Abstract
Cadmium ions are notorious environmental pollutants. To adapt to cadmium-induced deleterious effects plants have developed sophisticated defense mechanisms. However, the signaling pathways underlying the plant response to cadmium are still elusive. Our data demonstrate that SnRK2s (for SNF1-related protein kinase2) are transiently activated during cadmium exposure and are involved in the regulation of plant response to this stress. Analysis of tobacco (Nicotiana tabacum) Osmotic Stress-Activated Protein Kinase activity in tobacco Bright Yellow 2 cells indicates that reactive oxygen species (ROS) and nitric oxide, produced mainly via an l-arginine-dependent process, contribute to the kinase activation in response to cadmium. SnRK2.4 is the closest homolog of tobacco Osmotic Stress-Activated Protein Kinase in Arabidopsis (Arabidopsis thaliana). Comparative analysis of seedling growth of snrk2.4 knockout mutants versus wild-type Arabidopsis suggests that SnRK2.4 is involved in the inhibition of root growth triggered by cadmium; the mutants were more tolerant to the stress. Measurements of the level of three major species of phytochelatins (PCs) in roots of plants exposed to Cd2+ showed a similar (PC2, PC4) or lower (PC3) concentration in snrk2.4 mutants in comparison to wild-type plants. These results indicate that the enhanced tolerance of the mutants does not result from a difference in the PCs level. Additionally, we have analyzed ROS accumulation in roots subjected to Cd2+ treatment. Our data show significantly lower Cd2+-induced ROS accumulation in the mutants’ roots. Concluding, the obtained results indicate that SnRK2s play a role in the regulation of plant tolerance to cadmium, most probably by controlling ROS accumulation triggered by cadmium ions.
Cadmium is one of the most toxic soil pollutants. Cadmium ions accumulate in plants and affect, via the food chain, animal and human health. In plants, cadmium is taken up by roots and is transported to aerial organs, leading to chromosomal aberrations, growth reduction, and inhibition of photosynthesis, transpiration, nitrogen metabolism, nutrient and water uptake, eventually causing plant death (for review, see DalCorso et al., 2008). Plants are challenged not only by cadmium ions themselves, but also by Cd2+-induced harmful effects including oxidative stress (Schützendübel et al., 2001; Olmos et al., 2003; Cho and Seo, 2005; Sharma and Dietz, 2009). The extent of the detrimental effects on plant growth and metabolism depends on the level of cadmium ions present in the surrounding environment and on the plant’s sensitivity to heavy metal stress.
Tolerant plants avoid heavy metal uptake and/or induce the expression of genes encoding products involved, directly or indirectly, in heavy metal binding and removal from potentially sensitive sites, by sequestration or efflux (Clemens, 2006). The best-characterized heavy metal binding ligands in plants are thiol-containing compounds metallothioneins and phytochelatins (PCs), whose production is stimulated by Cd2+. PCs bind metal ions and transport them to the vacuole, thus reducing the toxicity of the metal in the cytosol (for review, see Cobbett, 2000; Cobbett and Goldsbrough, 2002). PCs are synthesized from reduced glutathione (GSH). Therefore, production of compounds involved in cadmium detoxification and, at the same time, in cadmium tolerance closely depends on sulfur metabolism. So far, our knowledge on the cellular processes induced by cadmium that lead to changes in sulfur metabolism in plants has been rather limited.
Protein kinases and phosphatases are considered major signal transduction elements. However, until now only a few of them have been described to be involved in cadmium stress response or sulfur metabolism. For instance, excessive amounts of cadmium or copper activate mitogen-activated protein kinases (MAPKs) in Medicago sativa (Jonak et al., 2004), rice (Oryza sativa; Yeh et al., 2007), and Arabidopsis (Arabidopsis thaliana; Liu et al., 2010). Studies on rice MAPKs involved in heavy metal stress response indicate that the activity of these kinases depends on the oxidative stress induced by Cd2+. Moreover, Yeh et al. (2007) suggested that the activation of MAPKs in rice by cadmium or copper required the activity of calcium-dependent protein kinase (CDPK) and PI3 kinase, since the MAPK pathways involved in cadmium and copper stress response could be inhibited by a CDPK antagonist (W7) or a PI3 kinase inhibitor (wortmannin). However, so far the function of the identified kinases in plant adaptation to heavy metal pollution has not been established. There is some information concerning an involvement of CDPK in sulfur metabolism (Liu et al., 2006). Soybean (Glycine max) Ser acetyltransferase (GmSerat2;1), the enzyme that catalyzes the first reaction in the biosynthesis of Cys from Ser, is phosphorylated by CDPK. The phosphorylation has no effect on GmSerat2;1 activity, but it renders the enzyme insensitive to the feedback inhibition by Cys (Liu et al., 2006). There is growing evidence that SnRK2s (for SNF1-related protein kinase2) play a role in the regulation of sulfur metabolism. Most information showing a connection between SnRK2s and sulfur metabolism comes from experiments on the lower plant Chlamydomonas reinhardtii (Davies et al., 1999; Irihimovitch and Stern, 2006; González-Ballester et al., 2008, 2010). SNRK2.1 is considered a general regulator of S-responsive gene expression in C. reinhardtii (González-Ballester et al., 2008).
In higher plants the SnRK2 family members are known to be involved in plant response to drought, salinity, and in abscisic acid (ABA)-dependent plant development (Boudsocq and Laurière, 2005; Fujii et al., 2007, 2011; Fujii and Zhu, 2009; Fujita et al., 2009; Nakashima et al., 2009; Kulik et al., 2011). Ten members of the SnRK2 family have been identified in Arabidopsis and in rice (Boudsocq et al., 2004; Kobayashi et al., 2004). All of them, except SnRK2.9 from Arabidopsis, are rapidly activated by treatment with different osmolytes, such as Suc, mannitol, sorbitol, and NaCl, and some of them also by ABA. Results presented by Kimura et al. (2006) suggest that in Arabidopsis, similarly to C. reinhardtii, some SnRK2s are involved in the regulation of S-responsive gene expression and O-acetyl-l-Ser accumulation under limited sulfur supply, indicating that also higher plants’ SnRK2s could be involved in sulfur metabolism.
As it was mentioned before, oxidative stress induced by cadmium ions significantly contributes to the metal toxicity. Reactive oxygen species (ROS) can be produced in many different reactions in various compartments of the cell in response to cadmium (Romero-Puertas et al., 2004; Heyno et al., 2008; Tamás et al., 2009). The best-characterized ROS-generating enzymes that take part in the response to cadmium are the plasma-membrane-bound NADPH oxidases (Olmos et al., 2003; Romero-Puertas et al., 2004; Garnier et al., 2006). There are some indications that plant NADPH oxidases are phosphorylated by SnRK2s (Sirichandra et al., 2009), therefore it is highly plausible that SnRK2s play a role in the regulation of ROS accumulation in plants subjected to cadmium stress. Taking into consideration all facts mentioned above we hypothesized that SnRK2s could be involved in the plant response to stress induced by cadmium ions. To verify this conjecture, we analyzed the activity and potential role of selected SnRK2s, in tobacco (Nicotiana tabacum) cells and Arabidopsis plants, in the response to cadmium ions.
RESULTS
CdCl2 Activates MAPK and Tobacco Osmotic Stress-Activated Protein Kinase Pathways in Tobacco Bright Yellow 2 Cells
To identify protein kinases involved in the plant response to cadmium ions, as a first approach we monitored the activity of protein kinases phosphorylating myelin basic protein (MBP) in Bright Yellow 2 (BY-2) tobacco cells exposed to increasing concentrations of CdCl2 (0–1,000 μm). The kinase activity in protein extracts prepared from BY-2 cells, not treated and treated with CdCl2 for 30 min, was analyzed by in-gel kinase activity assay. In tobacco cells exposed to Cd2+ at concentrations higher than 10 μm, at least two different protein kinases phosphorylating MBP, with molecular masses of 47 and 42 kD were activated (Fig. 1A). Since the activity of both kinases was relatively high after cell treatment with 50 or 100 μm Cd2+ and these concentrations were frequently used by other researchers investigating the activity of protein kinases potentially involved in plant response to Cd2+ (Jonak et al., 2004; Yeh et al., 2007; Liu et al., 2010), 100 μm CdCl2 was chosen for further experiments. The time course of the activation revealed that the kinases exhibited maximal activity when the cells were treated with 100 μm CdCl2 for about 15 to 60 min (Fig. 1B). Because earlier reports indicated that kinases belonging to the MAPK family were activated in similar conditions (Jonak et al., 2004; Yeh et al., 2007), we checked whether the kinases activated by Cd2+ in our experimental conditions were in fact MAPKs. For this purpose, we performed an immunoblotting assay using specific antibodies recognizing the double phosphorylated motif TEY present in active MAPKs. As shown in Figure 1C, cadmium ions cause activation of a 47-kD MAPK.
Figure 1.
MAPK and SnRK2 are activated in plant cells in response to cadmium stress. Six-day-old BY-2 suspension cell cultures were treated with various concentrations of CdCl2 (0–1,000 μm; A) or CdSO4 (0–200 μm; G) for 30 min, or with 100 μm CdCl2 for various times (0–90 min; B–F). Protein kinase activity in cells untreated and treated with the stressor was monitored by in-gel kinase activity assay with MBP as a substrate (A, B, D, and G), or by western blotting (C and E). D and G, Activity of NtOSAK analyzed by immunocomplex kinase activity assay using specific anti-C-terminal NtOSAK antibodies. C, Immunoblot probed with antiphospho-p44/42 MAPK antibodies. E, Immunoblot probed with anti-Ser-158(P) antibodies. F, NtOSAK protein level determined by western blotting with specific anti-C-terminal NtOSAK antibodies.
Tobacco Osmotic Stress-Activated Protein Kinase (NtOSAK) is a member of the SnRK2 family that has been shown to be strongly activated in response to osmotic stress (Kelner et al., 2004). Because CdCl2 treatment triggers oxidative (Schützendübel et al., 2001; Olmos et al., 2003; Martínez Domínguez et al., 2010) and osmotic stresses (Perfus-Barbeoch et al., 2002) in plant cells, we suspected that the 42-kD kinase activated by cadmium ions could be a SnRK2 protein kinase, most probably NtOSAK. To verify this hypothesis we monitored the activity of NtOSAK in BY-2 cells exposed to 100 μm CdCl2 by immunocomplex-kinase activity assay using specific anti-NtOSAK antibodies (Fig. 1D). Additionally, we analyzed the phosphorylation of Ser-158 in NtOSAK, which is required for the kinase activity (Burza et al., 2006), using specific antibodies recognizing phosphorylated Ser-158 (Fig. 1E). In parallel, the protein level of NtOSAK was monitored by western blotting with specific anti-NtOSAK antibodies recognizing a C-terminal peptide of the kinase (Fig. 1F). Results of those experiments confirmed that NtOSAK is transiently activated in BY-2 cells subjected to CdCl2 treatment. To be sure that NtOSAK activation depends on the presence of cadmium ions and not on the type of cadmium salt applied we analyzed the kinase activity in BY-2 cells treated with CdSO4 in parallel to CdCl2 treatment (Fig. 1G). In both cases we observed similar activation of NtOSAK.
Additionally, we checked the effect of Cu2+ ions on NtOSAK activity by monitoring NtOSAK activation in BY-2 cells exposed to CuSO4 (in concentration up to 200 μm). In this case the kinase activation was significantly lower than in cells treated with cadmium salts (Supplemental Fig. S1).
