. The hypocotyls were stimulated with unilateral blue light at the optimal fluence. The phototropic curvatures of segregated pin3 pin7 double mutants were also analyzed. The data shown are the means ± se from 13 to 22 seedlings. The asterisk shows a statistically significant difference between the segregated pin3 pin7 double mutants and the pin1 pin3 pin7 triple mutants (Student’s t test, P < 0.001).
In wild-type seedlings, red-light pretreatment induced not only the enhancement of phototropic curvatures but also a shift of the fluence-response curve to higher fluences during pulse-induced phototropism (Fig. 3B). The maximum curvature was 2-fold higher, and the fluence-response curve was shifted about one order higher compared with the results obtained without pretreatment (Fig. 2B). On the other hand, the enhancement of phototropic curvatures and a shift in the fluence-response curves occurred also in the pin3, pin7, and pin3 pin7 mutants following red-light pretreatment, indicating that enhancement and desensitization mechanisms are not impaired in the mutants. However, the enhanced curvatures were still reduced significantly in the pin3 mutant and slightly in the pin7 mutant. Furthermore, the pin3 pin7 double mutant showed enhanced impairment of the phototropic response compared with the pin3 mutant. These results suggest that PIN3 and PIN7 function additively in the enhanced pulse-induced phototropism by red-light pretreatment.
To clarify the participation of other PINs in the enhanced pulse-induced phototropism, the hypocotyls of dark-grown pin mutants were stimulated with unilateral blue light at the optimum total fluence (i.e. 1 μmol m−2) 2 h after red-light pretreatment, and curvatures were determined 3 h after the onset of this stimulation (Fig. 3C). The impairment of phototropic curvatures in the pin3, pin7, and pin3 pin7 mutants was confirmed in their allelic mutants. The pin1 mutants showed defects in the pulse-induced phototropism, but none of the other pin mutants showed a clear impairment. The results further indicated that PIN1, PIN3, and PIN7 play central roles in pulse-induced phototropism. The impairment observed in the pin1 mutants was more obvious following red-light pretreatment, indicating that red light preferentially enhances PIN1-mediated phototropism. To confirm the contribution of PIN1, PIN3, and PIN7 to the enhancement of pulse-induced phototropism, the pin1 pin3 pin7 triple mutant was analyzed (Fig. 3D). Although marginal levels of pulse-induced phototropism remained in the triple mutants, the phototropic curvature was reduced to 25% of wild-type levels, again suggesting that PIN1, PIN3, and PIN7 have critical roles in pulse-induced phototropism. The pin3 pin7 double mutants showed less impairment further indicating that these PINs function additively.
Time-Dependent Phototropism in pin Mutants
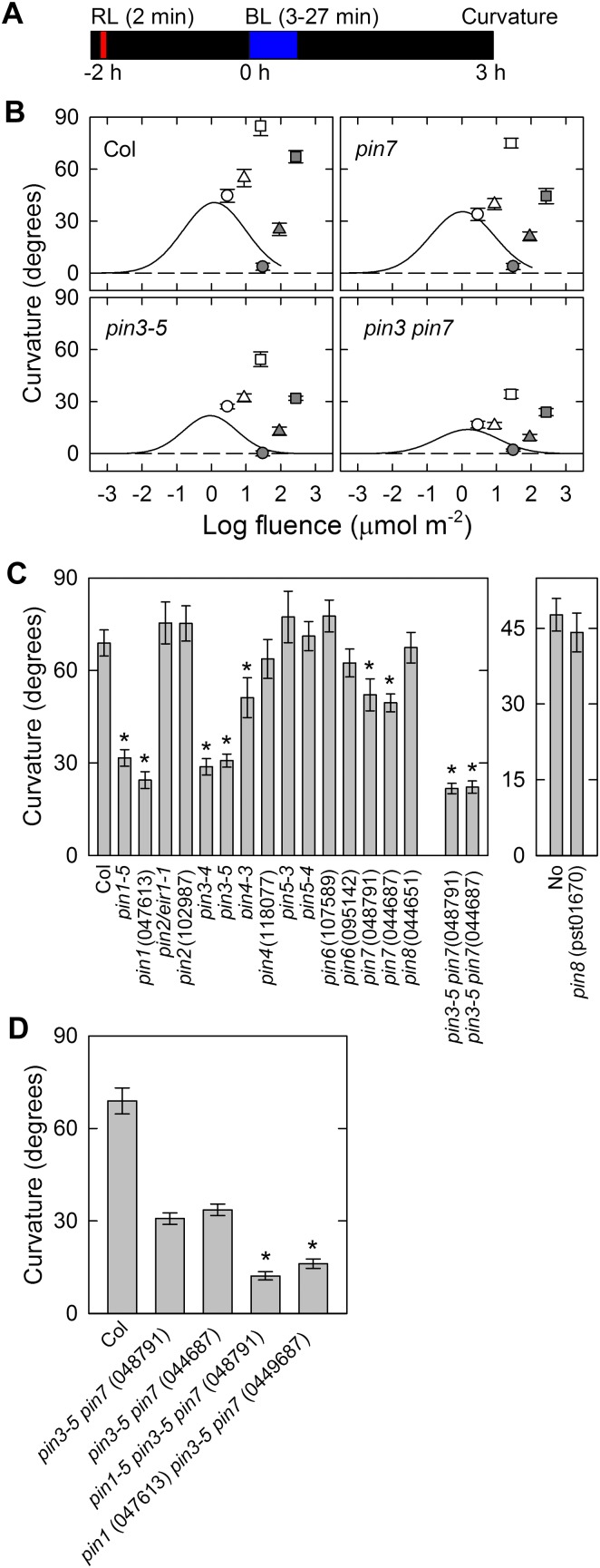
Because our data indicated that the contributions of the PINs differ between continuous-light-induced, second positive phototropism and pulse-induced, first positive phototropism, we analyzed the time-dependent second positive phototropism, which is induced by prolonged stimulation (around 3–30 min) to further clarify this. Under such conditions, the phototropic stimulation ceased before the phototropic responses were saturated. After stimulation, the seedlings were incubated under dark conditions to develop phototropic responses, and the phototropic curvatures were determined 3 h after the onset of the stimulation (Fig. 4A). In wild-type seedlings, the magnitudes of the phototropic curvatures increased as the duration of the stimulation increased (Fig. 4B). When the irradiation time exceeded 3 min, the curvature responses separated largely from the fluence-response curve, indicating that time-dependent phototropism was induced. Similar to the wild type, the pin3, pin7, and pin3 pin7 mutants showed time-dependent phototropism when the duration of the stimulation went beyond 3 min. However, the magnitudes of time-dependent phototropism were significantly reduced in the pin3 mutant and marginally in the pin7 mutant. The impairment of phototropic curvatures in the pin3 pin7 mutant were greater than in the pin3 mutant. The results suggest that PIN3 and PIN7 function additively, but that PIN3 has the major role in time-dependent phototropism.
