Abstract
Early embryogenesis in Arabidopsis (Arabidopsis thaliana) is distinguished by a predictable pattern of cell divisions and is a good system for investigating mechanisms of developmental pattern formation. Here, we identified a gene called LONO1 (LNO1) in Arabidopsis in which mutations can abolish the first asymmetrical cell division of the zygote, alter planes and number of cell divisions in early embryogenesis, and eventually arrest embryo development. LNO1 is highly expressed in anthers of flower buds, stigma papilla of open flowers, and embryo and endosperm during early embryogenesis, which is correlated with its functions in reproductive development. The homozygous lno1-1 seed is not viable. LNO1, a homolog of the nucleoporin NUP214 in human (Homo sapiens) and Nup159 in yeast (Saccharomyces cerevisiae), encodes a nucleoporin protein containing phenylalanine-glycine repeats in Arabidopsis. We demonstrate that LNO1 can functionally complement the defect in the yeast temperature-sensitive nucleoporin mutant nup159. We show that LNO1 specifically interacts with the Arabidopsis DEAD-box helicase/ATPase LOS4 in the yeast two-hybrid assay. Furthermore, mutations in AtGLE1, an Arabidopsis homolog of the yeast Gle1 involved in the same poly(A) mRNA export pathway as Nup159, also result in seed abortion. Our results suggest that LNO1 is a component of the nuclear pore complex required for mature mRNA export from the nucleus to the cytoplasm, which makes LNO1 essential for embryogenesis and seed viability in Arabidopsis.
In Arabidopsis (Arabidopsis thaliana), early embryogenesis follows a predictable pattern of cell divisions. For example, the zygote first divides asymmetrically to form a small apical cell and a large basal cell after fertilization, establishing two cell lineages with fundamentally different growth patterns and developmental fates (Scheres and Benfey, 1999). The apical cell then undergoes two rounds of longitudinal cell divisions and one round of transverse division to form eight cells, which divide periclinally to produce a 16-cell proembryo. The spherical proembryo continues to develop and passes through a series of stages that can be defined morphologically as globular, transition, heart, torpedo, and walking stick stages (Goldberg et al., 1994). The basal cell elongates and divides transversely to form a structure of seven to nine cells called the suspensor, which follows an extraembryonic cell fate. However, mutations in some genes can make the basal lineage cells adopt an embryonic cell fate by undergoing longitudinal cell divisions (Vernon and Meinke, 1994; Zhang and Somerville, 1997; Lotan et al., 1998; Lukowitz et al., 2004).
The normal pattern formation is important in embryogenesis, and many genes have been shown to regulate the process. The PIN-FORMED (PIN) genes encode transporter-like membrane proteins that function as auxin efflux carriers (Friml, 2003). PIN proteins control polar auxin transport and establish auxin gradients during embryogenesis, which are required for early embryo pattern formation in Arabidopsis (Friml et al., 2003; Weijers et al., 2005). Genes encoding transcription factors, such as AUXIN RESPONSE FACTORS, MONOPTEROS, BODENLOS, TARGET OF MONOPTEROS5 (TMO5), TMO7, HANABA TARANU, PLETHORA1 (PLT1), and PLT2, are also involved in pattern formation and/or embryonic root initiation in early embryogenesis (Berleth and Jurgens, 1993; Hamann et al., 1999; Aida et al., 2004; Nawy et al., 2010; Schlereth et al., 2010).
Determination of the apical and basal cell fates requires the signaling pathways regulated by YODA (a MEK kinase; Lukowitz et al., 2004), SHORT SUSPENSOR (an IL-1 receptor-associated kinase/Pelle-like kinase; Bayer et al., 2009), WUSCHEL-LIKE HOMEOBOX (Haecker et al., 2004; Breuninger et al., 2008), and GROUNDED or RKD4, a putative transcription factor of the RWP-RK motif family (Jeong et al., 2011; Waki et al., 2011). RECEPTOR-LIKE PROTEIN KINASE1 and TOADSTOOL2 are also critical for embryonic pattern formation (Nodine et al., 2007). These genes function in diverse pathways in plants, but how the signal events regulated by these genes converge during early embryogenesis to regulate pattern formation is not well understood. Recently, genomic approaches have been employed to understand how many genes are expressed in seeds and gametophyte as well as which genes are essential for making a seed (Meinke et al., 2008; Le et al., 2010; Autran et al., 2011; Drews et al., 2011; Hsieh et al., 2011; Nodine and Bartel, 2012), but it seems that there is no definitive answer to what the minimal gene set is for making a seed in plants.
In eukaryotic cells, the nuclear envelope forms a physical barrier to separate the cytosol from the nucleus. The nuclear pore complexes (NPCs) are channels embedded between the inner and outer nuclear membranes and consist of multiple copies of approximately 30 different proteins (Hetzer and Wente, 2009; Strambio-De-Castillia et al., 2010). NPCs are the only channels that all nucleocytoplasmic traffic must go through, such as mRNA export from the nucleus to the cytoplasm and protein import from the cytoplasm to the nucleus. As a macromolecular complex, NPC is not easy to study, but recent progress has shed light on its structure, assembly, and functions. Nucleoporins, the building blocks of NPCs, can be generally divided into three classes: a small group of membrane-anchored proteins, a large group of barrier proteins containing Phe-Gly (FG) repeats, and a large group of scaffold proteins that form a stable architectural framework of the NPC (Brohawn et al., 2009; Onischenko and Weis, 2011). NPC biogenesis occurs in both mitosis and interphase (Hetzer and Wente, 2009). NPCs disassemble into subcomplexes during mitosis. Subcomplexes and endoplasmic reticulum membranes are then recruited to chromatin at the end of mitosis (Dultz et al., 2008; Lupu et al., 2008). For example, it has been shown that recruitment of the Nup107/160 subcomplex initiates the NPC assembly during nuclear envelope reformation in Xenopus spp. and is followed by the recruitment of endoplasmic reticulum membranes containing POM121 and Ndc1 and the subsequent integration of Nup155 and Nup53 (Rasala et al., 2008). During interphase, other newly synthesized nucleoporins are targeted and incorporated into the nuclear envelope to form NPCs (Rasala et al., 2008). While the main function of NPCs is to mediate trafficking between the nucleus and the cytoplasm, functions of individual nucleoporins in growth and development have only begun to be understood. For example, the nucleoporin Nup98 stimulates the expression of cell cycle genes in Drosophila spp.(Kalverda et al., 2010).
CAN/NUP214 was originally discovered due to its involvement in myeloid leukemia in humans (Homo sapiens; von Lindern et al., 1992a, 1992b). NUP214 is an FG repeat-containing nucleoporin that is likely to serve as a docking site in the receptor-mediated import of substrates across the NPC (Bastos et al., 1997). It has been shown that nup214−/− mouse embryonic stem cells are not viable and that embryos die early in utero (van Deursen et al., 1996). Interestingly, these defective nup214−/− embryos do not display any obvious abnormal morphology in their nuclear envelopes or NPCs (van Deursen et al., 1996). The Nup159/RAT7 gene (for RNA trafficking) was identified in a screen for genes required for nucleocytoplasmic transport of mRNA in yeast (Saccharomyces cerevisiae; Gorsch et al., 1995). Nup159p/Rat7p contains FG repeats, is the yeast homolog of human NUP214, and is located on the cytoplasmic side at the nuclear rim (Kraemer et al., 1995). mRNA export in the yeast temperature-sensitive rat7-1 mutant was quickly stopped when the temperature was shifted from 23°C to 37°C, while the protein import from the cytoplasm to the nucleus did not seem to be affected (Gorsch et al., 1995). Recent studies in yeast have revealed the mechanism of the sequential interaction between DEAD-box protein5 (Dbp5) and Gle1 and Nup159 in regulating mRNA export (Hodge et al., 2011; Noble et al., 2011). The association of Dbp5 with NPCs is dynamic and correlated with translocation rates of mRNA protein (mRNP) through NPCs (Hodge et al., 2011; Noble et al., 2011). Inositol hexakisphosphate (IP6)-bound Gle1 promotes ATP binding to Dbp5 and activates its ATPase activity, and hydrolysis of Dbp5-ATP to Dbp5-ADP mediates the remodeling of mRNP complexes. Nup159 then functions to promote ADP release from Dbp5-ADP, and the resulting nucleotide-free Dbp5 is then ready for ATP loading upon stimulation from Gle1-IP6, starting a new round of nucleotide cycling, mRNP remodeling, and export (Hodge et al., 2011; Noble et al., 2011).
Although the structure of NPCs is conserved in eukaryotes, not much is known about the structure of NPCs and functions of nucleoporins in plants (Meier and Brkljacic, 2009). Ten nucleoporins have been isolated and functionally characterized in plants: NUP160/SAR1, NUP133, NUP96/SAR3/MOS3, NUP88/MOS7, NUP75/NUP85, NUP1/NUP136, RAE1, TPR/NUA, NUP107, and NUP62 (Zhang and Li, 2005; Dong et al., 2006; Kanamori et al., 2006; Parry et al., 2006; Jacob et al., 2007; Saito et al., 2007; Xu et al., 2007; Lee et al., 2009; Tamura et al., 2010; Tamura and Hara-Nishimura, 2011; Wiermer et al., 2010, 2012; Zhao and Meier, 2011). These nucleoporins have been shown to regulate cell division, hormone signaling, response to stress, rhizobial and fungal symbiosis, flowering, and other aspects of plant development. Recently, 22 nucleoporins have been identified through the mass spectrometry-based interactive proteomics in Arabidopsis, including Arabidopsis NUP214 (Tamura et al., 2010), but the biological functions of most nucleoporins in plant growth and development remain unknown.
Here, we report the isolation and characterization of the lono1 (lno1) mutant during early embryogenesis and seed development. LNO1 encodes nucleoporin-containing FG repeats in Arabidopsis, a homologous protein to the nucleoporin NUP214 in humans, and Nup159 in yeast. We show that LNO1 can functionally complement the yeast nucleoporin temperature-sensitive mutant nup159. We also show that the nucleoporin LNO1 interacts with Arabidopsis DEAD-box helicase/ATPase, CRYOPHYTE/LOS4, in the yeast two-hybrid assay. Mutations in LNO1 can abolish the first zygotic asymmetrical cell division, alter normal pattern formation in early embryogenesis, and lead to aborted seed. Remarkably, AtGLE1, an Arabidopsis homolog of the yeast Gle1 functioning in the same pathway as Nup159, is also required for seed viability. Our studies suggest that LNO1 is a functionally conserved nucleoporin protein that is required for embryogenesis and seed development in Arabidopsis.