ROS and Nitric Oxide Contribute to NtOSAK Activation in Response to CdCl2
It has previously been shown that cadmium treatment induces ROS and nitric oxide (NO) production in plant cells (Olmos et al., 2003; Garnier et al., 2006; Besson-Bard et al., 2009; De Michele et al., 2009; Arasimowicz-Jelonek et al., 2011). To check whether NO or ROS participate in the NtOSAK activation in response to cadmium, the kinase activity was analyzed in BY-2 cells pretreated with the NO scavenger 2-(4-carboxyphenyl)-4,5-dhydro-4,4,5,5-tetramethyl-1H-imidazol-1yl-oxy-3-oxide (cPTIO) or with the antioxidant enzyme catalase, prior to 100 μm CdCl2 exposure. We observed a substantial inhibition of the Cd2+-dependent NtOSAK activation in those cells, when compared with the control experiment (without cPTIO or catalase pretreatment). The results indicate that both ROS and NO contribute to the NtOSAK activation in response to cadmium stress (Fig. 2, A–C).
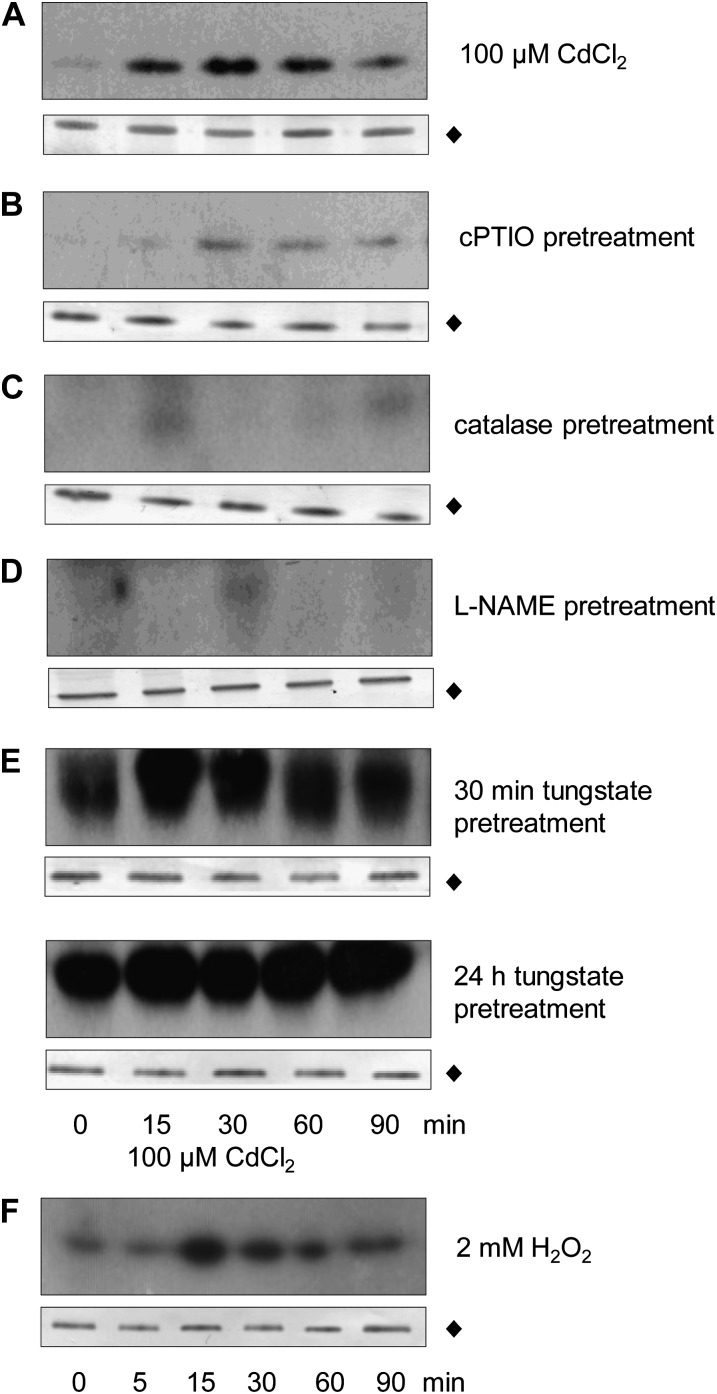
Figure 2.
Cadmium induces NtOSAK activation in NO- and ROS-dependent manner. BY-2 cells were treated with 100 μm CdCl2 without (A) or with pretreatment with: 500 μm cPTIO for 1 h (B), 1,000 units/mL catalase for 30 min (C), 300 μm l-NAME for 10 min (D), 1 mm sodium tungstate for 30 min or 24 h (E). NtOSAK activity in BY-2 cells was detected by immunocomplex in-gel kinase activity assay using specific anti-C-terminal NtOSAK antibodies and MBP as a substrate. Additionally, NtOSAK activity was monitored by immunocomplex kinase activity assay in BY-2 cells treated with 2 mm H2O2 for various times (F). NtOSAK protein level determined by western blotting with specific anti-C-terminal NtOSAK antibodies in protein extracts before immunoprecipitation (black diamond).
In plant cells NO can be produced via nonenzymatic reaction(s) or through at least two enzymatic pathways: from nitrate/nitrite by nitrate reductase (NR), and from l-Arg by enzyme(s) similar to mammalian NO synthase (NOS; del Río et al., 2004; Besson-Bard et al., 2008). To characterize the route that leads to the Cd2+-dependent NO generation and NtOSAK activation in BY-2 cells, we analyzed the kinase activity induced by 100 μm CdCl2 in BY-2 cells pretreated with the mammalian NOS inhibitor N-nitro-l-Arg-methyl ester (l-NAME) or with the NR inhibitor tungstate before CdCl2 addition. Results of those experiments revealed that l-NAME inhibited the Cd2+-induced NtOSAK activation (Fig. 2D), whereas this effect was not observed in cells treated with tungstate. Unexpectedly, in cells treated with tungstate, the NtOSAK activity was stimulated even in the absence of cadmium (Fig. 2E). These results indicate that NtOSAK activation in response to cadmium ions involves l-Arg-derived NO.
NtOSAK Undergoes Activation in Tobacco Cells in Response to Oxidative Stress
Considering that NO and ROS are involved in the NtOSAK activation in response to cadmium ions, we addressed the question whether the kinase activity in tobacco cells is triggered by these signaling molecules. As it had previously been established that NtOSAK is activated in response to treatment with a NO donor (diethylamine/NO; Lamotte et al., 2006; Wawer et al., 2010), we focused our studies on the effect of ROS on the kinase activity. To determine whether NtOSAK is activated by oxidative stress, the kinase activity was monitored in tobacco BY-2 cells exposed to 2 mm hydrogen peroxide (H2O2). Addition of H2O2 to BY-2 cells in suspension caused activation of NtOSAK (Fig. 2F). The maximal activity of NtOSAK was observed 15 min after the addition of H2O2 and returned to the control value within 90 min. It should be mentioned that in some experiments the elevation of NtOSAK activity took place as late as 30 min after the stressor application and reached a maximum by 60 min (data not shown). This variability could be due to slightly different culture conditions of BY-2 cells and also to H2O2 instability.
SnRK2.4 and SnRK2.10 Are Activated in Response to Cadmium
To investigate the role of SnRK2s in the plant response to cadmium, we turned to Arabidopsis as a model species, since its whole genome is sequenced and knockout mutants of several SnRK2s are available.
According to phylogenetical analysis, the closest homologs of NtOSAK in Arabidopsis are SnRK2.4 and SnRK2.10 (Supplemental Fig. S2A). Therefore, we first studied the activity of these enzymes in Arabidopsis seedlings exposed to CdCl2. The kinase activity was analyzed by immunocomplex kinase activity assay using anti-NtOSAK/SnRK2.4/SnRK2.10 antibodies raised against an N-terminal peptide of SnRK2.4, SnRK2.10, and NtOSAK. Indeed, the first 11 N-terminal amino acids of SnRK2.4, SnRK2.10, and NtOSAK are identical (Supplemental Fig. S2B). Since in other SnRK2s, but not in any other Arabidopsis protein, sequences exhibiting high similarity to the antigen are present, the antibodies can to some extent recognize also other SnRK2s. The specificity of the antibodies used is presented in Supplemental Figure S3.
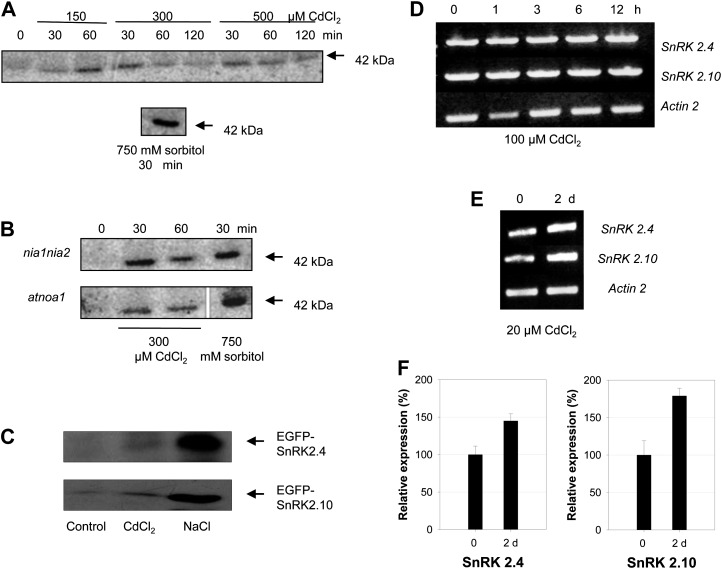
Analysis of the kinase activity by immunocomplex-activity assay showed activation of SnRK2(s) in 10-d-old Arabidopsis seedlings exposed to CdCl2 and to sorbitol, used as a positive control (Fig. 3A). To investigate whether the activation is due to NO produced via the NR or AtNOA1 pathways, we studied Cd2+-dependent SnRK2 activation in hydroponically grown seedlings of nia1nia2 (double knockout mutant of NR) and atnoa1 Arabidopsis T-DNA insertion mutants. The atnoa1 and nia1nia2 mutants had previously been shown to produce less NO in response to several biotic as well as abiotic stresses (Besson-Bard et al., 2008; Zhao et al., 2009; Hao et al., 2010; Lozano-Juste and León, 2010; Sun et al., 2010; Xuan et al., 2010). In both nia1nia2 and atnoa1 seedlings, we observed activation of SnRK2(s) in response to CdCl2 treatment (Fig. 3B). These results indicate that neither NR nor AtNOA1 contribute to the SnRK2 activation in Arabidopsis seedlings exposed to Cd2+.
Figure 3.
Effect of cadmium ions on SnRK2.4 and SnRK2.10 activity and on SnRK2.4 and SnRK2.10 transcript level. Ten-day-old seedlings of Arabidopsis wild-type Col-0, as well as insertion mutants nia1nia2 and atnoa1 were treated with 750 mm sorbitol or with different concentration of CdCl2: 150, 300, or 500 μm Col-0 (A) and 300 μm nia1nia2 and atnoa1 (B). Activity of SnRK2.4/SnRK2.10 in seedling extracts was monitored by immunocomplex in-gel kinase activity assay using specific antibodies against N-terminal peptide of both kinases and MBP as a substrate. C, EGFP-SnRK2.4 and EGFP-SnRK2.10 were transiently expressed in Arabidopsis T87 protoplasts. Protoplasts were treated with 200 μm CdCl2 or 300 mm NaCl for 30 min and activity of EGFP-SnRK2.4 and EGFP-SnRK2.10 was analyzed by in-gel kinase activity assay with MBP as a substrate. D, Ten-day-old Col-0 Arabidopsis seedlings were treated with 100 μm CdCl2 for various time (0–12 h), total RNA was isolated and transcript level of SnRK2.4 and SnRK2.10 was analyzed by semiquantitative RT-PCR. As a control Actin2 transcript level was monitored. E, Four-week-old Col-0 Arabidopsis plants grown in hydroponic culture were treated with 20 μm cadmium chloride for 2 d and transcript level of SnRK2.4 and SnRK2.10 was monitored in plant roots by RT-PCR. The results were confirmed by qRT-PCR (F).