Figure 4.
Time-dependent phototropism. A, Experimental scheme used to analyze phototropism. The seedlings were pretreated with overhead red light, and after 2 h, they were stimulated with unilateral blue light. Phototropic curvatures were determined 3 h after the onset of blue light. B, Fluence-response relationship. Hypocotyls were irradiated with unilateral blue light at fixed fluence rates (open symbols, 0.017 μmol m−2 s−1; gray symbols, 0.17 μmol m−2 s−1) for 3 (circles), 9 (triangles), and 27 (squares) min. The data shown are the means ± se from 16 seedlings. The solid lines show the fitted curves obtained from the data in Figure 3B. C, Time-dependent phototropism in pin mutants. The hypocotyls were stimulated with unilateral blue light at 0.17 μmol m−2 s−1 for 27 min. The data shown are the means ± se from 12 to 16 seedlings. The asterisk shows a statistically significant difference between the wild type and pin mutants (Student’s t test, P < 0.05). In the case of pin3 pin7 double mutants, the statistical analysis was conducted between pin3-5 and the double mutants. D, Time-dependent phototropism in pin1 pin3 pin7 triple mutants. The hypocotyls were stimulated with unilateral blue light at 0.17 μmol m−2 s−1 for 27 min. The phototropic curvatures of segregated pin3 pin7 double mutants were also analyzed. The data shown are the means ± se from 15 to 22 seedlings. The asterisk shows a statistically significant difference between the segregated pin3 pin7 double mutants and the pin1 pin3 pin7 triple mutants (Student’s t test, P < 0.001).
To clarify whether any other PINs participate in time-dependent phototropism, the hypocotyls of pin mutants were stimulated with blue light at a fluence rate of 0.17 μmol m−2 s−1 for 27 min, and the curvatures were determined 3 h after the onset of the stimulation (Fig. 4C), at which time point the curvature responses reached steady-state levels (Supplemental Fig. S1C). The curvature impairment in the pin3, pin7, and corresponding double mutants were confirmed in their allelic counterparts. The pin1 mutants showed time-dependent phototropism defects similar to those observed in the pin3 mutants. These results indicate that PIN1 and PIN3 have the major role, and PIN7 is a minor component, during time-dependent phototropism. To further clarify the functions of PIN1, PIN3, and PIN7 in time-dependent phototropism, the pin1 pin3 pin7 triple mutants were analyzed (Fig. 4D). Although a slight time-dependent phototropic response remained in the triple mutants, the phototropic curvature was reduced to 20% of the wild type, suggesting the crucial roles of these PINs in this process. Furthermore, the impairment of phototropism was less in the pin3 pin7 double mutants indicating that the PINs also function additively in time-dependent, second positive phototropism.
Distribution of Auxin during Phototropism
The aforementioned results demonstrated that PIN1, PIN3, and PIN7 are involved in pulse-induced first positive phototropism and time-dependent second positive phototropism, but not in continuous-light-induced second positive phototropism. If impairment of the different phototropic responses in the pin mutants results from defects in auxin gradients, it would be expected that the asymmetrical distribution of auxin required for pulse-induced and time-dependent phototropism would be dependent on PIN1, PIN3, and PIN7, but that the auxin asymmetry found in continuous-light-induced phototropism would not.
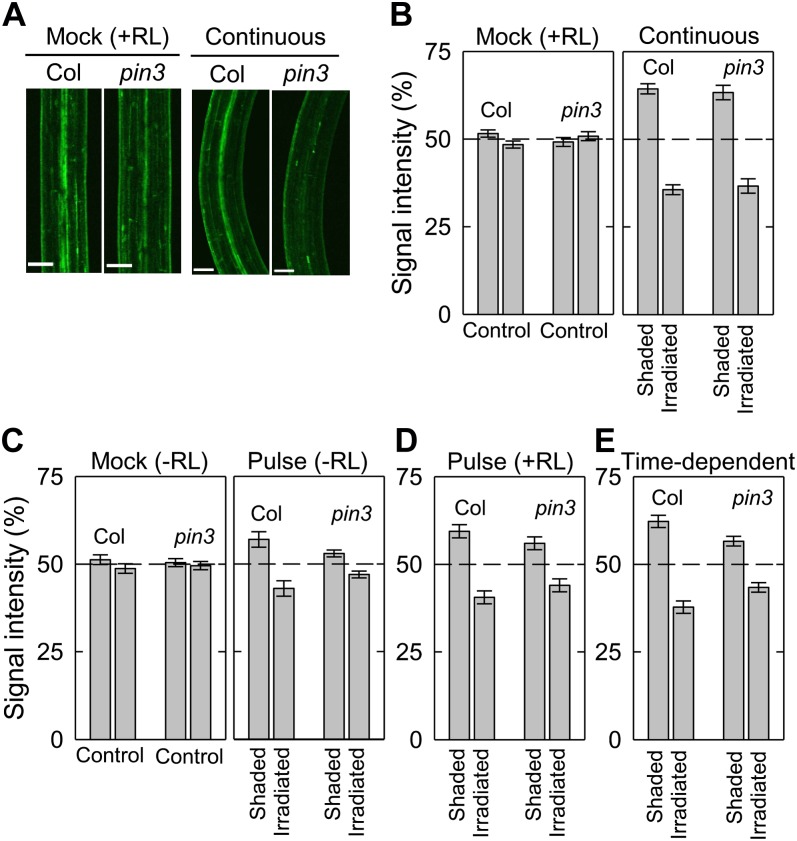
To test this, we investigated the PIN3-dependent auxin gradients during each phototropic response using an auxin-responsive reporter gene DR5rev:GFP (Fig. 5). In a similar manner to the curvature measurements shown in Figures 1 to 4, GFP signals around the bending parts of the hypocotyls were analyzed 3 h after the onset of blue-light irradiation, and were measured on the irradiated side and the shaded side of the epidermis. Regardless of red-light pretreatment, GFP signals were found to be evenly distributed in hypocotyls of both the wild type and the pin3 mutant without phototropic stimulation (Fig. 5, B and C, left). When the hypocotyls of wild-type seedlings were continuously stimulated with unilateral blue light, GFP signals on the shaded side were stronger than those of the irradiated side (Fig. 5, A and B, right), indicating that auxin asymmetrical distribution occurs in response to phototropic stimulation. The pin3 mutant also showed similar auxin gradients. This result is consistent with the findings for continuous stimulation shown in Figure 1.
Figure 5.