RESULTS
Identification of the LNO1 Gene and T-DNA Insertional Mutants
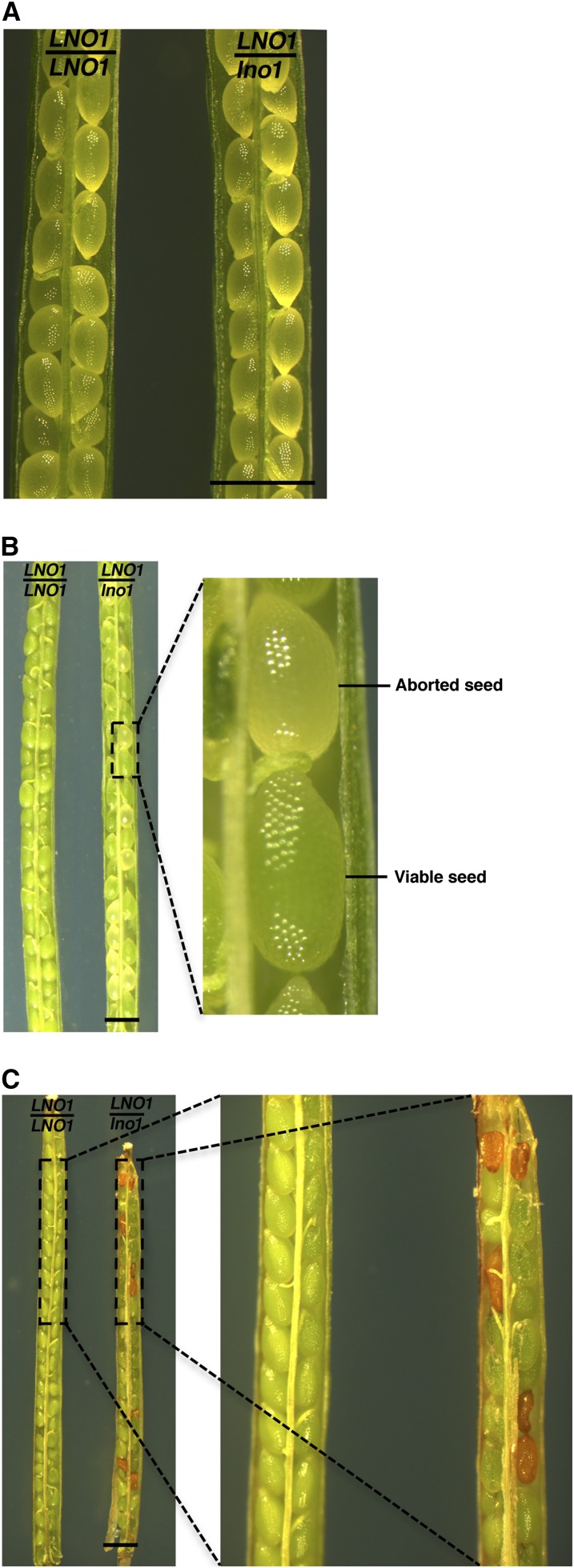
To understand embryogenesis and seed development, we took a genetic approach (see “Materials and Methods”) and identified genetic mutations that interrupt seed development. By screening a set of T-DNA insertional mutant lines, we identified a few mutants that had abnormal embryogenesis or seed abortion. We named these genes LONO, after a god who was associated with fertility, agriculture, rainfall, and music in Hawaiian mythology. We found that LNO1 (At1G55540) was required for seed viability in Arabidopsis. When lno1-1 became homozygous, the seed was not viable (Fig. 1), and we were not able to obtain viable lno1-1/lno1-1 plants. In self-pollinated heterozygous LNO1/lno1-1 plants, we counted seed abortion and found 357 aborted seeds among a total of 1,439 seeds (1,082:357, χ2 = 0.009 for the 3:1 ratio, P > 0.9; Fig. 1; Table I), suggesting that the lno1-1 mutation results in seed abortion and follows simple Mendelian inheritance. We backcrossed the lno1-1 mutant to wild-type plants five times, and the result showed that the seed abortion phenotype was correlated with the lno1-1 mutation in each progeny and each generation. To further confirm that the seed abortion phenotype was caused by the T-DNA insertional mutation in LNO1, we transformed the LNO1 transgene under the control of the 35S promoter into LNO1/lno1-1 heterozygous plants, and the result showed that the transgene was able to complement the mutant phenotype and restored the seed viability in the homozygous mutant lno1-1/lno1-1 background.
Figure 1.
Effect of the lno1-1 mutation on seed viability in Arabidopsis. Siliques of wild-type (LNO1/LNO1) and heterozygous (LNO1/lno1-1) plants were dissected and photographed at 6 DAP (A), at 12 DAP (B), and at 18 DAP (C). Brown and green seeds in the silique on the right in C were aborted and viable seeds, respectively.
Table I. The lno1-1 mutation affects embryogenesis and seed viability in Arabidopsis.
| Self-Pollinated | Abnormal F1 Embryo |
F1 Seed Abortion |
||||
|---|---|---|---|---|---|---|
| Abnormal | Total | Percentage | Aborted | Total | Percentage | |
| Wild type | 0 | 386 | 0 | 2 | 467 | 0.4 |
| LNO1/lno1-1 | 57 | 483 | 11.8 | 357 | 1439 | 24.8 |
| lno1-2/lno1-2 | 0 | 437 | 0 | 6 | 875 | 0.7 |
We did not detect any morphological phenotype or defect in ovule or pollen development of the lno1-1 mutant allele. We further examined whether the lno1-1 mutant allele has any defects in transmission through female or male by reciprocally crossing the heterozygous LNO1/lno1-1 with wild-type plants. In the F1 progeny, there were no empty positions in siliques, suggesting that there were no degenerated ovules or unfertilized ovules, and almost all the seeds were viable whether the LNO1/lno1-1 plants were used as female or male in the cross (Table II). After the F1 progeny were planted, we genotyped each plant in the F1 and found that the lno1-1 allele was transmitted to the next generation as efficiently as the wild-type allele either by female or male (Table II).
Table II. The lno1-1 mutant has neither parent-of-origin effect nor defects in transmission.
LNO1 Encodes a Nucleoporin Protein in Arabidopsis
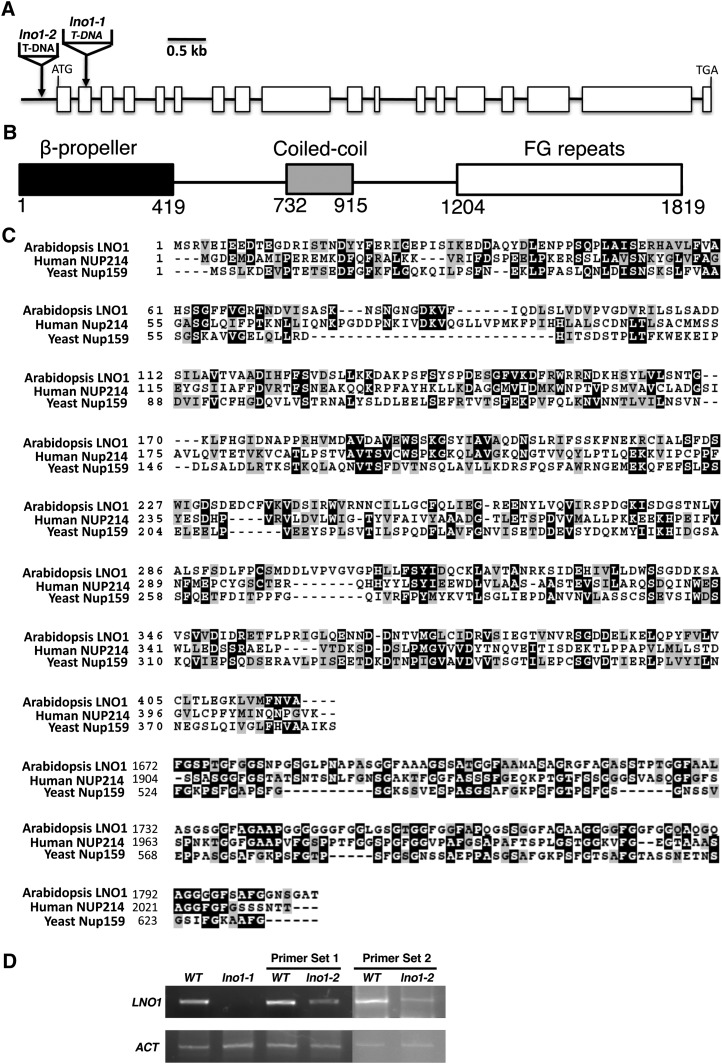
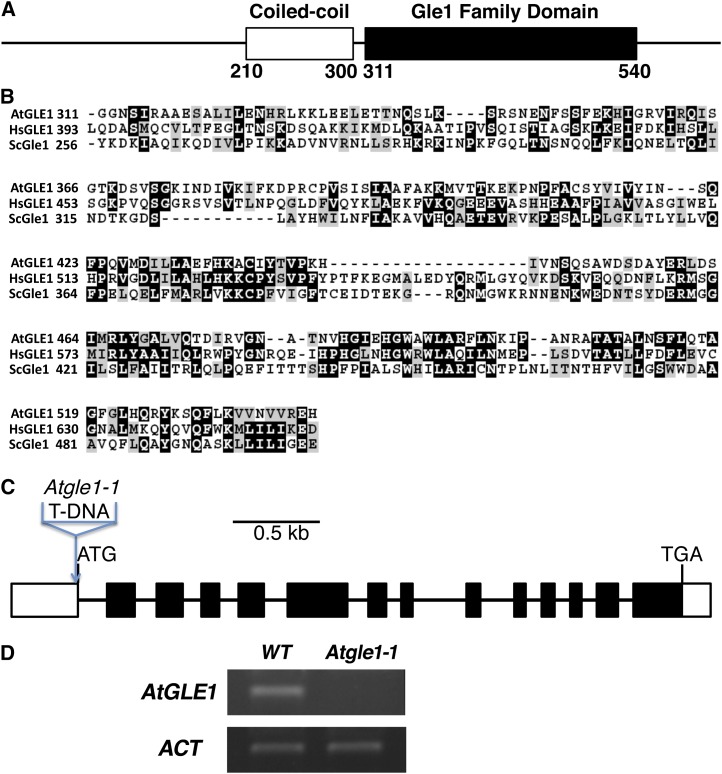
In Arabidopsis, LNO1 (At1G55540) has 18 exons and 17 introns (Fig. 2A) and encodes a protein with 1,819 amino acids that can be divided into an N-terminal β-propeller domain, a coiled-coil domain in the middle, and C-terminal FG repeats (Fig. 2B). LNO1 encodes a nucleoporin protein with the FG repeats and is a homolog of NUP214/CAN in humans and Nup159 in yeast (Fig. 2C). In the N-terminal β-propeller domain, LNO1 shares 23% and 14% amino acid sequence identity with NUP214 and Nup159, respectively, and the C-terminal FG repeats of LNO1 show 33% and 30% sequence identity to those of NUP214 and Nup159, respectively (Fig. 2C). We have identified two lno1 mutant alleles: lno1-1 and lno1-2. lno1-1 and lno1-2 had a T-DNA insertion in the second exon (+609 bp relative to the transcription start) and in the promoter (−187 bp relative to the transcription start), respectively. To examine whether the lno1 mutant is a knockout allele, we used semiquantitative reverse transcription (RT)-PCR to examine LNO1 expression in seeds. We found that lno1-1 was a null allele (Fig. 2D) and homozygous lethal (Fig. 1, B and C), whereas lno1-2 was a knockdown allele (Fig. 2D) that had no obvious defect in growth and development (Table I). This result indicates that a low expression of the nucleoporin LNO1 is sufficient to sustain normal embryogenesis and seed development, although LNO1 is essential.