To individually analyze the activation of SnRK2.4 and SnRK2.10, the closest homologs of NtOSAK, a transient expression system was applied. We produced either of the two kinases as an EGFP-fusion protein (EGFP-SnRK2.4 or EGFP-SnRK2.10), in Arabidopsis protoplasts. Changes in the kinase activity of each fusion protein in response to 200 μm CdCl2 or 300 mm NaCl were monitored by in-gel kinase activity assay using MBP as a substrate. The results indicated that both kinases were slightly activated by cadmium, albeit their activation was much lower than the activation observed in response to salinity stress (Fig. 3C).
SnRK2.4 and SnRK2.10 Expression in Response to Cd2+
The level of SnRK2.4 and SnRK2.10 transcripts was analyzed by reverse transcription (RT)-PCR in 10-d-old Arabidopsis seedlings untreated and treated with 100 μm CdCl2 for various time (0, 1, 3, 6, 12 h). We did not observe any significant changes in the amount of the SnRK2.4 or SnRK2.10 transcripts in plants exposed to cadmium stress for up to 12 h (Fig. 3D). Therefore, the observed transient Cd2+-dependent activation of these kinases is not due to changes in the expression level of the respective genes. We also analyzed the level of SnRK2.4 and SnRK2.10 transcripts in roots of 4-week-old Arabidopsis grown in hydroponic culture and subjected to 20 μm CdCl2 for 48 h. In this case, the level of SnRK2.4 and SnRK2.10 transcripts was significantly higher than in untreated plants (Fig. 3E). These results were confirmed by real-time RT-PCR (Fig. 3F); the expression level of SnRK2.4 and SnRK2.10 was about 150% and 175% of control, respectively.
NtOSAK and SnRK2.4 Are Localized to Cytoplasm and Nucleus, Whereas SnRK2.10 Is Exclusively Cytoplasmic; Localization Is Not Influenced by Cadmium Stress
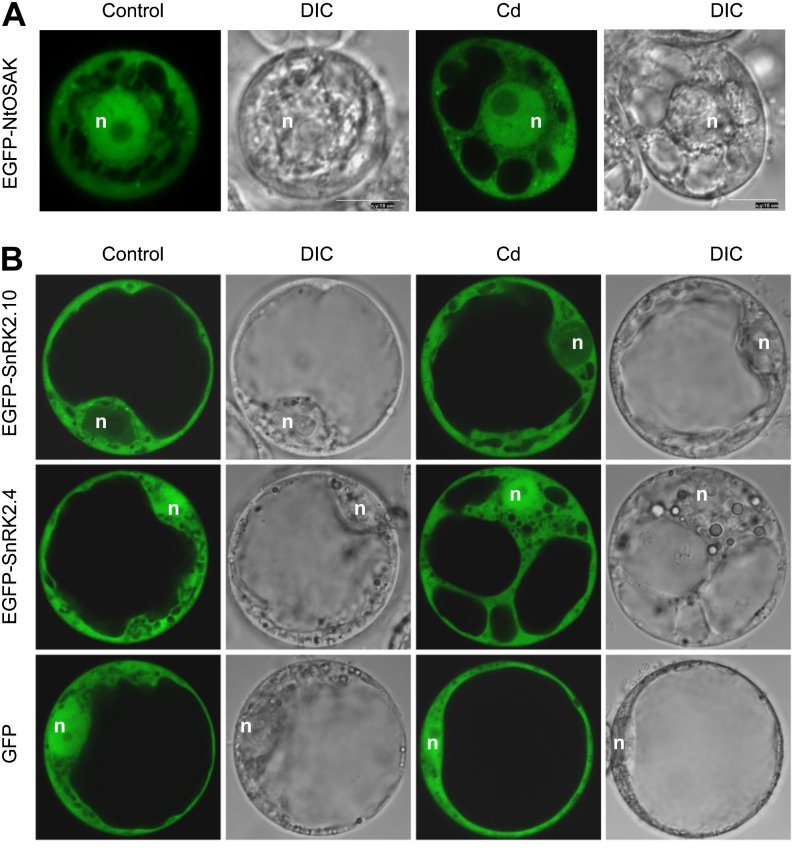
To determine the subcellular distribution of the tobacco and Arabidopsis kinases studied we investigated the localization of the EGFP-NtOSAK, EGFP-SnRK2.4, and EGFP-SnRK2.10 fusion proteins in plant protoplasts (tobacco or Arabidopsis, respectively), treated with 50 or 100 μm CdCl2 for various time periods (0, 30, 60, 120 min). As shown in Figure 4, EGFP-SnRK2.10 was found exclusively in the cytoplasm, whereas EGFP-SnRK2.4 and EGFP-NtOSAK were present in the nucleus and cytoplasm. The localization of the kinases studied was not altered in response to cadmium salt. These data suggest that SnRK2.4 rather than SnRK2.10 is a functional homolog of NtOSAK in Arabidopsis. Therefore, we focused our further studies on SnRK2.4.
Figure 4.
Localization of NtOSAK, SnRK2.10, and SnRK2.4 before and after cadmium treatment. Tobacco protoplast were prepared from BY-2 cells and transformed with EGFP-NtOSAK construct. The kinase localization was monitored before and after 30-min treatment with 50 μm CdCl2. In both conditions NtOSAK localized to cytoplasm and nucleus (A). Arabidopsis protoplasts were prepared from T87 cells and transformed with EGFP-SnRK2.4, EGFP-SnRK2.10, or EGFP constructs. Localization of the fluorescent proteins was observed before or after 30-min treatment with 50 μm CdCl2. EGFP-SnRK2.10 protein was observed only in cytoplasm in both conditions, whereas EGFP-SnRK2.4 was localized simultaneously to cytoplasm and nucleus in control and in cadmium-containing medium. n indicates position of the nucleus. Data represent one of several independent experiments showing similar results.
SnRK2.4 Contributes to Root Growth Sensitivity to CdCl2
Cadmium ions strongly impair the growth of Arabidopsis seedlings. Therefore, to gain more insight into the role of SnRK2.4 in the plant response to cadmium, we analyzed cadmium-response phenotypes of two lines of SnRK2.4 insertion mutants (snrk2.4-1 and snrk2.4-2), which do not express SnRK2.4 (Fig. 5, A and B), and of wild-type Arabidopsis (Columbia-0 [Col-0]). We measured root lengths of seedlings that were germinated for 10 d on control medium or medium supplemented with 30 or 100 μm CdCl2 (Fig. 5, C and D). In the absence of CdCl2, we did not observe any significant differences in root growth between the analyzed lines. As expected, increasing concentrations of CdCl2 progressively inhibited the growth of seedling roots in all the lines. However, the roots of both insertion mutants were about 20% longer than the roots of wild-type plants when grown on medium with 30 μm CdCl2. On plates with 100 μm cadmium, the roots of snrk2.4-1 and snrk2.4-2 were, respectively, about 20% and 45% (±5%) longer than the roots of wild-type seedlings. The data suggest that SnRK2.4 contributes to the inhibition of root elongation caused by cadmium ions.
Figure 5.
SnRK2.4 regulates root growth in response to cadmium ions. Sites of T-DNA insertions in SnRK2.4 gene (A). SnRK2.4 transcript levels in leaves of snrk2.4-1 (SALK_080588) and snrk2.4-2 (SALK_146522), and Col-0 determined by semiquantitative RT-PCR (B). Seeds of wild-type Arabidopsis and both mutants were sterilized and sown on square petri plates on control media or media supplemented with 30 or 100 μm CdCl2. Seeds were vernalized for 3 d at 4°C in darkness and germinated in standard germination condition. After 14 d of growth, root length was measured by ImageJ free software (C). Values correspond to means ± sd of three independent experiments. Asterisk means statistically significant difference between root length of the mutants and wild-type plants (P < 0.05). D, Image of seedlings after 14 d of growing on control media or media supplemented with 30 or 1,000 μm CdCl2 (bar = 1 cm). Data represent one of three independent experiments showing similar results. [See online article for color version of this figure.]
Enhanced Tolerance of the snrk2.4 Mutants to Cadmium Does Not Result from a Difference in the Level of Thiol-Containing Molecules
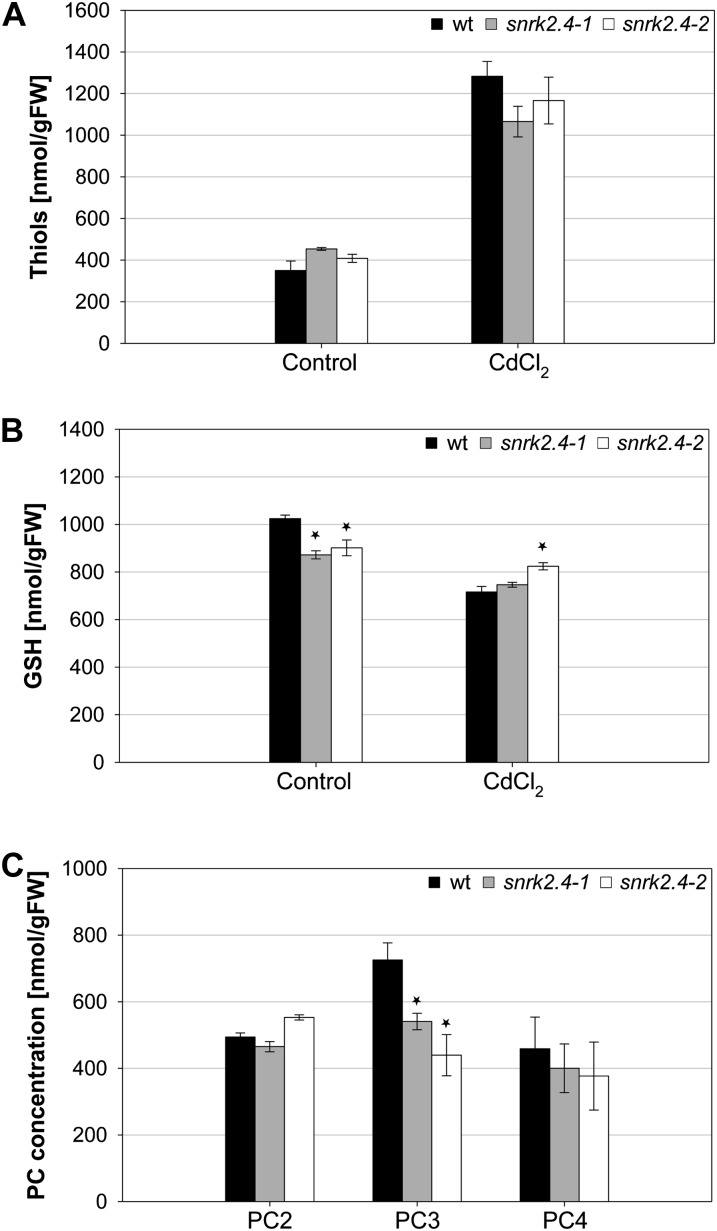
Plants’ tolerance to cadmium greatly depends on the level of thiol-containing molecules, both high Mr (e.g. PCs and methalothioneins) as well as low-molecular-weight compounds (e.g. glutathione), in plant tissues. Taking this into consideration, we analyzed the total amount of thiol groups in roots of snrk2.4 mutants and wild-type Arabidopsis plants not treated and treated with 20 μm CdCl2 for 2 d. The obtained results showed that the level of thiol groups is considerably higher in plants exposed to cadmium stress. However, our data did not show any significant differences between the lines studied, the two snrk2.4 mutants and wild-type plants (Fig. 6A).