Distribution of auxin during phototropism. Hypocotyls of Col and the pin3 mutant containing DR5rev:GFP were irradiated with unilateral blue light, and GFP signals were quantified 3 h after the onset of the phototropic stimulation. A, Representative confocal micrographs of DR5rev:GFP expression during the hypocotyl phototropism induced by continuous blue-light irradiation. Seedlings were pretreated with overhead red light, and they were stimulated with or without blue light from the right side at 0.17 μmol m−2 s−1 for 3 h. The images were obtained 3 h after stimulation. White bars, 100 μm. B, Distribution of DR5rev:GFP between the shaded and the irradiated sides of the hypocotyls during continuous-light-induced phototropism. The distribution was calculated as the percentage of the signal intensity obtained from the two sides. The data shown are the means ± se from 12 to 16 seedlings. C, Distribution of DR5rev:GFP between the shaded and the irradiated sides of hypocotyls during pulse-induced phototropism without red-light pretreatment. The optimum fluence was used for the phototropic induction (Fig. 2). The data shown are the means ± se from 13 to 16 seedlings. D, Distribution of DR5rev:GFP between the shaded and the irradiated sides of hypocotyls during the enhanced pulse-induced phototropism by red-light pretreatment. The optimum fluence was used for the phototropic induction (Fig. 3). The data shown are the means ± se from 13 to 16 seedlings. E, Distribution of DR5rev:GFP between the shaded and the irradiated sides of hypocotyls during time-dependent phototropism. Hypocotyls were stimulated with unilateral blue light at 0.17 μmol m−2 s−1 for 27 min. The data shown are the means ± se from 12 to 15 seedlings.
Unevenly distributed GFP signals were also found for pulse-induced phototropism without red-light pretreatment in wild-type hypocotyls (Fig. 5C), although the difference between the irradiated side and the shaded side was smaller than that found for continuous-light-induced phototropism (Fig. 5B). Red-light pretreatment exacerbated this uneven distribution of GFP signals observed during pulse-induced phototropism (Fig. 5D), and the reporter signal gradients during time-dependent phototropism were larger than those for pulse-induced phototropism (Fig. 5E). These results indicated that there is a clear correlation between the phototropic curvatures and the auxin gradients. On the other hand, the pin3 mutant showed partial impairment of the uneven distribution of GFP signals during pulse-induced and time-dependent phototropic responses, although an asymmetrical distribution was still observed (Fig. 5, C–E). Hence, PIN3 is involved in auxin lateral translocation during hypocotyl phototropisms. These results suggest that PIN3-mediated auxin asymmetrical distribution functions in both pulse-induced and time-dependent phototropism, and also that a PIN3-independent mechanism exists that controls auxin translocation during continuous-light-induced second positive phototropism.
Taken together with our results for phototropic curvature responses in pin mutants, it is possible that a PIN3-independent (maybe also PIN1- and PIN7-independent) mechanism plays a critical role in the regulation of auxin lateral translocation during continuous-light-induced phototropism, and that this regulatory mechanism does not work efficiently during pulse-induced phototropism. In addition, our present results demonstrate that red-light pretreatment promotes the asymmetrical distribution of auxin.
Involvement of ABCB19 in PIN-Mediated Hypocotyl Phototropism
Another type of auxin efflux carrier, ABCB19, has been shown to be a negative regulator of phototropism as hypocotyl phototropism is enhanced in abcb19 mutants in response to continuous phototropic stimulation when the etiolated seedlings are grown along the surface of vertically oriented agar medium (Noh et al., 2003; Nagashima et al., 2008b). In addition, red-light irradiation significantly reduces the protein levels of ABCB19 (Nagashima et al., 2008a), indicating that the inhibitory effects of this factor are likely involved in the enhancement of phototropic curvatures (Nagashima et al., 2008b). Because the participation of ABCB19 in pulse-induced, first positive phototropism has not been reported to date, we investigated the pulse-induced phototropism of the previously isolated abcb19 mutant (Nagashima et al., 2008a). Regardless of red-light pretreatment, pulse-induced phototropisms were enhanced in the abcb19 mutant compared with the wild type (Fig. 6). This indicated that ABCB19 functions as a negative regulator not only in continuous-light-induced phototropism, but also in pulse-induced phototropism. Furthermore, the enhancement of pulse-induced phototropism following red-light pretreatment was observed also in the abcb19 mutant (Fig. 6), suggesting that the red-light-preirradiation-induced phototropic enhancement is not dependent on ABCB19.
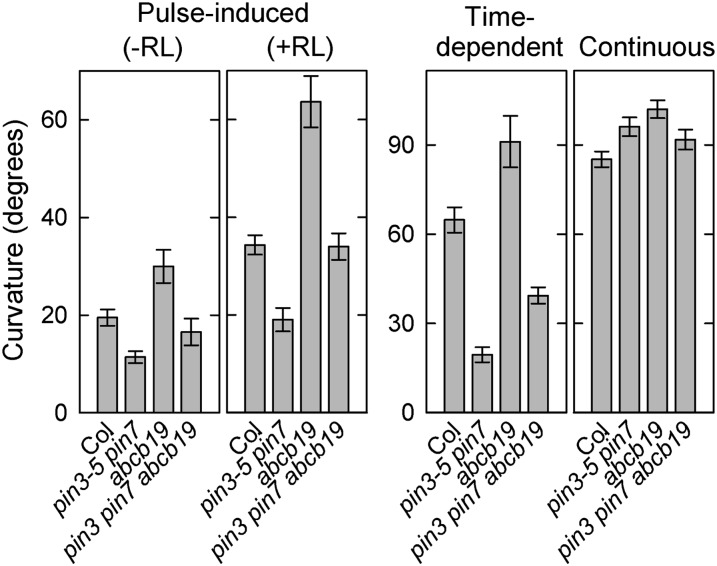
Figure 6.
Hypocotyl phototropism of the abcb19 single mutant and the pin3 pin7 abcb19 triple mutant. For pulse-induced phototropism with or without red-light pretreatment, hypocotyls were stimulated with blue light at the optimal fluence (see Figs. 2 and 3). For time-dependent phototropism, hypocotyls were stimulated with blue light at 0.17 μmol m−2 s−1 for 27 min. For phototropism induced by continuous irradiation, seedlings were irradiated with blue light at 0.17 μmol m−2 s−1 for 3 h. Phototropic curvatures were determined 3 h after the onset of stimulation. The data shown are the means ± se from 15 to 16 seedlings.
To clarify the possible involvement of ABCB19 in PIN-mediated phototropism, we generated a pin3 pin7 abcb19 triple mutant. The pulse-induced phototropisms in the triple mutant were enhanced compared with the pin3 pin7 double mutant, but were inhibited compared with the abcb19 single mutant (Fig. 6). The results indicate that PIN3- and PIN7-mediated positive functions are independent of ABCB19 in pulse-induced phototropism. This relationship was also applicable to the time-dependent phototropism pathway (Fig. 6). In contrast, it was difficult to clarify the possible involvement of ABCB19 in continuous-light-stimulated phototropism because the phototropic curvatures of the wild type and the pin3 pin7 double mutant were already saturated under our experimental conditions (Fig. 6).