Figure 2.
LNO1 encodes a nucleoporin protein containing FG repeats in Arabidopsis. A, Gene structure of LNO1 and locations of T-DNA insertions in two lno1 mutant alleles. Boxes represent exons, and lines represent promoter or introns. B, The three domains of the LNO1 protein in Arabidopsis. Numbers represent positions of amino acids in LNO1. C, Protein sequence alignments at the N terminus and FG repeats of Arabidopsis LNO1, human NUP214, and yeast Nup159. The alignment was performed using ClustalW2 and followed by using BOXSHADE 3.21. Identical residues are highlighted in dark gray, and similar residues are highlighted in light gray. D, Expression of LNO1 in the lno1-1 and lno1-2 mutant plants. Two sets of primers were used to examine LNO1 expression in lno1-2 and wild-type plants. ACT, ACTIN; WT, wild type.
LNO1 Is Important for Embryogenesis
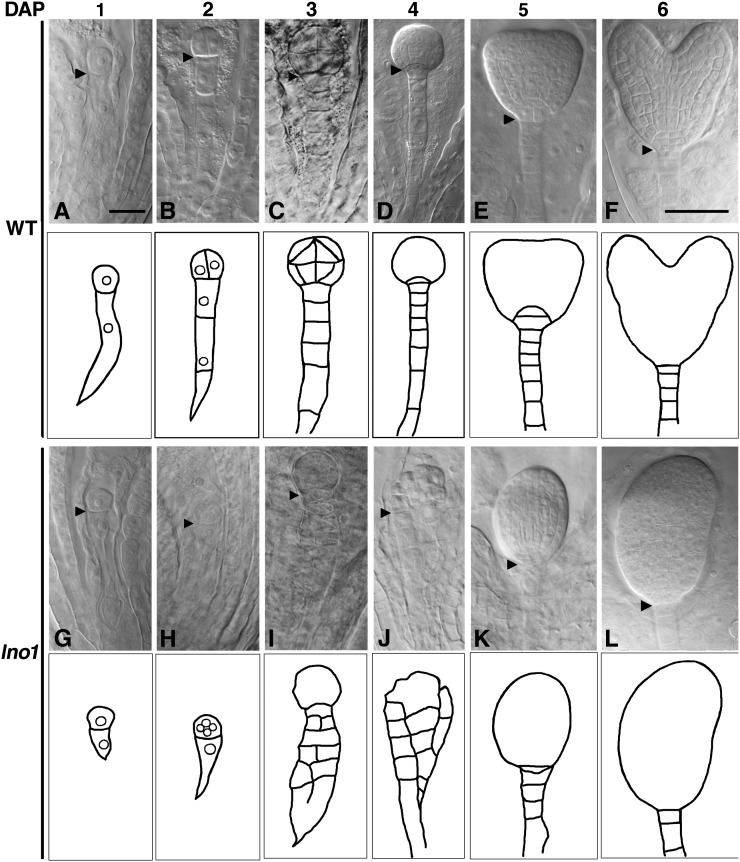
The seed with homozygous null allele lno1-1 was aborted before seed maturation (Fig. 1, B and C), but it appeared the same as the wild-type seed before 6 d after pollination (DAP) by regular light microscopy (Fig. 1A). To examine whether lno1-1 had any defects in early embryogenesis, we cleared the seed and observed that 47% (n = 121) of the putative homozygous lno1-1/lno1-1 embryos developed abnormally. As described below, these homozygous lno1-1 mutant embryos displayed a variety of developmental abnormalities that were consistently observed.
Abnormalities in the lno1-1 mutant embryos were first detected during the first zygotic division. At 1 DAP, the wild-type zygote usually elongates and divides asymmetrically to give rise to a small apical cell and a large basal cell (Fig. 3A). However, we observed that lno1-1 mutant zygotes divided almost symmetrically (Fig. 3G). Approximately 31% (n = 86) of the lno1-1/lno1-1 embryos had basal cells that failed to elongate (Fig. 3, G and H) as the wild-type embryos did (Fig. 3, A and B). This result shows that the lno1 mutation affects the earliest stage of embryogenesis in Arabidopsis. In wild-type embryos, the basal cell elongates and divides transversely to form a suspensor with seven to nine cells (Fig. 3, C and D), and longitudinal divisions do not occur in suspensors (Fig. 3, C and D). By contrast, longitudinal cell divisions in the basal cell lineage were detected in approximately 36% (n = 117) of the lno1-1/lno1-1 embryos at 3 to 4 DAP (Fig. 3, I and J). In wild-type embryos, there is a clear boundary between the spherical proembryo and the linear file of suspensor cells (Fig. 3, C and D) at 3 to 4 DAP. The hypophysis, the uppermost cell of the basal lineage, becomes prominent at 4 DAP (Fig. 3D). This clear demarcation between embryo and suspensor was often not detected in the lno1-1 mutant embryos because of many longitudinal cell divisions in the suspensor (Fig. 3, I and J). These results indicate that mutations in LNO1 can make the basal cell assume an embryonic cell fate that divides longitudinally.
Figure 3.
The lno1-1 mutation affects suspensor and embryo proper development. A to L, Nomarski photographs of wild-type (WT) and homozygous lno1-1 mutant embryos at 1 to 6 DAP. A to F show photographs of the wild type at 1, 2, 3, 4, 5, and 6 DAP, respectively, and outline drawings of individual embryos immediately below the embryo; G to L show the lno1-1 mutant embryos at 1, 2, 3, 4, 5, and 6 DAP, respectively, and outline drawings of individual embryos immediately below the embryo. Small circles inside the cells represent nuclei in the drawings for A, B, G, and H. Photographs A to C and G to I are on the same scale, and photographs D to F and J to L are on the same scale. Arrowheads indicate the plane of the first zygotic cell division in A and G and the boundary between the apical and basal lineage-derived cells in B to F and H to L. Bars = 20 μm in A and 50 μm in F.
Abnormalities in number and planes of cell division persisted throughout embryogenesis in the lno1-1 mutant. In wild-type Arabidopsis, the embryo passes through a series of stages that are defined morphologically as globular, transition, and heart stages at 4 to 6 DAP (Fig. 3, D–F), but in the abnormal lno1-1 mutant, the typical embryo morphology was not observed (Fig. 3, J–L). At the transition and early heart stages of wild-type embryos, two symmetrical cotyledons are initiated from lateral domains of the embryo and an embryonic shoot apical meristem is differentiated from the medial domain between the two cotyledons (Fig. 3F; Berleth and Chatfield, 2002; Prigge et al., 2005). Approximately 19% (n = 58) of lno1-1/lno1-1 embryos failed to differentiate two cotyledons (Fig. 3, K and L). Mutant embryos having one cotyledon also lacked a medial domain where the embryonic shoot meristem could be generated (Fig. 3, K and L). In summary, these results indicate that the knockout lno1 mutation alters the number and planes of cell divisions required for generating the normal embryo proper and suspensor, apical-basal axis, cotyledons, and meristem.
To examine whether the lno1-1 mutant allele has any parent-of-origin effects on embryogenesis, we crossed the heterozygous LNO1/lno1-1 female plants with wild-type (LNO1/LNO1) male plants and examined 271, 254, 423, and 316 embryos at 2, 3, 5, and 10 DAP, respectively, but we did not find any abnormal mutant embryos. In the reciprocal cross (LNO1/LNO1 × LNO1/lno1-1), we examined 246, 168, 374, and 281 embryos at 2, 3, 5, and 10 DAP, respectively, but we did not observe any abnormal mutant embryos either. This result showed that both the maternal and paternal mutant alleles are required for producing the abnormal embryo phenotype and that mutations in LNO1 do not have any detectable parent-of-origin effects on embryogenesis.