Figure 6.
Analysis of the level of thiol-containing compounds in roots of snrk2.4 mutants wild-type and Arabidopsis plants. Four-week-old Arabidopsis Col-0 wild-type and mutant plants grown in hydroponic culture were treated with 20 μm CdCl2 for 2 d. Nonprotein thiol level was measured by Ellman’s test (A). Concentration of GSH (B) and major PC species (C) in cadmium-treated plants was analyzed in roots according to method described by Wojas et al. (2008). Asterisk means statistically significant difference in the concentration of GSH and PCs between the wild type and mutants according to ANOVA one way followed by Dunnets test (P < 0.05). Values correspond to means ± sd (n = 3).
Subsequently, we estimated levels of GSH and of three major species of PCs (PC2, PC3, and PC4) in roots of snrk2.4 mutants and Col-0 Arabidopsis exposed or not (control) to 20 μm CdCl2 for 2 d. The GSH level was reduced after treatment with Cd2+ in all lines studied (Fig. 6B), however, the reduction was slightly less pronounced in the mutants than in wild-type plants. As expected, PCs were not detected in control plants (data not shown), they were detected only in plants subjected to cadmium ions. We observed some differences in the PCs level between roots of the mutants and wild-type plants exposed to the stressor (Fig. 6C). Surprisingly the PC3 level was lower in the mutants than in wild-type plants. These results indicate that lower sensitivity of snrk2.4 plants to cadmium does not result from alteration of the total amount of thiol-containing compounds or a higher accumulation of PCs. Additionally, the analysis showed that SnRK2.4 is most probably involved in regulation of PCs synthesis in plants exposed to cadmium ions.
SnRK2.4 Regulates Level of ROS Produced in Arabidopsis Roots in Response to Cadmium Ions
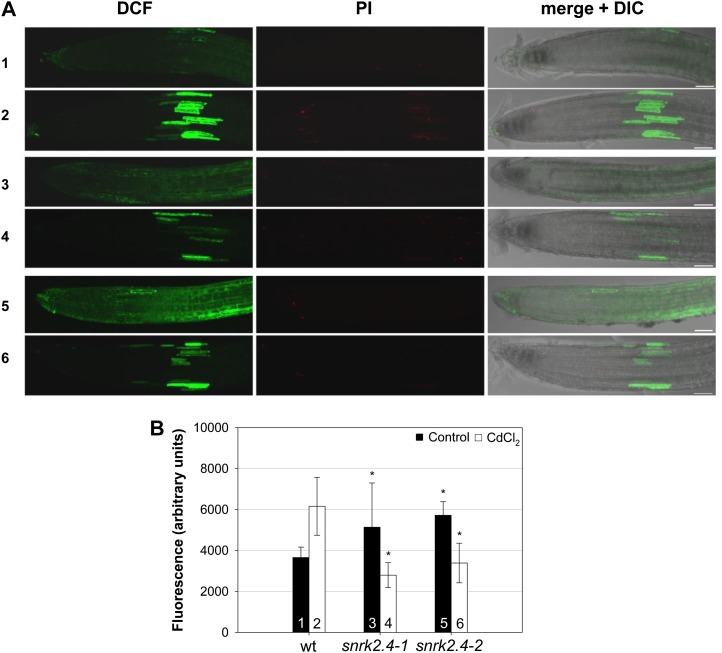
Cadmium ions induce oxidative stress in plants (Schützendübel et al., 2001; Olmos et al., 2003; Garnier et al., 2006), which contributes to the metal toxicity (Cho and Seo, 2005; Sharma and Dietz, 2009). Therefore, we analyzed the level of ROS induced by cadmium treatment in roots of 5-d-old Arabidopsis seedlings exposed or not to 50 μm CdCl2 for 30 min. ROS level was monitored in roots of snrk2.4-1, snrk2.4-2, and wild-type (Col-0) plants using the fluorescent dye 2’,7’-dichlorodihydrofluoresceine diacetate (H2DCFDA). In control conditions (no Cd2+) the level of ROS in the mutants’ roots was approximately 50% higher than in roots of the wild-type seedlings. These differences were unexpected. We can speculate that in control conditions the higher ROS level in mutants than in the wild-type plants can be due to the different sensitivity of the seedling lines studied to changes of the environment, mainly temperature and light, during progression of the experiment (e.g. transferring seedlings from incubator, placing them on slides, etc.). Under cadmium stress the responses of the wild type and the two mutants were dramatically different: While in Col-0 the stress brought about a substantial increase of ROS level (by approximately 80%), the mutants responded by an almost 2-fold reduction of ROS level in comparison to control conditions (Fig. 7).
Figure 7.
SnRK2.4 is involved in regulation of ROS level in Arabidopsis seedlings exposed to Cd2+. Five-day-old seedlings of snrk2.4-1 and snrk2.4-2 mutants and wild-type Col-0 were incubated for 30 min in one-half Murashige and Skoog medium (control) or medium with 50 μm CdCl2, rinsed with medium, and stained with PI (20 μg/mL) and H2DCFDA (30 μm), as described in “Materials and Methods.” Stained roots were observed in inverted epifluorescence microscope coupled with EZ-C1 confocal laser-scanning head. Fluorescent and differential interference contrast images are z-stacks projected from 10 collected images. A, Wild type (1, 2), snrk2.4.1 (3, 4), and snrk2.4.2 (5, 6) roots incubated in control (1, 3, 5) or cadmium-containing medium (2, 4, 6), stained with H2DCFDA (2',7'-dichlorofluorescein fluorescence in ROS presence), PI (nuclei in dead cells stained), and both images merged with differential interference contrast images. B, Total intensity of 2',7'-dichlorofluorescein fluorescence in all images rendered from z-stack projections calculated with NIS-Elements AR 3.0 software. Data represent the mean ± sd of 18 to 30 images collected from three independent experiments. Asterisk means statistically significant difference between ROS level of mutant and wild-type plants (P < 0.05).
SnRK2.4 Is Not Involved in Regulation of NO Production Induced by Cadmium Ions in Arabidopsis Roots
SnRK2s belong to the SNF1/AMP-activated protein kinase (AMPK)-related protein kinase family. The activity of mammalian AMPK is regulated by NO (Zhang et al., 2008). Moreover, AMPK itself plays a role in the regulation of NO production in response to several different stresses by phosphorylating eNOS and thus enhancing its activity (Chen et al., 1999; Morrow et al., 2003; Ritchie et al., 2010). Therefore, we decided to check whether SnRK2.4 is involved in the regulation of NO production in response to cadmium stress using 4,5-diaminofluorescein diacetate (DAF-2DA). NO level was investigated in roots of 5-d-old Arabidopsis seedlings (snrk2.4-1, snrk2.4-2, and wild-type Col-0), after 7-h treatment with 200 μm CdCl2, with or without NO scavenger (250 μm cPTIO). A strong increase of NO level was observed in Cd2+-treated seedlings, when compared with control plants and the induction effect was strongly reduced in seedlings cotreated with cPTIO. However, we did not observe any differences in NO production in response to Cd2+ between the studied mutant lines and wild-type Arabidopsis (Supplemental Fig. S4).
SnRK2.4 Regulates the Expression of Genes Involved in Iron Homeostasis in Plant Cells
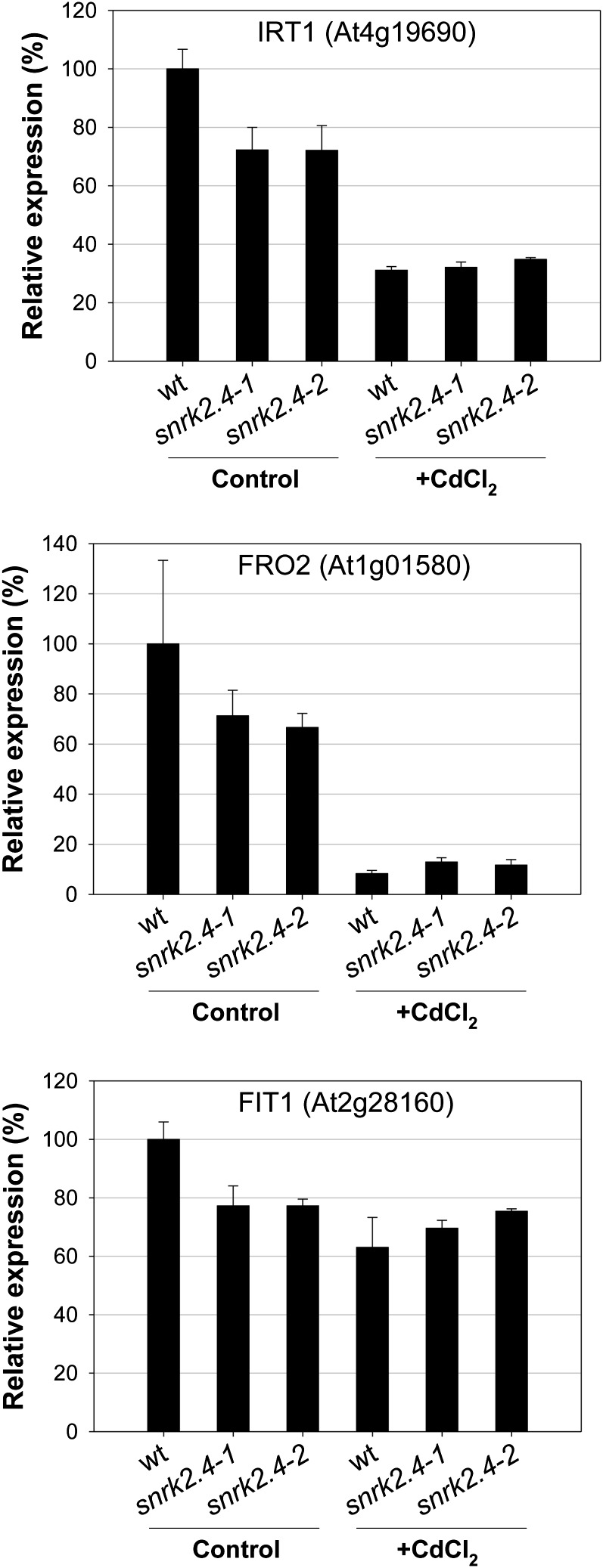
There are strong data indicating that cadmium ions influx is closely connected with iron homeostasis. Cadmium is absorbed into plant roots mainly by iron transporter IRON-RESPONSIVE TRANSPORTER1 (IRT1; Clemens 2001). IRT1 is a plasma-membrane cation transporter responsible for the uptake of ferrous ions, which can also transport several heavy metal ions including Cd2+. IRT1 works in concert with the Fe3+ reductase FERRIC REDUCTASE OXIDASE2 (FRO2) and their expression is under the control of the transcription factor FE-DEFICIENCY INDUCED TRANSCRIPTION FACTOR1 (FIT1). An earlier article reported that the expression of several genes was modulated by NO during Cd2+ treatment (Besson-Bard et al., 2009). Among them there were genes whose products are crucial for iron homeostasis: IRT1, FRO2, FIT1, and NICOTIANAMINE SYNTHASE4.