Involvement of PID in Hypocotyl Phototropism
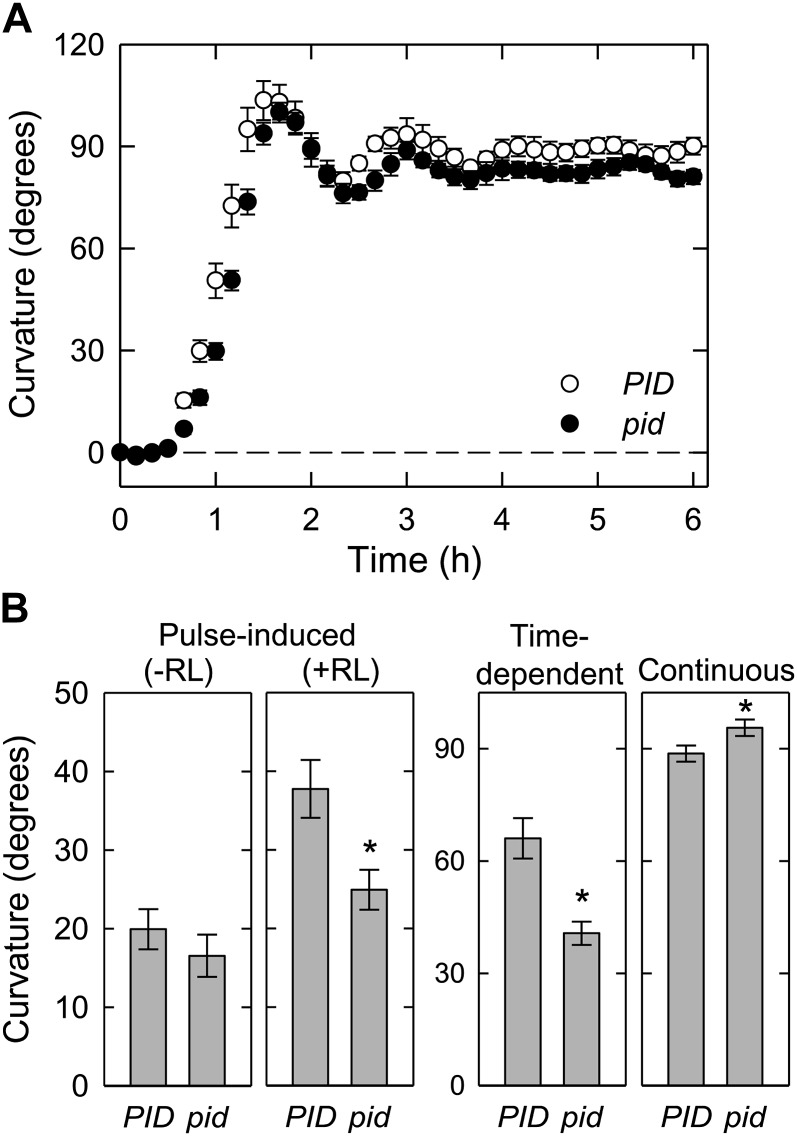
Recently, it has been shown that PID is involved in the asymmetrical localization of PIN3 during hypocotyl phototropism, and that the phototropic response is impaired in the pid mutant (Ding et al., 2011). To further clarify the participation of PID in hypocotyl phototropism in our current experiments, we analyzed the pid mutant (Fig. 7). Similar to the pin1 null mutant, the pid null mutant displays severe sterility (Bennett et al., 1995). Hence, we used similar strategy used for analysis of the pin1 mutants (see above). When the hypocotyls of the pid mutant were stimulated continuously, the kinetics of hypocotyl phototropism were similar to the wild type (Fig. 7A). These results were nearly comparable to those obtained from the pin3 mutant (Fig. 1B). On the other hand, enhanced pulse-induced and time-dependent phototropism, both of which are pretreated with red light, were partially impaired in the pid mutant (Fig. 7B). Similar results were also observed in the pin3 mutants (Figs. 3 and 4). Interestingly, pulse-induced phototropism without red-light pretreatment was not affected in the pid mutant, suggesting that PID is not necessary for pulse-induced phototropism without red-light pretreatment. Furthermore, the enhancement of pulse-induced phototropism by red-light pretreatment was partially impaired in the pid mutant; a 1.4-fold increase in the mutant versus a 1.9-fold increase in the wild type. The result indicates that PID is involved in the enhanced phototropic responses following this pretreatment.
Figure 7.
Hypocotyl phototropism of the pid-14 single mutant. A, Time course analyses of hypocotyl phototropism induced by continuous stimulation. Phototropic curvatures of the pid-14 mutant and the segregated wild type (PID) were analyzed. The data shown are the means ± se from 11 to 13 seedlings. Other details are as described for Fig. 1B. B, Dark-grown seedlings were irradiated with blue light, and phototropic curvatures were determined 3 h after the onset of stimulation. The data shown are the means ± se from 11 to 21 seedlings. The asterisk indicates a statistically significant difference (Student’s t test, P < 0.05). Other details are as described for Figure 6.