LNO1 Is Highly Expressed in Reproductive Tissues
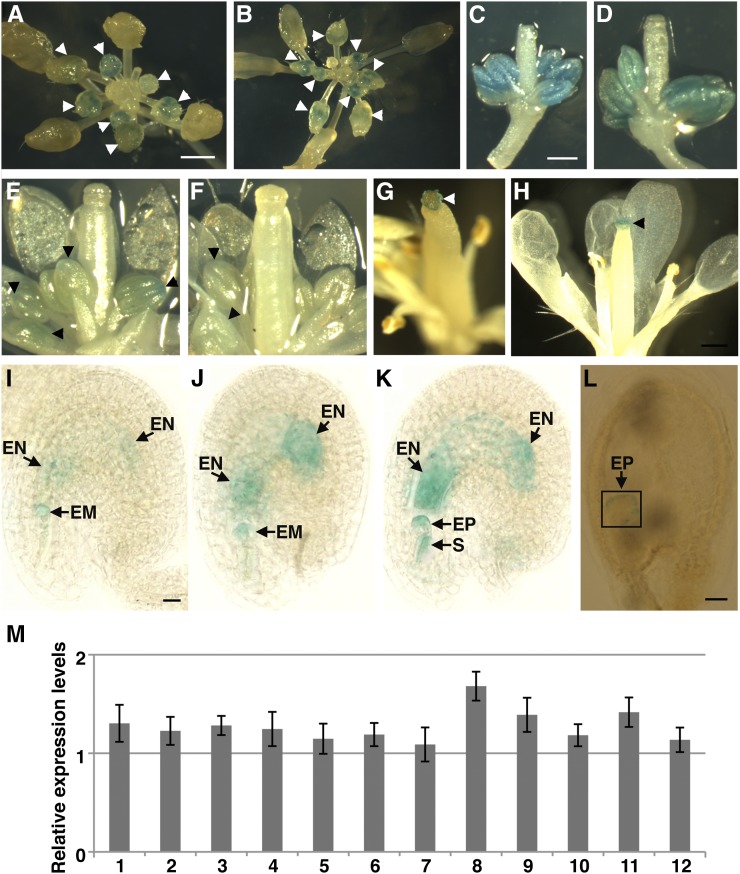
To obtain a preliminary overview of the microarray expression data, we searched a database (https://www.genevestigator.com/gv/) and found that LNO1 has very low expression in all the tissues examined except sperm cells. To examine the expression of LNO1 in Arabidopsis, we fused the LNO1 promoter with the reporter gene GUS (LNO1 promoter:GUS) and examined the temporal and spatial expression patterns of the transgene in Arabidopsis. We did not detect expression of the transgene LNO1 promoter:GUS in any vegetative tissues, such as leaves, shoots, stems, or roots, although we employed several GUS staining protocols as described (Debrouwe and De Block, 1992; Sessions et al., 1999; Luo et al., 2000; Yu et al., 2005). However, we were able to observe expression of the transgene LNO1 promoter:GUS in reproductive tissues, such as flowers and early developing seeds (Fig. 4). We examined more than 70 flowers from seven independent transgenic lines and observed a consistent expression pattern (Fig. 4, A and B). When we dissected out the flowers, we found that LNO1 promoter:GUS was expressed in anthers but not in other floral organs before flower stage 11 (Fig. 4, C–F). The LNO1 promoter:GUS transgene was not detected in early flower buds at stages 1 to 5 (Fig. 4, A and B), then started to express in flower buds at stages 6 and 7 (Fig. 4, A and B), reached the highest expression in flower buds at stages 8 and 9 (Fig. 4C), had reduced expression in flowers at stages 10 and 11 (Fig. 4, D and E), and was almost undetected in its expression in flowers at stage 12 (Fig. 4F). Interestingly, when flowers reached stage 13, LNO1 promoter:GUS was specifically expressed in stigma papilla (Fig. 4G) and reached its highest expression in the stage 14 flower (Fig. 4H). We further checked the expression of LNO1 promoter:GUS in the developing seed and observed that the transgene was expressed in both embryo and endosperm as early as 2 DAP (Fig. 4I), continually expressed at 3 DAP (Fig. 4J), and reached its highest expression at 4 DAP in embryo and endosperm (Fig. 4K). Then, its expression decreased, but it could still be detected at the outer layer cells of the embryo proper at the transition stage (Fig. 4L). In short, the temporal and spatial expression patterns of the LNO1 promoter:GUS transgene are correlated with its functions in reproductive development in Arabidopsis.
Figure 4.
Temporal and spatial expression of LNO1 in Arabidopsis. A to L, Expression of the transgene LNO1 promoter:GUS in Arabidopsis reproductive tissues. A and B, Vertical views of the apex of floral buds (stages 1–12) of the transgenic plant. C and D, Side views of floral buds at stages 9 and 10, respectively, with sepals and petals dissected away. E to H, Side views of floral buds at stages 11, 12, 13, and 14, respectively, with sepals removed. I to L, Seed photographs of the LNO1 promoter:GUS transgenic plant after GUS staining at 2, 3, 4, and 6 DAP, respectively. EM, Embryo; EN, endosperm; EP, embryo proper; S, suspensor. Arrowheads indicate locations of GUS expression. M, Temporal and spatial expression of LNO1 examined by quantitative RT-PCR in Arabidopsis. The mean relative expression levels from three independent replicates for a sample are shown. Error bars indicate sd. Lane 1, 4-d-old seedlings; lane 2, 7-d-old seedlings; lane 3, 14-d-old seedlings; lane 4, 21-d-old plants; lane 5, rosette leaves; lane 6, cauline leaves; lane 7, flower buds at flower stages 1 to 4; lane 8, flower buds at flower stages 7 to 11; lane 9, stamens at flower stages 10 and 11; lane 10, carpels at flower stage 12; lane 11, embryo at 7 DAP; lane 12, endosperm at 7 DAP.
Although we tried several GUS staining protocols (Debrouwe and De Block, 1992; Sessions et al., 1999; Luo et al., 2000; Yu et al., 2005) and also modified some parameters, we were not able to detect any expression of the LNO1 promoter:GUS transgene in vegetative tissues. To examine whether LNO1 was expressed in vegetative tissues, we employed more sensitive quantitative RT-PCR to examine LNO1 temporal and spatial expression. We found that LNO1 was expressed in all the tissues we examined (Fig. 4M). We could detect the expression of LNO1 in young seedlings (4-, 7-, and 14-d-old seedlings), mature plants (21-d-old plants), and rosette and cauline leaves. Expression of LNO1 was relatively low in very early stage flowers (stage 1–4 flowers) but was increased in later stages (stage 7–11 flowers). LNO1 was also expressed in stamens during flower stages 10 and 11, carpels at flower stage 12, and embryo and endosperm at 7 DAP. This quantitative RT-PCR result is consistent with the expression pattern of the LNO1 promoter:GUS transgene (high expression in flowers at flower stages 8 and 9) but also shows that LNO1 expression was detected in the tissues where expression of the transgene LNO1 promoter:GUS was not detected.
LNO1 Can Complement the Temperature-Sensitive Yeast Mutant nup159
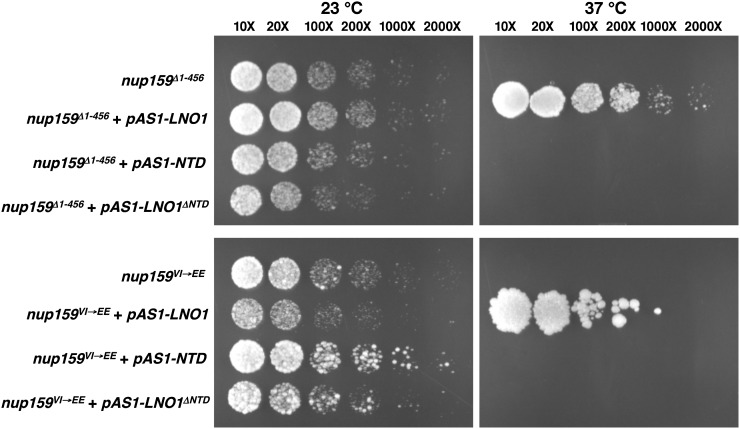
Nup159, a homolog of Arabidopsis LNO1, has been well studied in yeast. The crystal structure shows that the N terminus of Nup159 forms an asymmetric seven-bladed β-propeller that is conserved in eukaryotes (Weirich et al., 2004). The yeast mutant nup159Δ1-456, which has a deletion of the entire N-terminal domain and part of the central domain, has growth defects and is not able to grow at 37°C (Del Priore et al., 1997). The mutant nup159VI→EE has also been shown to be temperature sensitive (Weirich et al., 2004). To test whether LNO1 can functionally complement the growth defects in the yeast mutant, we cloned the Arabidopsis LNO1 full-length complementary DNA (cDNA) into the yeast expression vector pAS1 (Durfee et al., 1993; Wang et al., 2005) and transformed the construct into the yeast mutants nup159Δ1-456 and nup159VI→EE. Both mutants (nup159Δ1-456 and nup159VI→EE) with the LNO1 construct (pAS1-LNO1) grew well at the nonpermissive temperature (37°C; Fig. 5). We further did the complementation experiment by using the N-terminal domain of LNO1 (pAS1-NTD) alone or LNO1 sequence with deletion of NTD (pAS1-LNO1ΔNTD). The result showed that neither of the constructs can complement the growth defect of nup159 at the nonpermissive temperature (37°C; Fig. 5). These results demonstrate that the full-length LNO1 can functionally complement the growth defects of the yeast mutant nup159 with the N-terminal deletion or mutations at residues Val-323 and Ile-326 but not the N-terminal or C-terminal domain of LNO1 by itself. These experiments suggest that LNO1 is a functionally conserved nucleoporin in eukaryotes.
Figure 5.
LNO1 functions as a nucleoporin protein in Arabidopsis. LNO1 can functionally complement the defect in the yeast mutant nup159. The full-length LNO1, the N-terminal domain (NTD), and LNO1 without the N-terminal domain (LNO1ΔNTD) were cloned into the vector pAS1 and transformed into the nup159Δ1-456 and nup159VI→EE mutants. Yeast cultures were serially diluted (10×, 20×, 100×, 200×, 1,000×, and 2,000×) and spotted on YPD plates.