Since this and also other studies (Lamotte et al., 2006; Wawer et al., 2010) showed that NO participates in the activation of SnRK2s, we wondered whether SnRK2s play a role in controlling the expression of IRT1, FRO2, and FIT1. Therefore, we decided to analyze the expression of these genes in roots of snrk2.4-1, snrk2.4-2, and wild-type Arabidopsis plants, untreated and treated with cadmium ions. Gene expression was analyzed by real-time RT-PCR in roots of 4-week-old snrk2.4-1, snrk2.4-2, and wild-type Arabidopsis plants, untreated and treated with 20 μm CdCl2 for 48 h. In untreated mutant plants, the expression of all three genes was lower by approximately 20% to 30%, compared with the wild-type line (Fig. 8). Upon cadmium treatment, the level of mRNA of IRT1 and FRO2 in Arabidopsis roots decreased significantly, to a different extent in each line studied, resulting in a similar level of expression of these genes in wild-type and mutant plants.
Figure 8.
Effect of SnRK2.4 gene mutation on expression of genes involved in iron transport and homeostasis. Transcript level was monitored by qRT-PCR in roots of four-week-old plants, not treated or treated with 20 μm CdCl2 for 48 h (+CdCl2) and normalized against geometric mean of four housekeeping genes. In snrk2.4-1 and snrk2.4-2 knockout lines expression of each gene is plotted relative to that in nontreated wild-type plants (Col-0). sd bars represent sd of three replicates of each sample within experiment.
DISCUSSION
Our understanding of the role of protein kinases in signaling pathways involved in the plant response to heavy metal stress is still rather elusive. The only convincing evidence is that MAPK cascades take part in the signal transduction triggered by heavy metal stress. Activation of several MAPKs in response to treatment with copper or cadmium ions has been observed in M. sativa (Jonak et al., 2004), Arabidopsis (Liu et al., 2010), and rice (Yeh et al., 2007). Some MAPKs have been suggested to improve cadmium tolerance of rice plants, since cadmium-tolerant cultivars have a significantly increased MAPK activity (Yeh et al., 2007). However, there is no experimental data showing the specific role of MAPK(s) in the regulation of plant tolerance/sensitivity to heavy metal stress.
Our results indicate that treatment of BY-2 tobacco cells with Cd2+ at concentrations that cause MAPKs activation also stimulates SnRK2 activity. The time course of the SnRK2 activation is very similar to that observed for MAPKs. The MAPK activation in response to cadmium stress depends on ROS generation (Jonak et al., 2004; Yeh et al., 2007; Liu et al., 2010). The same is true for SnRK2 activation; pretreatment of tobacco BY-2 cells with catalase before addition of CdCl2 significantly reduced NtOSAK (tobacco SnRK2) activity. Our results revealed that oxidative stress by itself induces SnRK2 activity in plant cells. Moreover, also NO plays a significant role in SnRK2s activation (Lamotte et al., 2006; Wawer et al., 2010; and present results). The NtOSAK activation by cadmium ions in BY-2 cells can be abolished by pretreatment of the cells with cPTIO or inhibitors of mammalian NOS, which indicates that the NO responsible for the Cd2+-dependent induction of SnRK2 activity is generated mainly via an l-Arg-dependent pathway involving a putative plant NOS-like enzyme. This suggestion is supported by the fact that the induction of SnRK2 activity in response to cadmium ions was not decreased in the Arabidopsis mutants nia1nia2 and atnoa1, in which enzymatic pathways involved in NO production, other than the l-Arg-dependent one, are nonfunctional. In fact the activation was even stronger in nia1nia2 and atnoa1 than in wild-type Col-0 Arabidopsis. Our present data confirmed previous results indicating that NO production in response to cadmium ions in Arabidopsis does not involve NR or NOA1 and is suppressed by mammalian NOS inhibitors (Besson-Bard et al., 2009; De Michele et al., 2009), suggesting that the Cd2+-dependent NO synthesis is catalyzed by a so-far-unidentified enzyme. Concluding, the above results indicate that NtOSAK activation in response to Cd2+ treatment is triggered by ROS and NO produced in response to this stressor.
Since SnRK2s are activated in plants exposed to cadmium ions and because there are strong indications that in the lower plant C. reinhardtii SnRK2s are involved in sulfur metabolism (Davies et al., 1999; Irihimovitch and Stern, 2006; González-Ballester et al., 2008), which is closely connected with regulation of heavy metal tolerance, we addressed the question of the role of SnRK2.4, a putative NtOSAK functional homolog in Arabidopsis, in the plant response to cadmium stress. For this purpose we applied a reverse genetics approach. First, we analyzed the effect of cadmium ions on root growth of wild-type Col-0 and insertion mutants snrk2.4-1 and snrk2.4-2. Roots of the studied mutants were significantly longer than roots of wild-type plants grown on medium supplemented with 30 or 100 μm CdCl2, indicating that snrk2.4 plants are more tolerant to the stress. This result suggested that SnRK2.4 is an important element of plant response to cadmium. In plants, mainly thiol-containing compounds (PCs, metallothioneins, and glutathione) are responsible for reduction of metal toxicity. Moreover, glutathione plays the role of a reducing agent in plant defense against oxidative stress, one of the most serious contributors to cadmium toxicity (for review, see Jozefczak et al., 2012; Seth et al., 2012). Therefore, we analyzed the total amount of thiol groups as well as the level of major PC species and glutathione in roots of the mutants studied and in wild-type plants exposed to cadmium ions. The results indicate that the snrk2.4 mutants’ enhanced tolerance to cadmium in comparison to wild-type plants, observed in root growth test, is not due to a difference in the total level of thiol-containing compounds accumulating in plant tissues exposed to cadmium ions or even in the PCs concentration. The GSH level, after cadmium treatment, was only slightly higher in roots of the mutants, whereas surprisingly, levels of some PC species (especially, PC3) were lower in mutants than in Col-0 plants. These results suggest that PC synthase might be regulated by SnRK2.4 either at the level of transcription of PCS(s) or at the activity level. It was previously suggested that PCS could be positively controlled by posttranslational modifications e.g. phosphorylation (Wang et al., 2009). Therefore, it is highly plausible that SnRK2s, activated in response to heavy metal ions, are involved in PCSs regulation.
Since oxidative stress is one of the major components of cadmium toxicity, to investigate the contribution of SnRK2.4 in Cd2+-induced oxidative stress we monitored ROS level generated in response to cadmium in roots of snrk2.4-1 and snrk2.4-2 mutants and in Col-0 Arabidopsis. This analysis revealed that the ROS level is significantly lower in snrk2.4 mutants than in wild-type seedlings. This is in accordance with a slightly higher GSH level in the mutants, however the differences in the GSH level seem to be too small to be responsible for the observed disparity. Therefore, we presume that there are some other factors accountable for differences in ROS accumulation in snrk2.4 mutants and wild-type Arabidopsis. Previous work showed that the Arabidopsis SnRK2 family member OST1/SnRK2.6 acts upstream of ROS production induced by ABA in guard cells (Mustilli et al., 2002). In a loss-of-function mutant (ost1), ABA treatment failed to increase the ROS level in guard cells, as it was observed in wild-type guard cells, and consequently did not induce stomatal closure. The impairment of the ABA-induced ROS production in the ost1 mutant could be bypassed by externally applied H2O2. Recently, it was shown that NADPH oxidases could be SnRK2s’ cellular substrates (for review, see Hubbard et al., 2010; Umezawa et al., 2010; Joshi-Saha et al., 2011). A recombinant cytosolic part of one of the Arabidopsis NADPH oxidases, AtrbohF, produced in a bacterial system is phosphorylated by SnRK2.6/OST1 in vitro. Moreover, the kinase interacts with AtrbohF in bimolecular fluorescence complementation assay, suggesting that NADPH oxidase could be a SnRK2.6/OST1 substrate in vivo (Sirichandra et al., 2009). AtrbohF and AtrbohD (another NADPH oxidase), which are abundant in plant roots, are involved in ROS production in response to several abiotic and biotic stresses (Sagi and Fluhr, 2006; Suzuki et al., 2011; Ma et al., 2012; Marino et al., 2012). It was shown that phosphorylation and Ca2+ binding are needed for AtrbohD and AtrbohF activation (Ogasawara et al., 2008; Kimura et al., 2012). Therefore, it is possible that SnRK2.4, and maybe also other SnRK2 kinases, participate in AtrbohD and/or AtrbohF phosphorylation and activation in plant roots exposed to a toxic concentration of cadmium salt. In this way SnRK2s can be involved in a positive feedback loop in ROS accumulation: The kinases are activated by ROS and in turn they most probably participate in ROS production.
It is well established that ROS produced by NADPH oxidases are required for activation of Ca2+ channels (Murata et al., 2001; Foreman et al., 2003; Kwak et al., 2003), and Ca2+ channels are implicated in Cd2+ uptake (for review, see Clemens, 2006). Considering this, SnRK2 could have an impact on Cd2+ uptake by influencing ROS accumulation. However, we did not see any significant differences in cadmium level between the snrk2.4 mutants and Col-0 Arabidopsis grown on medium with a toxic level of CdCl2 (30 μm; data not shown). This lack of differences in cadmium accumulation between the lines studied could be explained, e.g. by the relatively high Ca2+ concentration in the medium; Ca2+ is a potent inhibitor of Cd2+ uptake (via Ca2+ channels; Rodríguez-Serrano et al., 2009).
As previously mentioned, Cd2+ enters plant cells efficiently through iron transporters (for review, see Clemens, 2006). There is strong evidence that IRT1 is responsible for Cd2+ uptake by plant roots. Therefore, iron deficiency, which induces IRT1 expression, stimulates heavy metal transport into plants (Cohen et al., 1998; Besson-Bard et al., 2009). Our results indicate that the snrk2.4 mutants studied have a significantly lower expression of IRT1 in comparison with wild-type plants, but only in the absence of Cd2+. In roots of the snrk2.4 mutants we observed not only reduction of IRT1 expression, but also reduction of expression of two other genes involved in iron homeostasis and iron uptake, FRO2 and FIT. We did not see this phenomenon in Cd2+-treated roots; the expression of genes mentioned was basically the same in the snrk2.4 mutants and wild-type plants exposed to a toxic concentration of cadmium. Right now, we can only speculate about the reasons responsible for the lack of those differences in stressed plants. It is highly likely that in plants growing in medium with a high concentration of cadmium, several different signaling pathways are induced that are involved in regulation of IRT1, FRO2, and FIT1 expression, and those are not connected or only slightly connected with the SnRK2 pathway. Considering the role of SnRK2 in the plant response to cadmium, we should keep in mind the localization of SnRK2. Our results showed that SnRK2.4 is present both in the nucleus and in the cytoplasm and its localization does not change upon stress. However, we do not know whether in response to cadmium both pools of the kinase (nuclear and cytoplasmic) are activated; it could well be that only the cytoplasmic pool is active, which is not involved in regulation of gene expression but is responsible for phosphorylation of cytoplasmic or membrane proteins, e.g. NADPH oxidase. On the other hand, it is possible that during growth in normal conditions a low activity of both nuclear and cytoplasmic SnRK2.4 and maybe also other SnRK2s is needed for normal plant development and iron homeostasis. Previous studies showed that NO positively regulates expression of IRT1, FRO2, and FIT1 (Besson-Bard et al., 2009). Since NO is a key element in NtOSAK activation (Lamotte et al., 2006; Wawer et al., 2010) and iron homeostasis is regulated by NO (for review, see Ramirez et al., 2010), we speculated that NO regulates expression of these genes at least partly by regulation of SnRK2’s activity. This might indicate that to some extent SnRK2 could be involved in cadmium uptake when the level of Cd2+ is very low, not recognized by the plant as toxic, but not when the Cd2+ concentration is relatively high (in the μm range).