Transcriptional Regulation of PID and PIN Genes
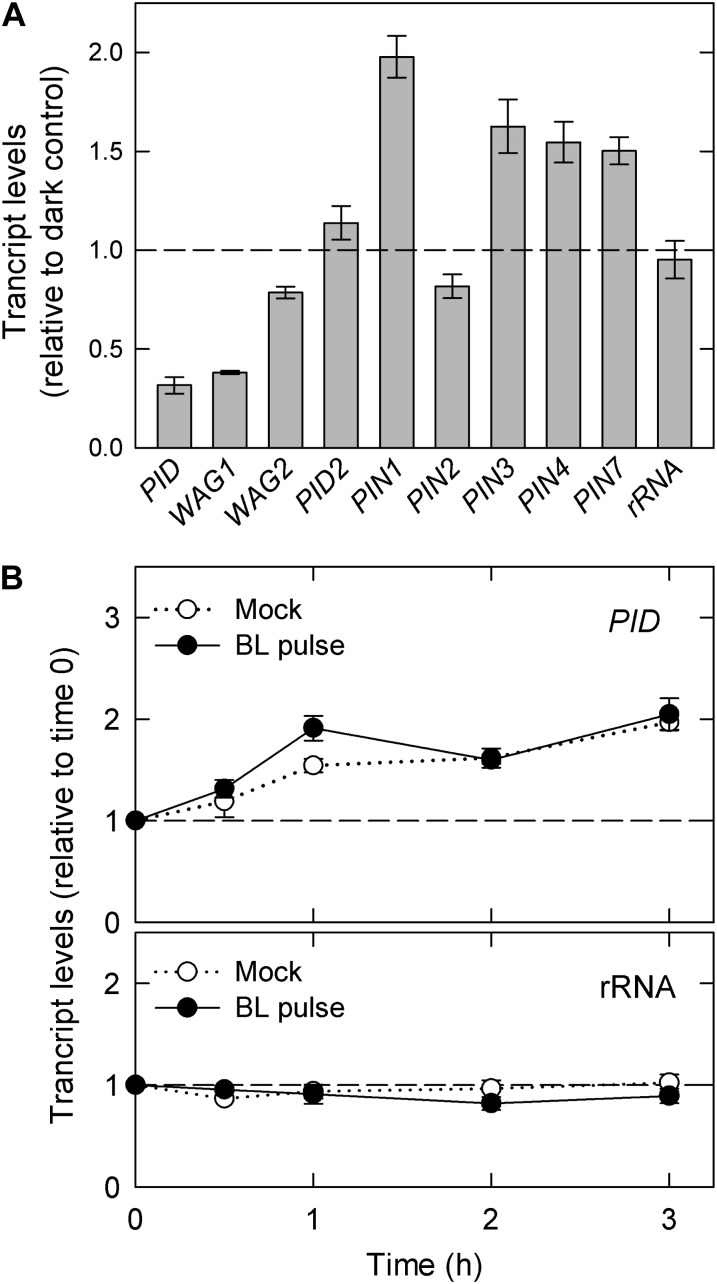
Because PID was found to be partially involved in the enhancement of pulse-induced phototropism (Fig. 7B), the effects of red-light pretreatment on the transcriptional levels of PID and its homologous genes (WAG1, WAG2, and PID2) were investigated (Fig. 8A). We also analyzed the transcript levels of PIN1, PIN3, PIN7, and the related homologous genes PIN2 and PIN4. Total RNA was extracted from wild-type hypocotyls 2 h after red-light treatment, and the transcript levels were determined using quantitative reverse transcription (RT)-PCR. Red-light pretreatment suppressed the mRNA levels of the PID family genes, most notably PID and WAG1. In contrast, red-light pretreatment enhanced the transcription of the PIN genes except PIN2. These results suggest that the transcriptional regulation of the PID and PIN family genes, particularly the transcriptional down-regulation of PID, by red-light pretreatment is involved in the enhancement of pulse-induced phototropic response to this pretreatment.
Figure 8.
Effects of light irradiation on the transcriptional levels of PID and PIN genes. Following light treatment, the hypocotyls were harvested and subjected to transcriptional analysis using quantitative RT-PCR. The data shown are the means ± se from three experiments using biological independent samples. 18s ribosomal RNA was used as an internal control. A, Effects of red-light pretreatment. Dark-grown seedlings were irradiated with or without overhead red light (20 μmol m−2 s−1) for 2 min. Following 2 h, the transcriptional levels were determined. The transcript amounts are expressed relative to the levels in the dark controls. B, Effects of a pulse of blue light. Two hours after red-light pretreatment, the hypocotyls were stimulated with (closed symbols) or without (open symbols) unilateral blue light at the optimum fluence (Fig. 3). Transcriptional levels of the PID gene were determined at the indicated time points after the onset of a pulse of blue light. The transcript levels are expressed relative to the amount at time 0.
Ding et al. (2011) have proposed that the phototropin-mediated transcriptional down-regulation of PID plays critical roles in the asymmetrical localization of PIN3 in endodermal cells. Thus, we next investigated whether the PID transcript levels are affected by blue-light irradiation under our experimental conditions (Fig. 8B and Supplemental Fig. S2). Independent of blue-light irradiation, the transcript levels of PID, which were once reduced by red-light pretreatment (corresponding to the levels at time 0), were gradually enhanced and nearly recovered to the original levels before pretreatment. Hence, the phototropic blue-light stimulation could not reduce PID mRNA, at least under our experimental conditions.
DISCUSSION
Phototropism Induced by Continuous Irradiation Is Not Mediated by PIN-Dependent Pathways in Arabidopsis Hypocotyls
In our present study, we did not observe any contribution of PIN and PID to the hypocotyl phototropism induced by continuous stimulation. Previous studies conducted by some groups including our laboratory have demonstrated that the pin3 mutants show statistically significant impairments of phototropic responses (Friml et al., 2002; Nagashima et al., 2008b; Ding et al., 2011). Differences in the experimental results of previous reports and our present study are probably the result of different experimental conditions. Previous studies have used Arabidopsis seedlings grown along the surface of a vertically oriented agar medium. Under such conditions, the hypocotyls, which are always in contact with the agar medium and produce some friction, will find it relatively hard to bend toward the light source. Hence, it takes 6 to 12 h to induce phototropic curvatures at steady-state levels (that is, the horizontal levels). Here, we employed a cultivation method using 0.2-mL tubes to grow the Arabidopsis seedlings, whose hypocotyls do not make contact with the agar medium (Fig. 1A). These conditions reduce the time it takes to induce the maximal curvatures from several hours to only 90 min (Fig. 1B). This is because the hypocotyls, which grow upwards without any friction between the agar surface and the shoots, can readily bend toward the light. Hence, it is possible that PIN3-mediated auxin gradients are required to overcome the friction that occurs when the hypocotyls are always in contact with the agar surface. Under such conditions, the involvement of PIN3 in the phototropic curvatures induced by continuous blue-light irradiation was found to be consistent with that observed in previous studies.
Another possibility is that the relationship between the direction of the phototropic stimulation and the position of the hooks affects the phototropic response (Khurana et al., 1989). Whereas the hypocotyls of dark-grown seedlings of the wild type and the pin3 mutant plants were stimulated with unilateral blue light irrespective of the hook position in the previous reports (Friml et al., 2002; Nagashima et al., 2008b; Ding et al., 2011), we stimulated these hypocotyls with unilateral blue light perpendicularly to the hook plane in our present experiments (Fig. 1A). Hence, it is possible that the impairment of the phototropic curvatures in the pin3 mutants is observed when phototropic stimulation is conducted from the asymmetrical plane of the hypocotyls. This would suggest that PIN proteins including PIN3 regulate auxin transport mainly between the abaxial side and the adaxial side of the shoots. In any case, we conclude that PIN1, PIN3, PIN7, and PID are not necessary for the continuous-light-induced second phototropism, at least in our experimental conditions, although our present data do not rule out a minor role for PIN3 in the phototropic responses.