LNO1 Interacts with the DEAD-Box Helicase/ATPase LOS4
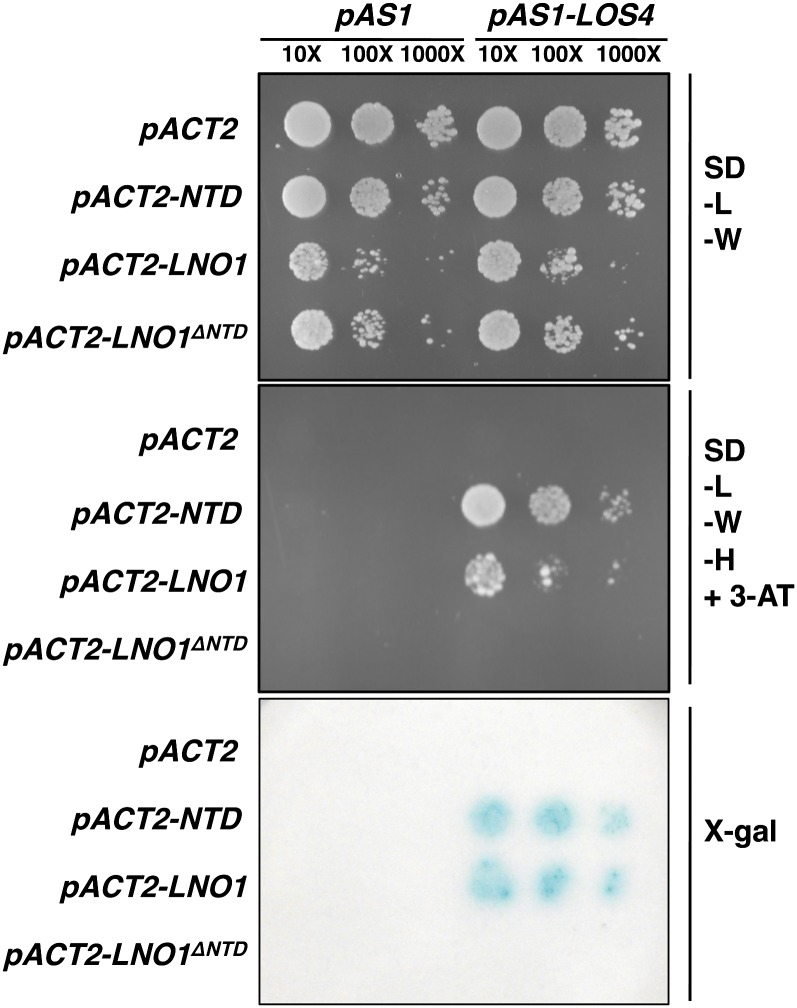
The yeast Nup159, localized to the cytoplasmic side of the NPC, plays a critical role in exporting mRNA from the nucleus to the cytoplasm. The N terminus of Nup159 forms a β-propeller, which mediates protein-protein interaction, and recruits Dbp5, which functions in mRNP export (Hodge et al., 1999; Schmitt et al., 1999). The N-terminal domain of the human nucleoporin NUP214 has also been shown to interact with the DEAD-box RNA helicase Ddx19 in the crystal structure (Napetschnig et al., 2009). We searched the database and found an Arabidopsis protein called LOS4 sharing high homology with the yeast Dbp5 and human Ddx19 (Gong et al., 2005). To examine whether LNO1 can interact with LOS4, we cloned the N-terminal domain, full-length, and C-terminal domain of LNO1 with the Gal4 DNA activation domain in pACT2 (pACT2-NTD, -LNO1, and -LNO1ΔNTD, respectively), fused LOS4 with the Gal4 DNA-binding domain in pAS1 (pAS1-LOS4), and examined the fusion protein interaction in a yeast two-hybrid assay. The result showed that the N-terminal domain and full-length LNO1 interacted with RNA helicase LOS4 in yeast, but the C-terminal domain of LNO1 alone (LNO1ΔNTD) did not (Fig. 6). When we swapped the Gal4 DNA-binding domain with the Gal4 DNA activation domain in the yeast two-hybrid constructs, we obtained the same result as that shown in Figure 6 (Supplemental Fig. S1): the N-terminal domain and full-length LNO1 specifically interact with RNA helicase LOS4. This result implies that LNO1 is part of the mRNA export machinery in nucleocytoplasmic transport across the nuclear membrane in eukaryotes.
Figure 6.
LNO1 interacts with Arabidopsis DEAD-box helicase/ATPase and LOS4 in yeast two-hybrid assays. The N-terminal domain of LNO1 (NTD), full-length LNO1, and LNO1 with deletion of NTD (LNO1ΔNTD) were fused with the GAL4 DNA activation domain (AD), and LOS4 was fused with the GAL4 DNA binding domain (BD). pACT2 (AD) and pAS1 (BD) empty vectors were included as negative controls. Yeast transformants were spotted on synthetic dropout plates without Leu (L) and Trp (W) or without Leu, Trp, and His (H) and with 20 mm 3-aminotriazole (3-AT). The 5-bromo-4-chloro-indolyl-β-d-galactopyranoside filter-lift assay was as described (Rea et al., 2012). Yeast cultures were serially diluted (10×, 100×, and 1,000×). [See online article for color version of this figure.]
AtGLE1 Is Also Critical for Seed Viability
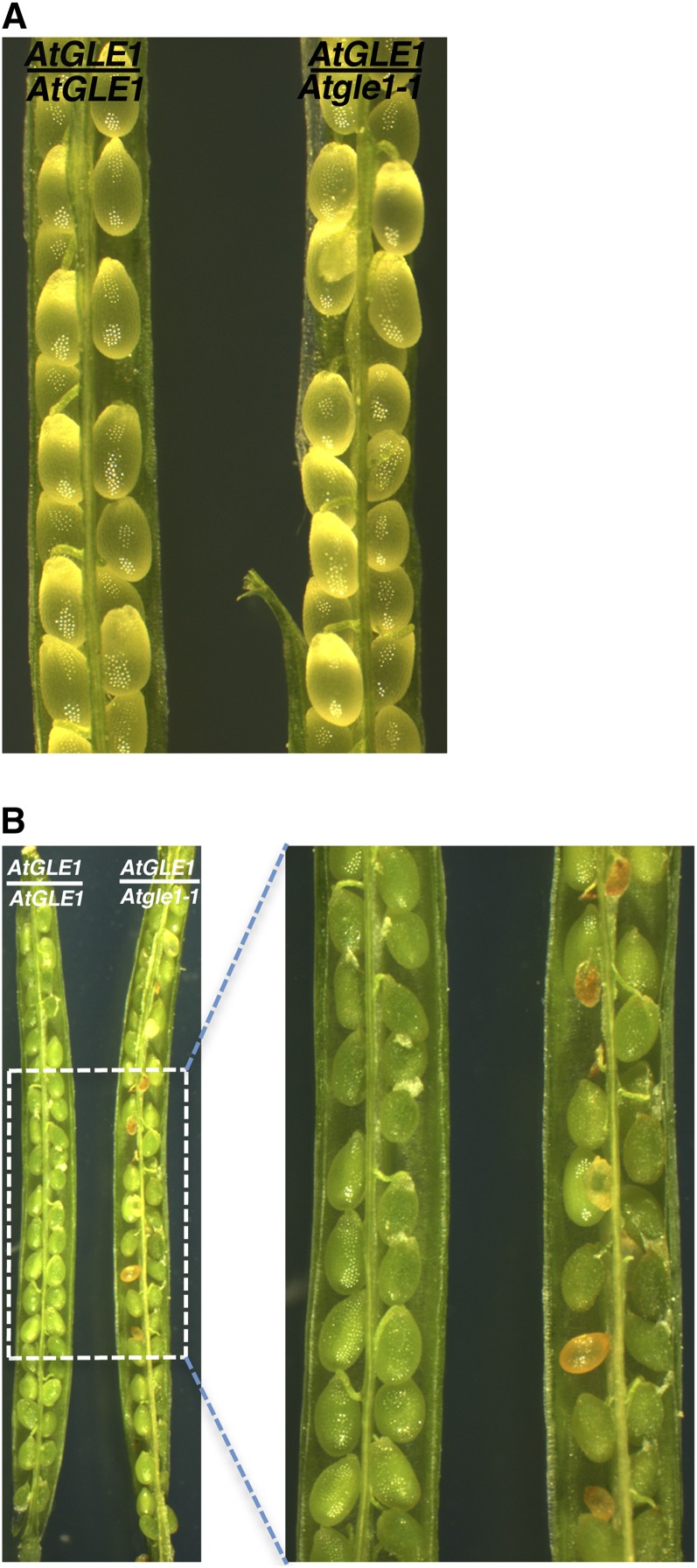
Nup159 functions together with other nucleoporins including Gle1 in exporting mRNAs from the nucleus to the cytoplasm (Alcázar-Román et al., 2006; Noble et al., 2011). It has been reported that there is a homolog of the yeast Gle1 in Arabidopsis, AtGLE1 (At1G13120; Tamura et al., 2010). AtGLE1 is a relatively small protein with 611 amino acids and has a putative coiled-coil domain in the middle and a GLE1 domain in the C terminus (Fig. 7A). The GLE1 domain of AtGLE1 shares 17% and 26% amino acid sequence identity with its homolog Gle1 in yeast and HsGle1 in humans, respectively (Fig. 7B). To examine if AtGLE1 is required for seed viability, we searched the Arabidopsis database (www.tair.org) and found a T-DNA insertional line, CS16088 or emb1745 (Tzafrir et al., 2004), and to be consistent with the nomenclature in Arabidopsis, we called the mutant Atgle1-1. Through genotyping, we found that there is a T-DNA insertion in the 5′ untranslated region (16 bp upstream of the translation start codon ATG) in the Atgle1-1 mutant (Fig. 7C). We used RT-PCR to examine AtGLE1 expression in seeds and showed that the expression of AtGLE1 was almost not detectable in the Atgle1 mutant seed (Fig. 7D). In self-pollinated heterozygous AtGLE1/Atgle1-1 plants, we found aborted seeds (Fig. 8). There were 127 aborted seeds among a total of 647 seeds (19.6% seed abortion). The Atgle1-1 mutant was backcrossed and self-pollinated, and we found that the seed abortion phenotype was cosegregating with the mutant allele in each generation and plant. This result indicates that AtGLE1, the Arabidopsis homolog of yeast Gle1 involved in the same poly(A) mRNA export pathway as Nup159, is also pivotal for seed viability in plants.
Figure 7.
AtGLE1 encodes an Arabidopsis putative homolog of yeast Gle1 and human HsGLE1. A, The two domains of the AtGLE1 protein in Arabidopsis. Numbers represent positions of amino acids in AtGLE1. B, Protein sequence alignment of the Gle1 domain in the C terminus of Arabidopsis AtGLE1, human HsGLE1, and yeast ScGle1. The alignment was performed using ClustalW2 and followed by BOXSHADE 3.21. Identical residues are highlighted in dark gray, and similar residues are highlighted in light gray. C, Gene structure of AtGLE1 and location of T-DNA insertion in the Atgle1-1 mutant (16 bp upstream of the ATG start codon). D, Expression of AtGLE1 in the Atgle1-1 mutant and wild-type plants. ACT, ACTIN; WT, wild type. [See online article for color version of this figure.]
Figure 8.
Effect of the Atgle1-1 mutation on seed viability in Arabidopsis. Wild-type (AtGLE1/AtGLE1) and heterozygous (AtGLE1/Atgle1-1) siliques were dissected and photographed at 6 DAP (A) and 18 DAP (B). Brown and green seeds in the silique on the right in B were aborted and viable seeds, respectively. [See online article for color version of this figure.]