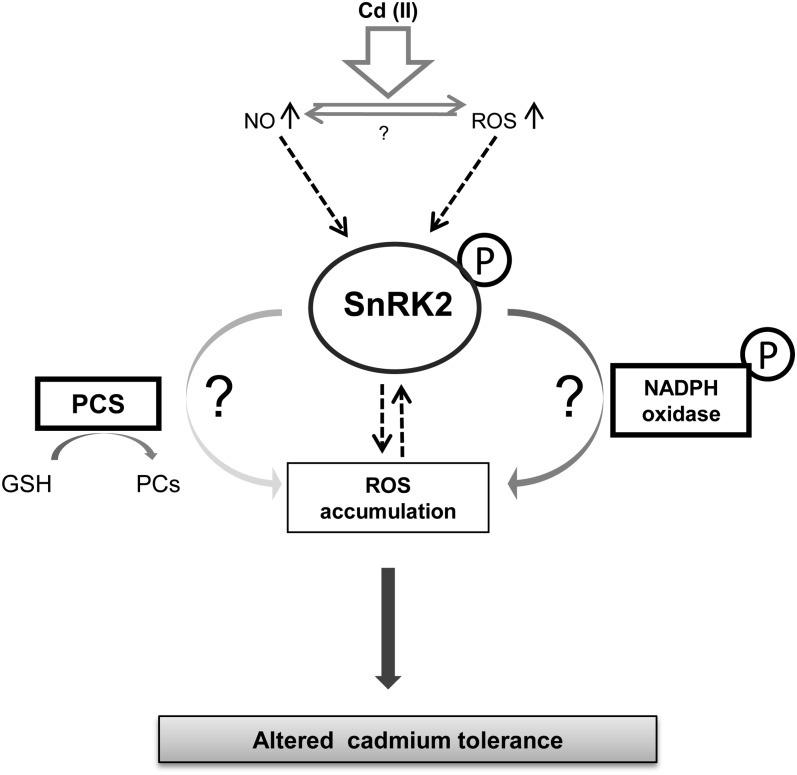
In conclusion, we show that SnRK2s, plant-specific kinases known so far to be involved in regulation of plant development and defense against osmotic stress (Fujii and Zhu, 2009; Nakashima et al., 2009; Fujii et al., 2011), also take part in the plant response to heavy metal stress probably by influencing ROS accumulation. The obtained results are summarized in a hypothetical model of the role of SnRK2 in plant response to the stress induced by cadmium ions (Fig. 9). We cannot exclude the role of SnRK2s in the regulation of other processes involved in the plant response to this stress, for example by controlling antioxidative enzymes’ and metal transporters’ activity, as well as expression of various stress-related genes. It is noteworthy that osmotic stress (water deficit and salinity) influences the plant response to heavy metal stress (Lefèvre et al., 2010; Xu et al., 2010) and that the Cd2+-response signaling pathways overlap and cross talk with different abiotic stress pathways (Dal Corso et al., 2010). It should be stressed out that the common signaling molecules, ROS and NO, triggering and regulating plant responses to various environmental conditions are key elements contributing to activation of SnRK2s studied by a variety of abiotic stresses (e.g. salinity and stress induced by heavy metal ions). Most probably therefore, SnRK2s similarly to MAPKs, which are activated by several different stresses also play a role in the plant response to heavy metal stress.
Figure 9.
A hypothetical model for SnRK2 role in Arabidopsis roots exposed to Cd2+. Cadmium ions induce NO and ROS accumulation in plant roots, which contribute to SnRK2 activation. Active SnRK2 stimulates Cd2+-dependent ROS accumulation in plant cells, most probably by phosphorylation and activation of NADPH oxidase(s). SnRK2 kinases contribute to the increase of the level of PCs presumably by regulating the activity or expression level of PCS. Enhanced Cd2+-induced PC synthesis resulting in an increased metal tolerance might, however, cause further accumulation of ROS, as a consequence of GSH depletion.
MATERIALS AND METHODS
Plant Lines and Growth Conditions
Tobacco (Nicotiana tabacum) BY-2 cell suspension culture, kindly provided by Dr. Witold Filipowicz (Friedrich-Miescher Institute, Basel, Switzerland), was cultured as described elsewhere (Nagata et al., 1992; Burza et al., 2006). The cells were subcultured every 7 d. BY-2 cells were treated with different stressors for indicated time, as described in “Results,” harvested by centrifugation, frozen in liquid nitrogen, and stored at −80°C until analyzed.
Several different Arabidopsis (Arabidopsis thaliana) lines were used: Arabidopsis ecotype Col-0, the wild type; atnoa1 (formerly atnos1, kindly provided by N.M. Crawford, University of California); the NR double mutant nia1nia2 (gift from C. Meyer, Institut National de la Recherche Agronomique Versailles, France); snrk2.4-1 (SALK_080588) and snrk2.4-2 (SALK_146522) T-DNA insertion lines were obtained from the Nottingham Arabidopsis Stock Center (NASC; http://signal.salk.edu/cgi-bin/tdnaexpress; Alonso et al., 2003). Homozygous plants were selected by PCR screening using gene-specific primers in combination with the left-border insert-specific primer (LBb1.3). The level of SnRK2.4 expression was analyzed by RT-PCR. All primers used in this study are listed in Supplemental Data S1.
Plants were grown at 24°C/22°C under long-day conditions (16-h-light/8-h-dark cycle) in hydroponic culture (Araponic system) using the following medium: 3.5 mm KNO3, 2.5 mm Ca(NO3)2, 1 mm Mg(NO3)2, 5 mm NH4NO3, 1 mm KH2PO4, 1 mm MgSO4, 0.9 mm MgCl2, 2 mm CaCl2, 0.1 mm NaCl, 50 μm Fe-Na-EDTA, 0.64 μm Cu(NO3)2, 10 μm Mn(NO3)2, 0.82 μm (NH4)2MoO4, 0.096 μm zinc acetate, 0.11 μm CoCl2, 25 μm H3BO3, 1 mm MES, pH 5.8. Roots of 4-week-old plants, not treated or treated with 20 μm CdCl2 for 2 d, were harvested, frozen in liquid nitrogen, and stored at −80°C until analyzed.
For aseptic cultures, seeds were sterilized by gentle shaking in 70% ethanol for 2 min and then incubating in water:bleach solution 13:1 (v:v) for 20 min. Finally, the seeds were extensively washed five times with sterile water. For sterile hydroponic culture about 100 seeds were imbibed at 4°C for 5 d to synchronize germination onset and grown for 10 d in 300-mL Erlenmeyer flasks containing 100 mL of one-half Murashige and Skoog medium supplemented with one-half Murashige and Skoog vitamin solution, 43 mg/L Fe-Na-EDTA, 500 mg/L MES, 10 g/L Suc, pH 5.7. Seedlings were treated with different stressors as indicated in “Results,” harvested, frozen in liquid nitrogen, and stored at −80°C until analyzed. For seedling culture on petri plates for use in microscopic observations, seeds were sterilized as described above and plants were grown vertically in medium containing one-half Murashige and Skoog salts, one-half Murashige and Skoog vitamin solution, 20 g/L Suc, and 8 g/L agar, pH 5.7. For determination of seedlings’ resistance to cadmium ions we plated sterile seeds on petri plates on medium prepared according to Besson-Bard et al. (2009) with or without addition of CdCl2 (final concentration 30 or 100 μm). For transient expression experiments protoplasts were isolated from 6-d-old Arabidopsis T87 or tobacco BY-2 cells. Arabidopsis suspension cultures were grown in Gamborg B5 medium as described by Yamada et al. (2004). Cells were subcultured every 7 d.
All chemicals were from Sigma/Aldrich with the exception of Suc, which was from Merck.
Protoplast Transient Expression Assay
Protoplasts were isolated and transformed via polyethylene glycol treatment according to the protocol provided by He et al. (2007) with minor modifications recently described (Bucholc et al., 2011). After transformation Arabidopsis T87 protoplasts were suspended in incubation solution (0.5 m mannitol, 4 mm MES [pH 5.7], 20 mm KCl), whereas BY-2 protoplasts were transferred into K4 medium (Murashige and Skoog salt, Murashige and Skoog vitamins, 0.4 m Suc, 250 mg/L Xyl, 0.1 mg/L 2,4-dichlorophenoxyacetic acid, 0.2 mg/L 6-benzylaminopurine, 1 mg/L 1-naphthaleneacetic acid) and incubated at 25°C in the dark for 2 d. Complementary DNAs (cDNAs) encoding NtOSAK, SnRK2.4, and SnRK2.10 were inserted into the pSAT6-EGFP-C1 vector provided by Prof. T. Tzfira (University of Michigan, Ann Arbor). cDNAs encoding SnRK2.4 and SnRK2.10 were PCR amplified using Pfu DNA polymerase and appropriate plasmids obtained from the NASC as templates and cloned into pCR II-TOPO (Invitrogen). Primers are listed in Supplemental Data S1. The sequences were verified by DNA sequencing and cloned as EcoRI/SalI (SnRK2.4 and SnRK2.10) or XhoI/SalI (NtOSAK) fragments into pSAT6-EGFP-C1 vector. In each transformation about 2 × 105 protoplasts were transfected with about 50 μg of plasmid DNA. The transfected protoplasts were subjected to 200 μm CdCl2 or 500 mm NaCl treatment. In control experiments, water instead of the stressor was added to the transfected protoplasts. The transformed protoplasts were used for analysis of the Cd2+ effect on EGFP-SnRK2.4 and EGFP-SnRK2.10 activities (measured by in-gel kinase activity assay) and for visualization of the cellular localization of EGFP-NtOSAK, EGFP-SnRK2.4, and EGFP-SnRK2.10 in response to Cd2+ treatment. Visualization was performed as described previously (Wawer et al., 2010; Bucholc et al., 2011).
RT-PCR Analysis
Total RNA was isolated from untreated and stressor-treated BY-2 cells or 3-week-old Arabidopsis seedlings (separately roots and leaves) using TRI REAGENT (MRC) according to the procedure recommended by the manufacturer. cDNA was synthesized from 1 μg of total RNA using HSRT 100 kit (Sigma-Aldrich). Briefly, RNA was reverse transcribed for 60 min at 47°C in 20 μL of reaction mixture containing 1 unit of enhanced avian reverse transcriptase, 500 μm each deoxynucleotide, 3.5 μm anchored oligo(dT) primer, 1 unit of RNase inhibitor. Two microliters of the RT reaction mixture was used for PCR in 20 μL containing 0.2 units of Taq DNA polymerase (EURX), 200 μm each deoxynucleotide, 1.5 mm MgCl2, and 625 nm appropriate primers. Routine PCR conditions were: 3 min, 94°C (first cycle); 30s, 94°C; 30s, 49°C or 54°C; 30 s, 72°C (27 cycles); and 10 min, 72°C (final cycle). PCR products were separated on 0.8% agarose gels and visualized by EtBr staining. Primers are listed in Supplemental Data S1.