Which kinds of regulatory molecular mechanisms underpin the emergence of phototropic responses in a PIN1-, PIN3-, PIN7-, and PID-independent manner is an obvious question arising from our present study. One possibility is that the PIN and PID family genes function redundantly in phototropism. It has been reported that PIN2 functions mainly in roots (Luschnig et al., 1998), and that pin1 pin3 pin4 pin7 quadruple mutants display severe growth and development defects (Benková et al., 2003; Blilou et al., 2005). Hence, we have not analyzed phototropic responses using such multiple pin mutants in the current study. However, to properly clarify the involvement of the PINs in phototropic responses, the functional specificities of each PIN, including PIN5, PIN6, and PIN8, which have the reduced central hydrophilic loop and are expected to function in the organelle (Mravec et al., 2009), will need to be elucidated in the future.
Another possibility is that phototropism is entirely a PIN-independent process. The pin3 mutant showed significant gradients of GFP signals derived from DR5rev:GFP between the irradiated side and the shaded side, as observed in the wild-type seedlings (Fig. 5B), suggesting that normal phototropic responses observed in the pin3 mutants result from asymmetrical distribution of auxin in response to unilateral blue light. Although it will be necessary to investigate whether auxin asymmetry is impaired in the pin1 pin3 pin7 triple mutants, we do not have any negative evidence for the involvement of auxin in the phototropic responses to date. Other types of auxin transporters, such as the AUX/LAX family and ABCB family, may participate in lateral auxin distribution in addition to the PIN family members. ABCB19, however, functions in the phototropic responses as a negative regulator in a PIN3- and PIN7-independent manner (Fig. 6), indicating that it is not an auxin transporter that functions positively to induce phototropism. We should, therefore, analyze not only auxin carrier proteins, but also the pathways of auxin biosynthesis/metabolism and the modification of auxin sensitivity by light, in future studies.
Phototropisms Induced by Transient Stimulations Are Mediated by a PIN-Dependent Pathway in Arabidopsis Hypocotyls
Our present study revealed that PIN1, PIN3, and PIN7 are involved in the pulse-induced first positive phototropism and the time-dependent second positive phototropism (Figs. 2–4). Because the phototropic curvatures began 30 min after the onset of the phototropic stimulations in all genotypes we examined, phototropic stimulation had been completed before the phototropic curvatures occurred (Supplemental Fig. S1). Therefore, the phototropic curvatures develop during dark incubation after phototropic stimulation, implying that PIN1-, PIN3-, and PIN7-mediated mechanisms to control auxin gradients somehow memorize transient phototropic signals for a certain period, and that the regulatory mechanisms achieve curvature development through the maintenance of auxin asymmetry without continuous phototropic stimuli.
The nature of how plants memorize a transient phototropic stimulation then becomes a key question. A possible mechanism is that the maintenance of a phototropic signal occurs downstream of phototropin signal transduction. That is, the functional status of PINs, which are once activated by phototropic stimuli and contribute to auxin asymmetrical distribution, persists during phototropic curvature development even when the activation status of phototropin is diminished. Ding et al. (2011) have reported that the PIN3 protein levels are significantly reduced by unilateral blue-light irradiation at the endodermis of the irradiated side in a phot1-dependent manner. It is thus possible that a similar mechanism functions during pulse-induced phototropism, where the phot1-dependent regulation of PIN3 localization somehow persists to maintain auxin gradients over a certain period even when the phototropic stimulation is diminished.
Another possible mechanism for phototropism induced by transient stimuli is that the activated status of phot1, which is expected to be a major photoreceptor in our phototropic conditions using a low fluence rate of blue light (Christie 2007), is somehow maintained during curvature development under dark conditions. It is known that phot1 is autophosphorylated at the C terminus via the activation of its kinase through conformational changes upon receiving blue-light signals (Christie et al., 2002). Furthermore, it has been reported that a 14-3-3 protein binds to activated phot1 by recognizing this autophosphorylation site (Kinoshita et al., 2003; Inoue et al., 2008), suggesting that 14-3-3 protein functions to stabilize activated phot1. Hence, the preservation of the activated status of phot1 may maintain phototropic signals under dark conditions.
The question of why pulse-activated phot1 induces the PIN1-, PIN3-, and PIN7-dependent phototropic response, but not the PIN1-, PIN3-, and PIN7-independent response, remains an unresolved issue from these data. It is possible that the activation status of phot1 is different between pulse-induced and continuous-light-induced phototropism. Alternatively, other photoreceptors such as phot2 may be involved in the phototropism induced by continuous stimulation at a low fluence rate of blue light under our experimental conditions, although this would conflict with previously reported evidence (Sakai et al., 2001). Such a mechanism possibly regulates the phototropic responses in a PIN1-, PIN3-, and PIN7-independent manner.
Effects of Red-Light Pretreatment on Fluence-Response Curves of Pulse-Induced Phototropism
Pretreatment with red light affects the fluence-response relationships of pulse-induced phototropism, such as the enhancement of phototropic curvatures, desensitization to phototropic stimulation, and induction of an additional peak at higher fluences (Iino, 2001; Whippo and Hangarter, 2006). The analyses from which these findings have arisen have been conducted mainly in coleoptiles of monocots. For Arabidopsis hypocotyls, however, only the enhancement response has been found to be induced by red-light pretreatment (Janoudi and Poff, 1991), and this response is known to be mediated by phytochromes (Janoudi et al., 1997; Whippo and Hangarter, 2004). Our present study also shows that pretreatment with red light enhances the phototropic curvature by about 2-fold (Figs. 2B and 3B). Although a previous report did not identify other responses (Janoudi and Poff, 1991), we have here observed that the total fluence required to induce maximal curvature is shifted to an almost 10-fold-higher level by red-light treatment, indicating that desensitization occurs (Fig. 3B). In addition, a small peak seems to be induced at a higher total fluence (Fig. 3B; 103 μmol m−2). Taken together, these data indicate that the effects of red-light pretreatment on the fluence-response relationships of pulse-induced phototropism are probably similar between Arabidopsis hypocotyls and the coleoptiles of monocots. This further suggests that common mechanisms exist in dicots and monocots that underlie pulse-induced phototropism.
The enhancement of pulse-induced phototropism by red-light pretreatment occurs not only in the wild type, but also in the pin1, pin3, pin7, and pid mutants (Figs. 2C, 3C, and 7B). Interestingly, the magnitude of the enhancement observed in the pin1 and pid mutants was slightly reduced compared with the pin3 and pin7 mutants. These results indicate that PIN1- and PID-mediated phototropisms are more affected by this pretreatment than PIN3- and PIN7-mediated phototropisms. In addition, whereas desensitization was induced in both the pin3 and pin7 mutants, the appearance of second small peak was not induced in the pin3 single mutant or the pin3 pin7 double mutant (Fig. 3B; 103 μmol m−2). Consequently, it is possible that PIN1, PIN3, and PID play roles in phytochrome-mediated phototropic regulation. On the other hand, ABCB19 was not involved in the enhancement of the pulse-induced phototropic response by red-light preirradiation (Fig. 6), though we previously proposed that a reduction of its expression is one of the underlying mechanisms that enhance the phototropic response by phytochromes (Nagashima et al., 2008a). Red-light preirradiation for 2 min in this study is not probably efficient for the reduction of the ABCB19 proteins.