DISCUSSION
The NPCs are important structures on the nuclear envelope that mediate trafficking between the nucleus and the cytoplasm. Nucleoporins participate in forming the nuclear pore structure and in nucleocytoplasmic transport. Genetic and biochemical evidence has shown that mutations in nucleoporins affect the structure of NPCs and the nuclear envelope (Doye and Hurt, 1997). NPCs can also affect growth and development. In Caenorhabditis elegans, 17 out of 20 nucleoporins are required for embryonic development (Galy et al., 2003). The C. elegans homologs of vertebrate Nup93 and Nup205 were found to be essential for normal NPC distribution in the nuclear envelope in vivo and for cell viability, and depletion of Nup93 or Nup205 caused defects in NPC exclusion, abnormal chromatin condensation, and early embryonic arrest (Galy et al., 2003). In mice, NUP214 is required for embryonic stem cell and embryo viability, and the nup214−/− null embryos die in utero between 4.0 and 4.5 d post coitum (van Deursen et al., 1996). However, its yeast homolog Nup159 is not essential: null mutant nup159 can grow at permissive temperature (Weirich et al., 2004). Here, we show that LNO1, a homolog of NUP214, is essential for embryogenesis and seed viability in plants. The lno1-1 mutation can affect cell divisions and pattern formation in early embryogenesis (Fig. 3). Approximately 47% of the homozygous lno1-1/lno1-1 embryos showed abnormal cell division and pattern formation in early embryogenesis, but more than half of the lno1-1/lno1-1 embryos could continue to develop without obvious morphologic defects to certain levels. There are no other genes showing high homology with LNO1 in Arabidopsis, so it is not clear why more than 50% of the null lno1-1/lno1-1 embryos could continue to develop until the heart or torpedo stage during early embryogenesis and then abort at a late stage. One possibility is that LNO1 plays a role in normal endosperm development in Arabidopsis. Since LNO1 was expressed in early endosperm tissues (Fig. 4), mutations in LNO1 might affect normal endosperm development in Arabidopsis, thus leading to late-stage seed abortion of those normal-looking lno1-1/lno1-1 embryos at an earlier stage. Alternatively, LNO1 is required for mRNA export, and mutations in LNO1 might abolish the export of mRNA of many essential genes in embryogenesis from the nucleus to the cytosol, thus causing seed abortion in all the lno1-1/lno1-1 embryos at the late stage.
The NPC consists of approximately 30 nucleoporins in eukaryotic cells. The nucleoporin CAN/NUP214 was originally found to be a putative oncogene product associated with myeloid leukemogenesis and is localized to the cytoplasmic side of the NPC (Kraemer et al., 1994). However, in cells overexpressing NUP214, NUP214 can bind to both the cytoplasmic and the nucleoplasmic sides of the NPC (Boer et al., 1997). NUP214 plays a role in nuclear protein import, mRNA export, and cell cycle progression and interacts with DDX19 (Napetschnig et al., 2009; von Moeller et al., 2009). In yeast, the nucleoporins Nup159 and Gle1 are both localized to the cytoplasmic side of the NPC and function in the same pathway in exporting mRNA. The N-terminal domain of Nup159 forms a β-propeller that functions in mRNA export by tethering the shuttling helicase Dbp5 at the nuclear periphery and locally concentrating this mRNA-remodeling factor at the cytoplasmic face of the NPC (Weirich et al., 2004). Nup159 and Nup82 form a cytoplasmically oriented subcomplex of the NPC that is essential for RNA export but not for classical nuclear localization sequence-mediated nuclear protein import (Hurwitz et al., 1998). LNO1 (AtNUP214) is an Arabidopsis homolog of human NUP214 and yeast Nup159. AtNUP214 was localized to the NPC in the root tip cells (Tamura et al., 2010). We showed that LNO1 complemented the yeast temperature-sensitive mutant nup159 (Fig. 5). LOS4, a homolog of ATPase DDX19 in human and Dbp5 in yeast, was shown to function in mRNA export in Arabidopsis (Gong et al., 2005), and we showed that LOS4 interacts with LNO1 in yeast (Fig. 6). Furthermore, the Gle1 homolog, AtGLE1, is also required for seed viability in Arabidopsis (Figs. 7 and 8). These results suggest that LNO1 (AtNUP214), LOS4, AtGLE1, and perhaps other plant nucleoporins might form a functionally conserved pathway as those in human and yeast, which plays an essential role in nuclear and cytoplasmic trafficking in plants.
Patterning formation in early embryogenesis is regulated by auxin gradients and other signaling pathways. Interestingly, SUPPRESSOR OF AUXIN RESISTANCE1 (SAR1) and SAR3, encoding nucleoporins NUP160 and NUP96, respectively, are involved in hormone auxin signaling and development (Parry et al., 2006). The defects in the mutant lno1 are similar to those embryogenesis mutants in the auxin signaling pathway. One possibility is that LNO1 as a nucleoporin might interact with other signaling pathways and regulate embryogenesis (e.g. the auxin signaling) or, at least in part, LNO1 can affect the transport of mRNAs of components of those signaling pathways involved in early embryogenesis, thus affecting early pattern formation. Since the homozygous lno1-1/lno1-1 seed is not viable, we are not able to examine the role of LNO1 at the seedling stage or in mature plants. Thus, it is possible that LNO1 can be an essential gene throughout the whole life cycle of plants. We expect that further biochemical characterization of LNO1 and genetic study will reveal its function in signal transduction or transporting other signaling components in early embryogenesis and perhaps in other developmental processes in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
We took a genetic approach to identify mutants that affect seed development or embryogenesis in Arabidopsis (Arabidopsis thaliana). We performed a cDNA microarray using the F1 seed of the DNA methyltransferase met1 mutant (Xiao et al., 2003, 2006) crossed with wild-type ecotype Columbia (Col-0) and a control wild-type cross and found over 1,300 genes that had altered expression in the met1 mutant cross compared with the wild type (W. Xiao, C. Braud, and W. Zhang, unpublished data). We then chose 116 putative genes that affect embryogenesis, seed development, epigenetics, transcription, translation, or chromatin remodeling based on annotations in The Arabidopsis Information Resource, and there were putative T-DNA insertional mutants available for these genes at the Arabidopsis Biological Resource Center. lno1-1 is one of the mutants that we obtained and characterized in this screen. We have ordered all available putative T-DNA insertional lines (CS16007, CS423371, CS804562, CS810290, CS810299, CS813163, CS813171, CS842534, CS 842651, SALK_037979, SALK_108182, and SALK_108189) in the LNO1 locus (At1G55540). However, we were only able to genotype and find T-DNA insertion in the At1G55540 locus in two lines: CS810299 (lno1-1) and CS842651 (lno1-2). CS16007 was identified as emb1011-1 (Tzafrir et al., 2004), but it was later withdrawn from the database (www.seedgenes.org). The T-DNA insertion in lno1-1 (CS810299) was detected in the second exon by amplifying a PCR fragment with the following primers: forward primer, 5′-CGGAAAGAAGGAGAACGACG-3′, and LB3, 5′-TAGCATCTGAATTTCATAACCAATCTC-3′. The T-DNA insertion in lno1-2 (CS842651) was detected in the promoter by amplifying a PCR fragment with two primers: 5′-CAGTCTTCATCGGAATCACC-3′ and LB3 (see above). Once T-DNA insertions were found in a plant, PCR amplification was used to determine homozygosity by amplifying an endogenous gene fragment across the T-DNA insertion. An endogenous fragment was amplified in lno1-1 with the following primers: forward, 5′-TACGATCTCGAAAACCCTCC-3′, and reverse, 5′-CAGTCTTCATCGGAATCACC-3′, and in lno1-2 with the following primers: forward, 5′-CGAAGCTTCCTTGTATCGTCCTTTGTAGG-3′, and reverse, 5′-CGTCTAGACTTTCAAACTCTTCTCAAGGT-3′. By using the same approach, we found a T-DNA insertion in the 5′ untranslated region and 14 bp upstream of ATG in Atgle1-1 (CS16088, emb1745) by amplifying a PCR fragment with two primers: 5′-GTCTGGTTTGGTTCGTGATT-3′ and LB1, 5′-GCCTTTTCAGAAATGGATAAATAGCC-3′. Arabidopsis plants were grown in growth chambers or a greenhouse under 16 h of light and 8 h of dark at 23°C.
Complementation Assay for the lon1-1 Mutant
The full-length coding region of LNO1 was amplified by PCR using forward primer 5′-CGCTCTAGAATGAGCAGAGTTGAGATTGAAGAA-3′ (XbaI site underlined) and reverse primer 5′-CGCGAGCTCTCATTTTCTCATCTGTGTGAAGAG-3′ (SacI site underlined) and cloned into pBI121 vector (Clontech). The resulting construct was transformed into Agrobacterium tumefaciens strain GV3101 and infiltrated into the LON1/lon1-1 heterozygous plants by floral dipping. Positive transformants were selected based on their resistance to kanamycin on Murashige and Skoog medium, and the transgenic plants in the lon1-1 mutant background were chosen for analysis of seed viability.
Whole-Mount Seed Clearing and Microscopy
Whole-mount immature seeds were dissected from siliques 1 to 6 DAP, cleared in Hoyer’s fluid (70% (w/v) chloral hydrate, 4% (v/v) glycerol, and 5% (v/v) gum arabic), and observed with a Zeiss Axioskop 50 microscope using differential interference contrast optics in the Department of Pathology of the Medical School at Saint Louis University.
Gene Expression Analysis
For examining the expression of LNO1 in the lno1-1 mutant allele, viable (LNO1/LNO1) and aborted (lno1-1/lno1-1) seeds at 10 DAP from the heterozygous LNO1/lno1-1 plants were isolated, and total RNA was extracted. For examining the expression of LNO1 in the lno1-2 mutant allele, seeds of wild-type (LNO1/LNO1) and homozygous mutant (lno1-2/lno1-2) seeds at 10 DAP were isolated, and total RNA was extracted. Gene expression was examined by using semiquantitative RT-PCR using 28 to 30 PCR amplification cycles or quantitative RT-PCR. First-strand cDNA was synthesized using the ProtoScript First Strand cDNA Synthesis Kit (New England Biolabs). PCR product was amplified using SABiosciences’ SYBR Green qPCR Mastermix, and ACTIN was used as an internal control. Quantitative RT-PCR was performed using MJ Research PTC-200 Thermal Cycler and MJ Opticon Monitor Analysis Software (Bio-Rad). The primer pairs for amplifying cDNA of LNO1 were as follows: forward 9, 5′-TACGATCTCGAAAACCCTCC-3′, and reverse 8, 5′-CAGTCTTCATCGGAATCACC-3′. The second set of primer pairs for amplifying cDNA of LNO1 were as follows: forward 4, 5′-GGATGCTTCTGCAGGAGCAA-3′, and reverse 3, 5′-GAGGCCTGATCAGATGACACTTGC-3′. The primer pairs for amplifying cDNA of AtGLE1 were as follows: forward 3, 5′-CCTATGAGTGAACCAAATTGT-3′, and reverse 3, 5′-AAGGAGGTTCCAAAACAATCC-3′.