Real-Time RT-PCR Analysis
Real-time PCR analysis of gene expression (SnRK2.4, SnRK2.10, IRT1, FIT, FRO2) was performed by two-step quantitative (q)PCR. Optimal reference gene set was selected according to data presented by Besson-Bard et al. (2009) and Remans et al. (2008). Optimal template and standards concentration for each target gene were evaluated using PREXCEL-Q software (Gallup and Ackermann, 2008). Total RNA was extracted from Arabidopsis roots (control or treated for 2 d with 20 μm CdCl2) with RNeasy plant mini kit (QIAGEN) and DNase-digested with TURBO DNA-free kit (Ambion), according to standard manufacturer’s protocol. RNA quality was checked on Nanodrop 1000 and 2100 Bioanalyzer (Agilent). RT of 1 μg of RNA was performed in 20-μL reaction using SuperScript III reverse transcriptase (Invitrogen) and random pentadecamers, with some modifications of standard manufacturer’s protocol. Following RNA digestion with RNase H (Ambion), each cDNA sample was diluted 5× and stored. Expression level was assayed by qPCR in a Rotorgene 3000 (Corbett Research) device, using MESA GREEN qPCR MasterMix plus for SYBR Green I No ROX (Eurogentec). Following amplification, a meltcurve was performed in the 60°C to 95°C range, with 0.5°C steps. For each target gene amplification, two gene-specific primers were used (listed in Supplemental Data S1) and all cDNA samples (three replicates) and standards (two replicates) were assayed in a single run. Relative gene expression in each sample was calculated using standard curve method (five point), normalized using a geometric mean of expression values for four reference genes (PDF2, SAND, YLS8, F-Box), and scaled to the calibrator sample (Col-0 control). RNA quality analysis and test qPCR reactions proved that the material obtained for comparative studies was of high quality and free of amplification inhibitors or genomic DNA contamination (Supplemental Figs. S5 and S6). Detailed description of the experiment setup, analysis, and primer design, according to MIQE guidelines (Bustin et al., 2009; http://www.rdml.org/miqe.php), is presented in Supplemental Protocol S1.
Preparation of Protein Extracts
Protein extracts were prepared as previously described (Mikołajczyk et al., 2000; Burza et al., 2006; Wawer et al., 2010).
Immunoblotting
Western blotting was performed as described previously (Burza et al., 2006). Antibodies used: polyclonal anti-NtOSAK antibodies raised against the C-terminal peptide (KQVKQAHESGEVRLT) of the kinase (BioGenes); polyclonal anti-NtOSAK/SnRK2.4/SnRK2.10 antibodies raised against the N-terminal peptide (MDKYELVKDIG) of these kinases (BioGenes); phospho-specific polyclonal antibodies raised in rabbit against the phosphopeptide (KS(P)TVGT) and purified by affinity chromatography (BioGenes); antibodies recognizing active MAPKs-phospho-p44/42 MAPK (Erk1/2; Thr-202/Tyr-204; Cell Signaling Technology).
Immunoprecipitation
Immunoprecipitation was performed as described previously (Mikołajczyk et al., 2000; Burza et al., 2006; Wawer et al., 2010) using anti-C-terminal NtOSAK or anti-N-terminal NtOSAK/SnRK2.4/SnRK2.10 antibodies. Briefly, Protein A-agarose (10 μL per sample) was incubated for 2 h with antibodies (24 μg for anti-C-terminal NtOSAK or 120 μg for anti-N-terminal NtOSAK/SnRK2.4/SnRK2.10 per sample) at 4°C with gentle shaking. After incubation agarose beads were pelleted by brief centrifugation and unbound antibodies were removed by triple-washing protein A-agarose with immunoprecipitation buffer. The equal volume of protein A-agarose with bound antibodies was added to protein extract (150 or 300 μg in the case of BY-2 cells or Arabidopsis plants, respectively) and incubated for 4 h at 4°C with gentle shaking. Agarose-beads-protein complexes were pelleted by brief centrifugation, washed three times with 1 mL of immunoprecipitation buffer and two times with 1 mL of 20 mm Tris-HCl pH 7.5 buffer.
Immunocomplex Kinase Activity Assay
After immunoprecipitation Laemmli sample buffer was added to the pelleted protein complexes attached to protein A-agarose and the sample was heated at 95°C for 3 min with vigorous shaking. After brief centrifugation the supernatant was analyzed by in-gel kinase activity assay.
In-Gel Kinase Activity Assay
In-gel kinase activity assays were performed according to Zhang and Klessig (1997).
Determination of Thiol-Group Content
The content of thiol groups [SH] was analyzed by modified Ellman test (Ellman, 1959) according to the procedure described by Wawrzynski et al. (2006). Roots of 4-week-old Arabidopsis plants of wild-type, snrk2.4-1, and snrk2.4-2 lines grown in hydroponic culture, nontreated and treated with 20 μm CdCl2 for 48 h, were analyzed. About 100 mg of root tissue was powdered in liquid nitrogen and after homogenization the tissue was treated with 500 μL of cold 0.1 m HCl with vigorous mixing. After treatment samples were centrifuged (10 min, 20,000g). Aliquots of 200 μL of supernatant, 775 μL of 0.5 m K2HPO4, and 25 μL of 5,5′-dithio-2-nitrobenzoic acid (Sigma) were mixed together and incubated for 2 min at room temperature. After incubation the optical density was measured at 412 nm. The amount of thiols was calculated from difference between the optical density of the sample with and without 5,5′-dithio-2-nitrobenzoic acid as nmol per g fresh weight.
The level of PCs and GSH was determined in plant leaves and roots according to the procedure described previously (Wojas et al., 2008). A total of 200 to 300 mg of previously frozen powdered plant material was homogenized in 1.78 mL of cold 6.3 mm diethylenetriaminepenta-acetic acid, 100 μL of 1 m NaOH, and 100 μL of 6 m NaBH4 (in 0.1 m NaOH). N-acetyl-l-Cys at final concentration 10 μm was added to each sample as an internal standard. The homogenized samples were centrifuged (5 min, 10,000g) and 250 μL of the obtained extracts were mixed with 10 μL of 20 mm monobromobimane and 450 μL of 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid (HEPPS) buffer, pH 8.2 containing 6.3 mm diethylenetriaminepenta-acetic acid. Derivatization was performed at 45°C in the dark. The reaction was stopped after 30 min with 300 μL of 1 m methanesulphonic acid. Finally the samples were filtered through 0.22-μm filters and stored at 4°C in the dark until HPLC analysis. Thiol compounds were separated on Nova-Pak C18 column (Waters) at 37°C and were eluted with methanol-water gradient, both with 0.1% trifluoro-acetic acid. The injection volume was 20 μL. For calibration GSH at concentration from 5 up to 20 μm was used.
The data were integrated using Waters Millenium Software.
ROS Detection
ROS production in Arabidopsis roots was analyzed using H2DCFDA (Invitrogen) basically according to the procedure described in Murata et al. (2001). Five-day-old Arabidopsis seedlings of snrk2.4-1 and snrk2.4-2 mutants and the wild type (Col-0) were incubated in one-half Murashige and Skoog medium without (control) or with 50 μm CdCl2 for 30 min, rinsed with medium for 2 min, stained with 20 μg/mL propidium iodide (PI; Invitrogen) for 2 min, rinsed with medium, and stained with H2DCFDA (30 μm) for 20 min. The excess of the dye was removed by three washes with the medium. Whole seedlings were carefully placed on slides with homemade chambers preventing damage and drying. Images were collected with a 20× (NA 0.75) Plan Fluor multiimmersion objective mounted on an inverted epifluorescence TE 2000E microscope (Nikon) coupled with an EZ-C1 confocal laser-scanning head (Nikon). Fluorescence images are z-stack projections assembled from 10 optical sections collected with 10 μm steps made with the standard EZ-C1 Nikon software. The fluorescence of H2DCFDA was excited with blue light at 488 nm emitted by a 40 mW argon-ion laser (Melles Griot). H2DCFDA fluorescence was detected with a 515/30-nm band-pass filter and rendered in false green. The fluorescence of PI was excited with green light at 543 nm emitted by a 1 mW He-Ne laser (Melles Griot) and was detected with a 590-nm long-pass filter and rendered in false red. All confocal parameters (laser power, gain, etc.) and conditions were the same during each experiment. NIS-Elements AR 3.0 software was used to quantify the total intensity of H2DCFDA fluorescence signal in all images rendered from z-stack projections. Each experimental variant was repeated at least three times with six to 10 z-stacks collected in each series.
Nitric Oxide Detection
NO production in Arabidopsis roots was analyzed according to the method described by Besson-Bard et al. (2009) using DAF-2DA (Sigma-Aldrich) fluorescent dye. Five-day-old seedlings were incubated in 1 μm DAF-2DA in 50 mm Tris-HCl pH 7.5 solution for 2 h in darkness. Then, roots were washed three times with distilled water (to remove excess of the dye) and transferred into 50 mm Tris-HCl pH 7.5 (control) or 50 mm Tris-HCl pH 7.5 supplemented with 200 μm CdCl2. The fluorescence of DAF-2T was observed after 30 min or 7 h of incubation using a Leica epifluorescence microscope model DMRB. Images were collected with an RGB 3CCD camera (Sony) and for DAF-2T fluorescence observation a standard GFP filter was used. Pictures were analyzed by visilog 5.4 (Noesi) software and the fluorescence was quantified using the NIS-Elements BR 3.0 software.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY081175 (NtOSAK); NM100969, AT1G10940 (SnRK2.4); and NM104774, AT1G60940 (SnRK2.10).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. MAPK And SnRK2 are activated in plant cells in response to stress induced by heavy metal ions.
Supplemental Figure S2. Comparative sequence analysis of SnRK2s from Arabidopsis and NtOSAK from tobacco.
Supplemental Figure S3. Analysis of the specificity of anti-N-terminal NtOSAK/SnRK2.4/SnRK2.10 antibodies.
Supplemental Figure S4. The effect of SnRK2.4 on NO levels in Arabidopsis roots.
Supplemental Figure S5. Gel electrophoresis of amplification products obtained after qRT-PCR analysis.
Supplemental Figure S6. Quality and purity of RNA used for two-step qRT-PCR gene expression studies.
Supplemental Data S1. List of primers used in this study.
Supplemental Protocol S1. Real time RT-PCR analysis.
Acknowledgments
We are grateful to Professor J. Fronk for critical reading of the manuscript. We also thank the Salk Institute Genomic Analysis Laboratory and NASC for providing sequence-indexed Arabidopsis T-DNA insertion mutants and cDNA clones, and all members of our laboratory for stimulating discussions. A.Z. thanks Jack M. Gallup from the College of Veterinary Medicine of Iowa State University for all great comments and advice on qPCR experiments as well as for sharing his excellent Excel-based qPCR set-up and analysis tools while F.M. thanks Essam Darwish for help with qPCRs. We are very thankful to Professor D.M. Antosiewicz for providing us with chemicals and for very valuable help in GSH and PCs analysis.