The nature of how PIN and PID function in phototropic enhancement is a key consideration to arise from our current analyses. We investigated the effects of red-light pretreatment on the transcriptional levels of PIN and PID family genes. It is clear that transcripts of PIN1, PIN3, PIN4, and PIN7 are enhanced and that PIN1 is up-regulated by the greatest degree (Fig. 8A). These results suggest that the transcriptional up-regulation of these PINs, especially PIN1, is involved in the phototropic enhancement by red light. On the other hand, the transcript levels of PID family genes were found to be reduced in response to red-light pretreatment (Fig. 8A). The pid mutant did not show a clear defect in pulse-induced phototropism without red-light pretreatment, whereas phototropic enhancement by this pretreatment was partially impaired in this mutant (Fig. 7B). This indicated that PID is not required for pulse-induced phototropism without red-light pretreatment, but is necessary for the enhanced phototropic responses following this pretreatment. Taken together, it is possible to suggest from these data that transcriptional down-regulation of PID genes is involved in phytochrome-mediated phototropic enhancement.
Our present study demonstrates that the transcriptional down-regulation of PID does not occur following a pulse of blue light sufficient to induce phototropic responses (Fig. 8B). Ding et al. (2011) have proposed that the phototropin-mediated transcriptional down-regulation of PID is a key reaction that induces phototropic responses because blue light reduces the transcript levels of PID in a phototropin-dependent manner. However, our current results do not support this proposed model. Our observation that red-light pretreatment reduces the PID transcripts strongly suggests that blue-light-dependent transcriptional down-regulation of PID reported by Ding et al. (2011) is mediated by phytochromes rather than by phototropin. Other previous reports have also indicated that the phototropins function in blue-light-dependent protein modification events rather than in blue-light-dependent transcriptional regulation (Ma et al., 2001; Ohgishi et al., 2004; Pedmale et al., 2010). Although it is not clear whether the phototropin-dependent PID-mediated functional regulation of PINs occurs, analysis of phototropin-mediated accumulation and/or modification of PID proteins should be conducted in the future to better understand the functions of phototropin and PID that are related to the functional regulation of the PINs.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds of pin1-5, pin1 (Salk_047613), pin2/eir1-1, pin2 (Salk_102987), pin3-4, pin3-5, pin4-3, pin4 (CS118077), pin5-3, pin5-4, pin6 (CS107589, Salk_095142), pin7 (Salk_044687, Salk_048791), pin8 (Salk_044651), and pid-14 mutants (Columbia background) were obtained from the Arabidopsis Biological Resource Center (ABRC). The abcb19-101 (SALK_033455) mutant has been described previously (Nagashima et al., 2008a). Seeds of pin8 (pst01670) and its wild type (Nossen) were obtained from the RIKEN Bio-Resource Center. The pin3 pin7 double mutants were produced by crossing pin3-5 with pin7 (Salk_044687) or pin7 (Salk_048791), and the pin1 pin3 pin7 triple mutants were produced by crossing the pin3 pin7 double mutants with pin1-5 or pin1 (Salk_047613). A Columbia strain containing DR5rev:GFP was obtained from ABRC, and the pin3 mutant harboring DR5rev:GFP was produced by crossing the pin3-4 mutant with this Columbia variant.
Seeds of Arabidopsis were sown in 0.2-mL plastic tubes filled with 1.5% (w/v) agar medium (Sakai et al., 2000), placed in a black plastic box, and kept at 4°C for 3 to 5 d. Following irradiation with overhead red light (3−5 μmol m−2 s−1) for 2 h, the prepared seeds were incubated at 22°C ± 1°C for 2 d under complete darkness. Seedlings were selected by length of the hypocotyls (3−5 mm) and, during the experiments, were kept in the black plastic box to maintain a high humidity until they were handled for treatments. Experimental manipulations were carried out under dim green light (less than 0.1 μmol m−2 s−1) to avoid any light responses (Fitzelle and Kiss, 2001).
Induction of Phototropism and Gravitropism
For phototropic stimulation, selected seedlings were irradiated using a blue-light-emitting diode light source (470 ± 30 nm, LED-B; Eyela) through two layers of a blue filter (no. 72 film; Tokyo Butai Shomei, Tokyo). The fluence rate was controlled with neutral-density plastic filters (Fujifilm) and by changing the distance from the light source to the seedlings. The direction of the phototropic stimulation was perpendicular to the plane of the hook (Fig. 1A). To investigate the effects of red-light pretreatment on the phototropic response, the seedlings were irradiated with overhead red light (660 ± 20 nm, LED-R; Eyela) at 20 μmol m−2 s−1 for 2 min. This fluence of red light is sufficient to saturate phytochrome phototransformation (Iino, 1982). For gravitropic stimulation, the seedlings were displaced by 90° from the vertical under darkness.
Measurement of Curvature and Growth
The images of dark-grown seedlings were recorded just before phototropic stimulation and at 3 h after the onset of the stimulation with a CCD camera (ORCA-ER; Hamamatsu Photonics) driven by IPLab under infrared illumination (IRDR-110; Nissen Electronics). For time course experiments, images of the seedlings were captured at 10- or 30-min intervals using the same equipment. The angles and lengths of the hypocotyls were measured with an e-Ruler (http://www.mycnknow.com/).
Laser Scanning Confocal Microscopy
GFP signals were detected with a Leica TCS-SP5 confocal laser scanning microscope (Leica Microsystems). GFP signals were excited with an argon laser at 488 nm, and the spectral detector was set at 500 to 530 nm. All scans were carried out at a 1,024 × 1,024-pixel resolution with repeated scanning of four lines. GFP signal intensities were quantified using LAS AF Lite software (Leica).
Transcript Analysis by Quantitative RT-PCR
Following light treatment, shoots were harvested from 100 to 120 dark-grown seedlings and immediately dipped in liquid nitrogen. The frozen samples were ground to fine powders with a mixer mill (TissueLyser II; Qiagen), and total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen). The extracts were treated with RNase-free DNase (Qiagen) to remove DNA. Quantitative RT-PCR was carried out using a PCR system (CFX96; Bio-Rad) with an iScript One-Step RT-PCR Kit (Bio-Rad). Triplicate PCR reactions were performed in each case and three biological independent samples were used for each gene. The primers used are listed in Supplemental Table S1 and 18s ribosomal RNA was amplified as an internal standard.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Time courses of the hypocotyl phototropisms in the pin mutants.