The LNO1 Promoter:GUS Construct and GUS Histochemical Staining
The 5′ promoter region of LNO1 was PCR amplified with forward primer 5′-CGCGTCGACCTAAACAATGGAGACAATAA-3′, containing a SalI restriction site, and reverse primer 5′-CGCGGATCCGATGAGCCGACGACGGTTCA-3′, containing a BamHI restriction site, and cloned into the binary vector pBI101. The construct was confirmed by sequencing, transformed into A. tumefaciens strain GV3101, and infiltrated into Col-0. Stable transgenic T2 plants were used for GUS staining analysis. GUS staining was performed as described with minor modifications (Stangeland and Salehian, 2002). In short, plant tissues were harvested and immersed into the GUS staining buffer (10 mm sodium phosphate buffer, pH 7.2, 0.5% Triton X-100, 1 mg mL−1 5-bromo-4-chloro-3-indolyl-β-glucuronic acid, and 2 mm potassium ferricyanide) on ice. Samples in the staining buffer were vacuum infiltrated on ice for 20 min and then incubated at 37°C with rotation for 24 h. Tissues were destained using 70% ethanol. Samples were imbibed in 50% glycerol on slides for imaging.
Yeast Two-Hybrid Assay
Yeast (Saccharomyces cerevisiae) two-hybrid experiments were performed using the system described previously (Rea et al., 2012). To make Gal4 DNA activation domain (AD) constructs, the full-length LNO1 was PCR amplified with primers LON1-ADF (5′-CCCGATATCGATGAGCAGAGTTGAGATTGAAGAA-3′; EcoRV site underlined) and LON1-ADR (5′-CCCGTCGACTCATTTTCTCATCTGTGTGAAGAG-3′; SalI site underlined) and inserted into pACT2 vector digested with SmaI and XhoI; the LNO1 N-terminal domain (1–761 amino acids; NTD) coding region was PCR amplified with primers LNO1-NTD-ADF (5′-CCCGGATCCGAATGAGCAGAGTTGAGATTGAAGAA-3′; BamHI site underlined) and LNO1-NTD-ADR (5′-CCCGTCGACTCATATAAACGCGCAAGAATCCTT-3′; SalI site underlined) and inserted into pACT2 digested with BamHI and XhoI; the LNO1ΔNTD fragment coding region was PCR amplified with primers LNO1-CCC-ADF (5′-CCCGATATCGCTGAAAAGCAATGTTGAAGAACTG-3′; EcoRV site underlined) and LON1-ADR and inserted into pACT2 digested with SmaI and XhoI. For the Gal4 binding domain (BD) constructs, the Arabidopsis LOS4 coding region was PCR amplified with primers LOS4-BDF (5′-CCCGATATCGATGGCGGATACGGTAGAGAAAGTT-3′; EcoRV site underlined) and LOS4-BDR (5′-CCCCTCGAGTCACTCGTCCAGCAGGCCAGCTTC-3′; XhoI site underlined) and inserted into pAS1 vector digested with SmaI and SalI. Positive transformants were selected on complete minimal (CM) dropout medium minus Trp and Leu. Positive interactions were selected on CM medium minus Trp, Leu, and His containing 20 mm 3-aminotriazole and were confirmed by filter lift assay (Rea et al., 2012).
To further confirm the interactions, a vector-swap experiment was performed. The full-length LNO1 PCR amplified using primers LNO1-BDF (5′-CCCCATATGAGCAGAGTTGAGATTGAAGAA-3′; NdeI site underlined) and LNO1-BDR (5′-CCCGTCGACTCATTTTCTCATCTGTGTGAAGAG-3′; SalI site underlined), the LNO1-NTD coding region PCR amplified using primers LNO1-BDF and LNO1-NTD-BDR (5′-CCCGTCGACTCATATAAACGCGCAAGAATCCTT-3′; SalI site underlined), and the LNO1ΔNTD coding region PCR amplified using primers LNO1-CCC-BDF (5′-CCCCATATGCTGAAAAGCAATGTTGAAGAACTG-3′; NdeI site underlined) and LNO1-BDR were cloned into pAS1 vector to make pAS1-LNO1, pAS1-NTD, and pAS1-LNO1ΔNTD constructs, respectively. The Arabidopsis LOS4 coding region was PCR amplified with primers LOS4-ADF (5′-CCCGAATTCGAATGGCGGATACGGTAGAGAAAGTT-3′; EcoRI site underlined) and LOS4-ADR (5′-CCCCTCGAGTCACTCGTCCAGCAGGCCAGCTTC-3′; XhoI site underlined) and inserted into pACT2 vector to make the pACT2-LOS4 construct. Positive transformants and positive interactions were tested as described above.
Yeast Complementation Test
The plasmid DNA of pAS1-LNO1, pAS1-NTD, or pAS1-LNO1ΔNTD was transformed into the yeast nup159 mutant strains KWY1267 (nup159Δ1-456) and KWY1269 (nup159VI→EE). Positive transformants were selected on CM medium minus Trp and Leu. Five microliters of liquid culture in yeast extract peptone dextrose (YPD) medium for each positive transformant was spotted on YPD plates after serial dilutions. Complementation tests were performed at 37°C (nonpermissive temperature). Control plates were incubated at 23°C for growth.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. LNO1 interacts with LOS4 in yeast.
Acknowledgments
We thank colleagues in the Xiao laboratory for discussion and Robert L. Fischer for critical reading of the manuscript and valuable comments. We thank Paul Smelcer for maintenance of Arabidopsis plants, the Arabidopsis Biological Resource Center at Ohio State University for providing seeds, Karsten Weis for providing the yeast temperature-sensitive mutant nup159, Jan Ryerse and Barbara Nagel for providing assistance with differential interference contrast microscopy, and Matthew Rea, Brian Downes, and Yuqi Wang for technical assistance and helpful discussions.
Glossary
- NPC
nuclear pore complex
- FG
Phe-Gly
- mRNP
mRNA protein
- RT
reverse transcription
- DAP
days after pollination
- SD
synthetic dextrose
- YPD
yeast peptone dextrose
- Col-0
ecotype Columbia
- cDNA
complementary DNA
References
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 109–120 [DOI] [PubMed] [Google Scholar]
- Alcázar-Román AR, Tran EJ, Guo SL, Wente SR. (2006) Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat Cell Biol 8: 711–716 [DOI] [PubMed] [Google Scholar]
- Autran D, Baroux C, Raissig MT, Lenormand T, Wittig M, Grob S, Steimer A, Barann M, Klostermeier UC, Leblanc O, et al. (2011) Maternal epigenetic pathways control parental contributions to Arabidopsis early embryogenesis. Cell 145: 707–719 [DOI] [PubMed] [Google Scholar]
- Bastos R, Ribas de Pouplana L, Enarson M, Bodoor K, Burke B. (1997) Nup84, a novel nucleoporin that is associated with CAN/Nup214 on the cytoplasmic face of the nuclear pore complex. J Cell Biol 137: 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M, Nawy T, Giglione C, Galli M, Meinnel T, Lukowitz W. (2009) Paternal control of embryonic patterning in Arabidopsis thaliana. Science 323: 1485–1488 [DOI] [PubMed] [Google Scholar]
- Berleth T, Jurgens G. (1993) The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118: 575–587 [Google Scholar]
- Boer JM, van Deursen JMA, Croes HJ, Fransen JAM, Grosveld GC. (1997) The nucleoporin CAN/Nup214 binds to both the cytoplasmic and the nucleoplasmic sides of the nuclear pore complex in overexpressing cells. Exp Cell Res 232: 182–185 [DOI] [PubMed] [Google Scholar]
- Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. (2008) Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell 14: 867–876 [DOI] [PubMed] [Google Scholar]
- Brohawn SG, Partridge JR, Whittle JR, Schwartz TU. (2009) The nuclear pore complex has entered the atomic age. Structure 17: 1156–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A, Chatfield S, Provart N, Berleth T. (2009) Embryogenesis: pattern formation from a single cell. The Arabidopsis Book 7: e0126, doi/10/1199/tab.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrouwe D, De Block M. (1992) In-situ enzyme histochemistry on plastic-embedded plant material: the development of an artefact-free beta-glucuronidase assay. Plant J 2: 261–266 [Google Scholar]
- Del Priore V, Heath C, Snay C, MacMillan A, Gorsch L, Dagher S, Cole C. (1997) A structure/function analysis of Rat7p/Nup159p, an essential nucleoporin of Saccharomyces cerevisiae. J Cell Sci 110: 2987–2999 [DOI] [PubMed] [Google Scholar]
- Dong CH, Hu X, Tang W, Zheng X, Kim YS, Lee BH, Zhu JK. (2006) A putative Arabidopsis nucleoporin, AtNUP160, is critical for RNA export and required for plant tolerance to cold stress. Mol Cell Biol 26: 9533–9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doye V, Hurt E. (1997) From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol 9: 401–411 [DOI] [PubMed] [Google Scholar]
- Drews GN, Wang D, Steffen JG, Schumaker KS, Yadegari R. (2011) Identification of genes expressed in the angiosperm female gametophyte. J Exp Bot 62: 1593–1599 [DOI] [PubMed] [Google Scholar]
- Dultz E, Zanin E, Wurzenberger C, Braun M, Rabut G, Sironi L, Ellenberg J. (2008) Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J Cell Biol 180: 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn AE, Lee W-H, Elledge SJ. (1993) The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev 7: 555–569 [DOI] [PubMed] [Google Scholar]
- Friml J. (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Galy V, Mattaj IW, Askjaer P. (2003) Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol Biol Cell 14: 5104–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, de Paiva G, Yadegari R. (1994) Plant embryogenesis: zygote to seed. Science 266: 605–614 [DOI] [PubMed] [Google Scholar]
- Gong Z, Dong CH, Lee H, Zhu J, Xiong L, Gong D, Stevenson B, Zhu JK. (2005) A DEAD box RNA helicase is essential for mRNA export and important for development and stress responses in Arabidopsis. Plant Cell 17: 256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsch LC, Dockendorff TC, Cole CN. (1995) A conditional allele of the novel repeat-containing yeast nucleoporin RAT7/NUP159 causes both rapid cessation of mRNA export and reversible clustering of nuclear pore complexes. J Cell Biol 129: 939–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668 [DOI] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jürgens G. (1999) The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 126: 1387–1395 [DOI] [PubMed] [Google Scholar]
- Hetzer MW, Wente SR. (2009) Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Dev Cell 17: 606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CA, Colot HV, Stafford P, Cole CN. (1999) Rat8p/Dbp5p is a shuttling transport factor that interacts with Rat7p/Nup159p and Gle1p and suppresses the mRNA export defect of xpo1-1 cells. EMBO J 18: 5778–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CA, Tran EJ, Noble KN, Alcazar-Roman AR, Ben-Yishay R, Scarcelli JJ, Folkmann AW, Shav-Tal Y, Wente SR, Cole CN. (2011) The Dbp5 cycle at the nuclear pore complex during mRNA export. I. dbp5 mutants with defects in RNA binding and ATP hydrolysis define key steps for Nup159 and Gle1. Genes Dev 25: 1052–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TF, Shin J, Uzawa R, Silva P, Cohen S, Bauer MJ, Hashimoto M, Kirkbride RC, Harada JJ, Zilberman D, et al. (2011) Regulation of imprinted gene expression in Arabidopsis endosperm. Proc Natl Acad Sci USA 108: 1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz ME, Strambio-de-Castillia C, Blobel G. (1998) Two yeast nuclear pore complex proteins involved in mRNA export form a cytoplasmically oriented subcomplex. Proc Natl Acad Sci USA 95: 11241–11245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y, Mongkolsiriwatana C, Veley KM, Kim SY, Michaels SD. (2007) The nuclear pore protein AtTPR is required for RNA homeostasis, flowering time, and auxin signaling. Plant Physiol 144: 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Bayer M, Lukowitz W. (2011) Taking the very first steps: from polarity to axial domains in the early Arabidopsis embryo. J Exp Bot 62: 1687–1697 [DOI] [PubMed] [Google Scholar]