Glossary
- ROS
reactive oxygen species
- PC
phytochelatin
- GSH
reduced glutathione
- MAPK
mitogen-activated protein kinase
- CDPK
calcium-dependent protein kinase
- ABA
abscisic acid
- cPTIO
2-(4-carboxyphenyl)-4,5-dhydro-4,4,5,5-tetramethyl-1H-imidazol-1yl-oxy-3-oxide
- NR
nitrate reductase
- NOS
nitric oxide synthase
- l-NAME
N-nitro-l-Arg-methyl ester
- H2O2
hydrogen peroxide
- RT
reverse transcription
- Col-0
Columbia-0
- AMPK
AMP-activated protein kinase
- NASC
Nottingham Arabidopsis Stock Center
- H2DCFDA
2’,7’-dichlorodihydrofluoresceine diacetate
- PI
propidium iodide
- DAF-2DA
4,5-diaminofluorescein diacetate
- q
quantitative
- qRT
quantitative reverse transcription
- BY-2
Bright Yellow 2
- NO
nitric oxide
- MBP
myelin basic protein
- cDNA
complementary DNA
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Gwóźdź EA. (2011) The message of nitric oxide in cadmium challenged plants. Plant Sci 181: 612–620 [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D. (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149: 1302–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D. (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59: 21–39 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Barbier-Brygoo H, Laurière C. (2004) Identification of nine SNF1-related protein kinase 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279: 41758–41766 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Laurière C. (2005) Osmotic signaling in plants: multiple pathways mediated by emerging kinase families. Plant Physiol 138: 1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholc M, Ciesielski A, Goch G, Anielska-Mazur A, Kulik A, Krzywińska E, Dobrowolska G. (2011) SNF1-related protein kinases 2 are negatively regulated by a plant-specific calcium sensor. J Biol Chem 286: 3429–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burza AM, Pękala I, Sikora J, Siedlecki P, Małagocki P, Bucholc M, Koper L, Zielenkiewicz P, Dadlez M, Dobrowolska G. (2006) Nicotiana tabacum osmotic stress-activated kinase is regulated by phosphorylation on Ser-154 and Ser-158 in the kinase activation loop. J Biol Chem 281: 34299–34311 [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622 [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. (1999) AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289 [DOI] [PubMed] [Google Scholar]
- Cho U-H, Seo N-H. (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168: 113–120 [Google Scholar]
- Clemens S. (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475–486 [DOI] [PubMed] [Google Scholar]
- Clemens S. (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88: 1707–1719 [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P. (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Cobbett CS. (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CK, Fox TC, Garvin DF, Kochian LV. (1998) The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol 116: 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Farinati S, Furini A. (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5: 663–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Farinati S, Maistri S, Furini A. (2008) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50: 1268–1280 [DOI] [PubMed] [Google Scholar]
- Davies JP, Yildiz FH, Grossman AR. (1999) Sac3, an Snf1-like serine/threonine kinase that positively and negatively regulates the responses of Chlamydomonas to sulfur limitation. Plant Cell 11: 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, Sanità di Toppi L, Lo Schiavo F. (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150: 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río LA, Corpas FJ, Barroso JB. (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry 65: 783–792 [DOI] [PubMed] [Google Scholar]
- Ellman GL. (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu J-K. (2007) Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu J-K. (2011) Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc Natl Acad Sci USA 108: 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu JK. (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA 106: 8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Nakashima K, Yoshida T, Katagiri T, Kidokoro S, Kanamori N, Umezawa T, Fujita M, Maruyama K, Ishiyama K, et al. (2009) Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol 50: 2123–2132 [DOI] [PubMed] [Google Scholar]
- Gallup JM, Ackermann MR. (2008) The ‘PREXCEL-Q Method’ for qPCR. Int J Biomed Sci 4: 273–293 [PMC free article] [PubMed] [Google Scholar]
- Garnier L, Simon-Plas F, Thuleau P, Agnel JP, Blein J-P, Ranjeva R, Montillet J-L. (2006) Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ 29: 1956–1969 [DOI] [PubMed] [Google Scholar]
- González-Ballester D, Casero D, Cokus S, Pellegrini M, Merchant SS, Grossman AR. (2010) RNA-seq analysis of sulfur-deprived Chlamydomonas cells reveals aspects of acclimation critical for cell survival. Plant Cell 22: 2058–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ballester D, Pollock SV, Pootakham W, Grossman AR. (2008) The central role of a SNRK2 kinase in sulfur deprivation responses. Plant Physiol 147: 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F, Zhao S, Dong H, Zhang H, Sun L, Miao C. (2010) Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J Integr Plant Biol 52: 298–307 [DOI] [PubMed] [Google Scholar]
- He P, Shan L, Sheen J. (2007) The use of protoplasts to study innate immune responses. Methods Mol Biol 354: 1–9 [DOI] [PubMed] [Google Scholar]
- Heyno E, Klose C, Krieger-Liszkay A. (2008) Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol 179: 687–699 [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irihimovitch V, Stern DB. (2006) The sulfur acclimation SAC3 kinase is required for chloroplast transcriptional repression under sulfur limitation in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 103: 7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Nakagami H, Hirt H. (2004) Heavy metal stress: activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol 136: 3276–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Saha A, Valon C, Leung J. (2011) Abscisic acid signal off the STARting block. Mol Plant 4: 562–580 [DOI] [PubMed] [Google Scholar]
- Jozefczak M, Remans T, Vangronsveld J, Cuypers A. (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13: 3145–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner A, Pękala I, Kaczanowski S, Muszyńska G, Hardie DG, Dobrowolska G. (2004) Biochemical characterization of the tobacco 42-kD protein kinase activated by osmotic stress. Plant Physiol 136: 3255–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K. (2012) Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta 1823: 398–405 [DOI] [PubMed] [Google Scholar]
- Kimura T, Shibagaki N, Ohkama-Ohtsu N, Hayashi H, Yoneyama T, Davies JP, Fujiwara T. (2006) Arabidopsis SNRK2.3 protein kinase is involved in the regulation of sulfur-responsive gene expression and O-acetyl-L-serine accumulation under limited sulfur supply. Soil Sci Plant Nutr 52: 211–220 [Google Scholar]
- Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T. (2004) Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Wawer I, Krzywińska E, Bucholc M, Dobrowolska G. (2011) SnRK2 protein kinases—key regulators of plant response to abiotic stresses. OMICS 15: 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D. (2006) Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic Biol Med 40: 1369–1376 [DOI] [PubMed] [Google Scholar]
- Lefèvre I, Marchal G, Edmond Ghanem M, Correal E, Lutts S. (2010) Cadmium has contrasting effects on polyethylene glycol-sensitive and resistant cell lines in the Mediterranean halophyte species Atriplex halimus L. J Plant Physiol 167: 365–374 [DOI] [PubMed] [Google Scholar]
- Liu F, Yoo B-C, Lee J-Y, Pan W, Harmon AC. (2006) Calcium-regulated phosphorylation of soybean serine acetyltransferase in response to oxidative stress. J Biol Chem 281: 27405–27415 [DOI] [PubMed] [Google Scholar]
- Liu X-M, Kim KE, Kim K-C, Nguyen XC, Han HJ, Jung MS, Kim HS, Kim SH, Park HC, Yun D-J, et al. (2010) Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry 71: 614–618 [DOI] [PubMed] [Google Scholar]
- Lozano-Juste J, León J. (2010) Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol 152: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na⁺/K⁺homeostasis in Arabidopsis under salt stress. J Exp Bot 63: 305–317 [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17: 9–15 [DOI] [PubMed] [Google Scholar]
- Martínez Domínguez D, Córdoba García F, Canalejo Raya A, Torronteras Santiago R. (2010) Cadmium-induced oxidative stress and the response of the antioxidative defense system in Spartina densiflora. Physiol Plant 139: 289–302 [DOI] [PubMed] [Google Scholar]
- Mikołajczyk M, Awotunde OS, Muszyńska G, Klessig DF, Dobrowolska G. (2000) Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12: 165–178 [PMC free article] [PubMed] [Google Scholar]
- Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. (2003) Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem 278: 31629–31639 [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei Z-M, Mori IC, Schroeder J. (2001) Abscisic acid activation of plasma membrane Ca(2+) channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J. (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S. (1992) Tobacco BY-2 cells line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132: 1–30 [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, Maruyama K, Yoshida T, Ishiyama K, Kobayashi M, et al. (2009) Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol 50: 1345–1363 [DOI] [PubMed] [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al. (2008) Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283: 8885–8892 [DOI] [PubMed] [Google Scholar]
- Olmos E, Martínez-Solano JR, Piqueras A, Hellín E. (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 54: 291–301 [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32: 539–548 [DOI] [PubMed] [Google Scholar]
- Ramirez L, Zabaleta EJ, Lamattina L. (2010) Nitric oxide and frataxin: two players contributing to maintain cellular iron homeostasis. Ann Bot (Lond) 105: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T, Smeets K, Opdenakker K, Mathijsen D, Vangronsveld J, Cuypers A. (2008) Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta 227: 1343–1349 [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Kohlhaas CF, Boyd AR, Yalla KC, Walsh K, Connell JM, Salt IP. (2010) Insulin-stimulated phosphorylation of endothelial nitric oxide synthase at serine-615 contributes to nitric oxide synthesis. Biochem J 426: 85–90 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, Del Río LA, Sandalio LM. (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150: 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas MC, Rodrígues-Serrano M, Compas FJ, Gómes M, Del Rio LA, Sandalio ML. (2004) Cadmium-induced subcellular accumulation of O2˙− and H2O2 in pea leaves. Plant Cell Environ 27: 1122–1134 [Google Scholar]
- Sagi M, Fluhr R. (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A. (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127: 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth CS, Remans T, Keunen E, Jozefczak M, Gielen H, Opdenakker K, Weyens N, Vangronsveld J, Cuypers A. (2012) Phytoextraction of toxic metals: a central role for glutathione. Plant Cell Environ 35: 334–346 [DOI] [PubMed] [Google Scholar]
- Sharma SS, Dietz K-J. (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14: 43–50 [DOI] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, et al. (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Sun LR, Hao FS, Lu BS, Ma LY. (2010) AtNOA1 modulates nitric oxide accumulation and stomatal closure induced by salicylic acid in Arabidopsis. Plant Signal Behav 5: 1022–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14: 691–699 [DOI] [PubMed] [Google Scholar]
- Tamás L, Valentovicová K, Halusková L, Huttová J, Mistrík I. (2009) Effect of cadmium on the distribution of hydroxyl radical, superoxide and hydrogen peroxide in barley root tip. Protoplasma 236: 67–72 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K. (2010) Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol 51: 1821–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Wu JS, Chia JC, Yang CC, Wu YJ, Juang RH. (2009) Phytochelatin synthase is regulated by protein phosphorylation at a threonine residue near its catalytic site. J Agric Food Chem 57: 7348–7355 [DOI] [PubMed] [Google Scholar]
- Wawer I, Bucholc M, Astier J, Anielska-Mazur A, Dahan J, Kulik A, Wysłouch-Cieszynska A, Zareba-Kozioł M, Krzywinska E, Dadlez M, et al. (2010) Regulation of Nicotiana tabacum osmotic stress-activated protein kinase and its cellular partner GAPDH by nitric oxide in response to salinity. Biochem J 429: 73–83 [DOI] [PubMed] [Google Scholar]
- Wawrzynski A, Kopera E, Wawrzynska A, Kaminska J, Bal W, Sirko A. (2006) Effects of simultaneous expression of heterologous genes involved in phytochelatin biosynthesis on thiol content and cadmium accumulation in tobacco plants. J Exp Bot 57: 2173–2182 [DOI] [PubMed] [Google Scholar]
- Wojas S, Clemens S, Hennig J, Sklodowska A, Kopera E, Schat H, Bal W, Antosiewicz DM. (2008) Overexpression of phytochelatin synthase in tobacco: distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J Exp Bot 59: 2205–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yin H, Liu X, Li X. (2010) Salt affects plant Cd-stress responses by modulating growth and Cd accumulation. Planta 231: 449–459 [DOI] [PubMed] [Google Scholar]
- Xuan Y, Zhou S, Wang L, Cheng Y, Zhao L. (2010) Nitric oxide functions as a signal and acts upstream of AtCaM3 in thermotolerance in Arabidopsis seedlings. Plant Physiol 153: 1895–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Koizumi N, Nakamichi N, Kiba T, Yamashino T, Mizuno T. (2004) Rapid response of Arabidopsis T87 cultured cells to cytokinin through His-to-Asp phosphorelay signal transduction. Biosci Biotechnol Biochem 68: 1966–1976 [DOI] [PubMed] [Google Scholar]
- Yeh C-M, Chien P-S, Huang H-J. (2007) Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J Exp Bot 58: 659–671 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xie Z, Dong Y, Wang S, Liu C, Zou M-H. (2008) Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem 283: 27452–27461 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang S, Klessig DF. (1997) Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9: 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Chen L, Zhang LL, Zhang WH. (2009) Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]