Supplemental Figure S2. Effects of a pulse of blue light on the transcript levels of the PID homologs.
Supplemental Table S1. Gene specific primers used for quantitative RT-PCR.
Acknowledgments
We thank Professor T. Mitsui (Niigata University) for the use of the Bio-Rad CFX96 facility. We also thank the Arabidopsis Biological Resource Center for providing the pin1-5, eir1-1, pin3-4, pin3-5, pin4-3, pin5-3, pin5-4, and pid-14 mutants, the SALK T-DNA insertion mutants (SALK_047613, SALK_102987, SALK_095142, SALK_048791, SALK_044687, and SALK_044651), the SM transposon insertion mutants (CS118077 and CS107589), and a transgenic line harboring the DR5rev:GFP gene. We thank the RIKEN Bio-Resource Center for providing the Ds transposon insertion mutant (pst01670) and the Nossen wild-type seeds.
Glossary
- phot1
phototropin blue-light photoreceptor 1
- phot2
phototropin blue-light photoreceptor 2
- RT
reverse transcription
References
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett SR, Alvarez J, Bossinger G, Smyth DR. (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505–520 [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Christie JM. (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Christie JM, Swartz TE, Bogomolni RA, Briggs WR. (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J 32: 205–219 [DOI] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, et al. (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski L, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Esmon CA, Tinsley AG, Ljung K, Sandberg G, Hearne LB, Liscum E. (2006) A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc Natl Acad Sci USA 103: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzelle KJ, Kiss JZ. (2001) Restoration of gravitropic sensitivity in starch-deficient mutants of Arabidopsis by hypergravity. J Exp Bot 52: 265–275 [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Haga K, Iino M. (2006) Asymmetric distribution of auxin correlates with gravitropism and phototropism but not with autostraightening (autotropism) in pea epicotyls. J Exp Bot 57: 837–847 [DOI] [PubMed] [Google Scholar]
- Haga K, Takano M, Neumann R, Iino M. (2005) The Rice COLEOPTILE PHOTOTROPISM1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin. Plant Cell 17: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han IS, Tseng TS, Eisinger W, Briggs WR. (2008) Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JJ, Roberts D, Liscum E. (2009) Understanding phototropism: from Darwin to today. J Exp Bot 60: 1969–1978 [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR. (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Iino M. (1982) Action of red light on indole-3-acetic-acid status and growth in coleoptiles of etiolated maize seedlings. Planta 156: 21–32 [DOI] [PubMed] [Google Scholar]
- Iino M. (1988) Desensitization of red and blue light of phototropism in maize coleoptiles. Planta 176: 183–188 [DOI] [PubMed] [Google Scholar]
- Iino M. (1990) Phototropism: mechanisms and ecological implications. Plant Cell Environ 13: 633–650 [Google Scholar]
- Iino M. (1991) Mediation of tropisms by lateral translocation of endogenous indole-3-acetic acid in maize coleoptiles. Plant Cell Environ 14: 279–286 [Google Scholar]
- Iino M. (2001) Phototropism in higher plants. In D Häder, M Lebert, eds, Photomovement: ESP Comprehensive Series in Photosciences, Vol 1. Elsevier, Amsterdam, The Netherlands, pp 659–811
- Iino M, Haga K. (2005) Roles played by auxin in phototropism and photomorphogenesis. In M Wada, K Shimazaki, M Iino, eds Light Sensing in Plants. Springer, Tokyo, Japan, pp 269–276
- Inoue S, Kinoshita T, Matsumoto M, Nakayama KI, Doi M, Shimazaki K. (2008) Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc Natl Acad Sci USA 105: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi AK, Konjević R, Whitelam G, Gordon WR, Poff KL. (1997) Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol Plant 101: 278–282 [Google Scholar]
- Janoudi A-K, Poff KL. (1991) Characterization of adaptation in phototropism of Arabidopsis thaliana. Plant Physiol 95: 517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana JP, Best TR, Poff KL. (1989) Influence of hook position on phototropic and gravitropic curvature by etiolated hypocotyls of Arabidopsis thaliana. Plant Physiol 90: 376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Shin J, Lee SH, Kweon HS, Maloof JN, Choi G. (2011) Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc Natl Acad Sci USA 108: 1729–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Emi T, Tominaga M, Sakamoto K, Shigenaga A, Doi M, Shimazaki K. (2003) Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol 133: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, De Carbonnel M, Alonso JM, Ecker JR, Liscum E, et al. (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW. (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13: 2589–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E. (1999) Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Mravec J, Skůpa P, Bailly A, Hoyerová K, Krecek P, Bielach A, Petrásek J, Zhang J, Gaykova V, Stierhof YD, et al. (2009) Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459: 1136–1140 [DOI] [PubMed] [Google Scholar]
- Nagashima A, Suzuki G, Uehara Y, Saji K, Furukawa T, Koshiba T, Sekimoto M, Fujioka S, Kuroha T, Kojima M, et al. (2008a) Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. Plant J 53: 516–529 [DOI] [PubMed] [Google Scholar]
- Nagashima A, Uehara Y, Sakai T. (2008b) The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-naphthyphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant Cell Physiol 49: 1250–1255 [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. (2003) Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgishi M, Saji K, Okada K, Sakai T. (2004) Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc Natl Acad Sci USA 101: 2223–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale UV, Celaya RB, Liscum E. (2010) Phototropism: Mechanisms and outcomes. The Arabidopsis Book 8: e0125, doi/10.1199/tab.0125 [DOI] [PMC free article] [PubMed]
- Petrášek J, Friml J. (2009) Auxin transport routes in plant development. Development 136: 2675–2688 [DOI] [PubMed] [Google Scholar]
- Rakusová H, Gallego-Bartolomé J, Vanstraelen M, Robert HS, Alabadí D, Blázquez MA, Benková E, Friml J. (2011) Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J 67: 817–826 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K. (2000) RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT. (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Uilecan IV, Assadi AH, Kozmik CA, Spalding EP. (2009) HYPOTrace: image analysis software for measuring hypocotyl growth and shape demonstrated on Arabidopsis seedlings undergoing photomorphogenesis. Plant Physiol 149: 1632–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW, Thimann KV. (1937) Phytohormones. Macmillan, New York [Google Scholar]
- Whippo CW, Hangarter RP. (2004) Phytochrome modulation of blue-light-induced phototorpism. Plant Cell Environ 27: 1223–1228 [Google Scholar]
- Whippo CW, Hangarter RP. (2006) Phototropism: bending towards enlightenment. Plant Cell 18: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zažímalová E, Murphy AS, Yang H, Hoyerová K, Hošek P. (2010) Auxin transporters—why so many? Cold Spring Harb Perspect Biol 2: a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]