- Kalverda B, Pickersgill H, Shloma VV, Fornerod M. (2010) Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 140: 360–371 [DOI] [PubMed] [Google Scholar]
- Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EM, Miwa H, Downie JA, James EK, Felle HH, Haaning LL, et al. (2006) A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA 103: 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer D, Wozniak RW, Blobel G, Radu A. (1994) The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc Natl Acad Sci USA 91: 1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer DM, Strambio-de-Castillia C, Blobel G, Rout MP. (1995) The essential yeast nucleoporin NUP159 is located on the cytoplasmic side of the nuclear pore complex and serves in karyopherin-mediated binding of transport substrate. J Biol Chem 270: 19017–19021 [DOI] [PubMed] [Google Scholar]
- Le BH, Cheng C, Bui AQ, Wagmaister JA, Henry KF, Pelletier J, Kwong L, Belmonte M, Kirkbride R, Horvath S, et al. (2010) Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc Natl Acad Sci USA 107: 8063–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee HS, Wi SJ, Park KY, Schmit AC, Pai HS. (2009) Dual functions of Nicotiana benthamiana Rae1 in interphase and mitosis. Plant J 59: 278–291 [DOI] [PubMed] [Google Scholar]
- Lotan T, Ohto M-A, Yee KM, West MAL, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ. (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93: 1195–1205 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. (2004) A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell 116: 109–119 [DOI] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A. (2000) Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci USA 97: 10637–10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu F, Alves A, Anderson K, Doye V, Lacy E. (2008) Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev Cell 14: 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier I, Brkljacic J. (2009) Adding pieces to the puzzling plant nuclear envelope. Curr Opin Plant Biol 12: 752–759 [DOI] [PubMed] [Google Scholar]
- Meinke D, Muralla R, Sweeney C, Dickerman A. (2008) Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci 13: 483–491 [DOI] [PubMed] [Google Scholar]
- Napetschnig J, Kassube SA, Debler EW, Wong RW, Blobel G, Hoelz A. (2009) Structural and functional analysis of the interaction between the nucleoporin Nup214 and the DEAD-box helicase Ddx19. Proc Natl Acad Sci USA 106: 3089–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy T, Bayer M, Mravec J, Friml J, Birnbaum KD, Lukowitz W. (2010) The GATA factor HANABA TARANU is required to position the proembryo boundary in the early Arabidopsis embryo. Dev Cell 19: 103–113 [DOI] [PubMed] [Google Scholar]
- Noble KN, Tran EJ, Alcázar-Román AR, Hodge CA, Cole CN, Wente SR. (2011) The Dbp5 cycle at the nuclear pore complex during mRNA export. II. Nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1. Genes Dev 25: 1065–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodine MD, Bartel DP. (2012) Maternal and paternal genomes contribute equally to the transcriptome of early plant embryos. Nature 482: 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodine MD, Yadegari R, Tax FE. (2007) RPK1 and TOAD2 are two receptor-like kinases redundantly required for Arabidopsis embryonic pattern formation. Dev Cell 12: 943–956 [DOI] [PubMed] [Google Scholar]
- Onischenko E, Weis K. (2011) Nuclear pore complex: a coat specifically tailored for the nuclear envelope. Curr Opin Cell Biol 23: 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Ward S, Cernac A, Dharmasiri S, Estelle M. (2006) The Arabidopsis SUPPRESSOR OF AUXIN RESISTANCE proteins are nucleoporins with an important role in hormone signaling and development. Plant Cell 18: 1590–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE. (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17: 61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasala BA, Ramos C, Harel A, Forbes DJ. (2008) Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol Biol Cell 19: 3982–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea M, Zheng W, Chen M, Braud C, Bhangu D, Rognan TN, Xiao W. (2012) Histone H1 affects gene imprinting and DNA methylation in Arabidopsis. Plant J 71: 776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Yoshikawa M, Yano K, Miwa H, Uchida H, Asamizu E, Sato S, Tabata S, Imaizumi-Anraku H, Umehara Y, et al. (2007) NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19: 610–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, Benfey PN. (1999) Asymmetric cell division in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 505–537 [DOI] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. (2010) MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916 [DOI] [PubMed] [Google Scholar]
- Schmitt C, von Kobbe C, Bachi A, Panté N, Rodrigues JP, Boscheron C, Rigaut G, Wilm M, Séraphin B, Carmo-Fonseca M, et al. (1999) Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p. EMBO J 18: 4332–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Weigel D, Yanofsky MF. (1999) The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J 20: 259–263 [DOI] [PubMed] [Google Scholar]
- Stangeland B, Salehian Z. (2002) An improved clearing method for GUS assay in Arabidopsis endosperm and seed. Plant Mol Biol Rep 20: 107–114 [Google Scholar]
- Strambio-De-Castillia C, Niepel M, Rout MP. (2010) The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol 11: 490–501 [DOI] [PubMed] [Google Scholar]
- Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I. (2010) Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell 22: 4084–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Hara-Nishimura I. (2011) Involvement of the nuclear pore complex in morphology of the plant nucleus. Nucleus 2: 168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D, et al. (2004) Identification of genes required for embryo development in Arabidopsis. Plant Physiol 135: 1206–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J, Boer J, Kasper L, Grosveld G. (1996) G2 arrest and impaired nucleocytoplasmic transport in mouse embryos lacking the proto-oncogene CAN/Nup214. EMBO J 15: 5574–5583 [PMC free article] [PubMed] [Google Scholar]
- Vernon DM, Meinke DW. (1994) Embryogenic transformation of the suspensor in twin, a polyembryonic mutant of Arabidopsis. Dev Biol 165: 566–573 [DOI] [PubMed] [Google Scholar]
- von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, Grosveld G. (1992a) The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol 12: 1687–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lindern M, van Baal S, Wiegant J, Raap A, Hagemeijer A, Grosveld G. (1992b) Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol Cell Biol 12: 3346–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moeller H, Basquin C, Conti E. (2009) The mRNA export protein DBP5 binds RNA and the cytoplasmic nucleoporin NUP214 in a mutually exclusive manner. Nat Struct Mol Biol 16: 247–254 [DOI] [PubMed] [Google Scholar]
- Waki T, Hiki T, Watanabe R, Hashimoto T, Nakajima K. (2011) The Arabidopsis RWP-RK protein RKD4 triggers gene expression and pattern formation in early embryogenesis. Curr Biol 21: 1277–1281 [DOI] [PubMed] [Google Scholar]
- Wang Y, Marotti LA, Jr, Lee MJ, Dohlman HG. (2005) Differential regulation of G protein alpha subunit trafficking by mono- and polyubiquitination. J Biol Chem 280: 284–291 [DOI] [PubMed] [Google Scholar]
- Weijers D, Sauer M, Meurette O, Friml J, Ljung K, Sandberg G, Hooykaas P, Offringa R. (2005) Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17: 2517–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich CS, Erzberger JP, Berger JM, Weis K. (2004) The N-terminal domain of Nup159 forms a beta-propeller that functions in mRNA export by tethering the helicase Dbp5 to the nuclear pore. Mol Cell 16: 749–760 [DOI] [PubMed] [Google Scholar]
- Wiermer M, Cheng YT, Imkampe J, Li M, Wang D, Lipka V, Li X. (2012) Putative members of the Arabidopsis Nup107-160 nuclear pore sub-complex contribute to pathogen defense. Plant J 70: 796–808 [DOI] [PubMed] [Google Scholar]
- Wiermer M, Germain H, Cheng YT, García AV, Parker JE, Li X. (2010) Nucleoporin MOS7/Nup88 contributes to plant immunity and nuclear accumulation of defense regulators. Nucleus 1: 332–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Custard KD, Brown RC, Lemmon BE, Harada JJ, Goldberg RB, Fischer RL. (2006) DNA methylation is critical for Arabidopsis embryogenesis and seed viability. Plant Cell 18: 805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Gehring M, Choi Y, Margossian L, Pu H, Harada JJ, Goldberg RB, Pennell RI, Fischer RL. (2003) Imprinting of the MEA Polycomb gene is controlled by antagonism between MET1 methyltransferase and DME glycosylase. Dev Cell 5: 891–901 [DOI] [PubMed] [Google Scholar]
- Xu XM, Rose A, Muthuswamy S, Jeong SY, Venkatakrishnan S, Zhao Q, Meier I. (2007) NUCLEAR PORE ANCHOR, the Arabidopsis homolog of Tpr/Mlp1/Mlp2/megator, is involved in mRNA export and SUMO homeostasis and affects diverse aspects of plant development. Plant Cell 19: 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, Hogan P, Sundaresan V. (2005) Analysis of the female gametophyte transcriptome of Arabidopsis by comparative expression profiling. Plant Physiol 139: 1853–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Somerville CR. (1997) Suspensor-derived polyembryony caused by altered expression of valyl-tRNA synthetase in the twn2 mutant of Arabidopsis. Proc Natl Acad Sci USA 94: 7349–7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X. (2005) A putative nucleoporin 96 is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1,constitutive 1. Plant Cell 17: 1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Meier I. (2011) Identification and characterization of the Arabidopsis FG-repeat nucleoporin Nup62. Plant Signal Behav 6: 330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]