Abstract
It is assumed that the refilling of drought-induced embolism requires the creation of an osmotic gradient between xylem parenchyma cells and vessel lumens to generate the water efflux needed to fill the void. To assess the mechanism of embolism repair, it is crucial to determine if plants can up-regulate the efflux of osmotically active substances into embolized vessels and identify the major components of the released osmoticum. Here, we introduce a new approach of sap collection designed to separate water from nonembolized (functional) and embolized (nonfunctional) vessels. This new approach made possible the chemical analysis of liquid collected from both types of vessels in plants subjected to different levels of water stress. The technique also allowed us to determine the water volumes in nonfunctional vessels as a function of stress level. Overall, with the increase of water stress in plants, the osmotic potential of liquid collected from nonfunctional vessels increased while its volume decreased. These results revealed the presence of both sugars and ions in nonfunctional vessels at elevated levels in comparison with liquid collected from functional vessels, in which only traces of sugars were found. The increased sugar concentration was accompanied by decreased xylem sap pH. These results provide new insight into the biology of refilling, underlining the role of sugar and sugar transporters, and imply that a large degree of hydraulic compartmentalization must exist in the xylem during the refilling process.
Long-distance water transport in vascular plants occurs in a conduit network of nonliving cells connecting roots to leaves (Sperry, 2003). In certain conditions, such as drought and/or high evaporative demand, the water column within the lumen of xylem vessel or tracheid can be subjected to tensions that result in cavitation and the subsequent formation of embolism, causing a decrease in stem hydraulic conductance and a loss of plant productivity (Tyree and Sperry, 1989; Hölttä et al., 2009; Zwieniecki and Holbrook, 2009). Plants have evolved several mechanisms in order to mitigate the loss of the water transport capacity. These include shading leaves or small branches (shrubs) to lower evaporative demand, generating root pressure (small herbaceous plants) to refill embolized conduits, and growing new vessels or tracheids to replace lost capacity (Sperry et al., 1987; Stiller and Sperry, 2002). However, these strategies are limited in their usefulness, since to be successful they require both relief from water stress/transpiration and prolonged time. The ability of plants to dynamically refill embolized conduits under adverse conditions, such as large soil water deficits or high transpiration rates, would allow for greater flexibility in plants’ response to water stress and the avoidance of temporal losses to photosynthetic capacity. How refilling can occur in the presence of large xylem tension has proved to be difficult to understand (Holbrook and Zwieniecki, 1999; Tyree et al., 1999), and only recently has in vivo imaging undoubtedly confirmed the ability of plants to refill embolized vessels (Holbrook et al., 2001; Clearwater and Goldstein, 2005) and that water droplets preferentially are formed on the vessel walls adjacent to parenchyma cells (Brodersen et al., 2010). However, despite significant scientific efforts (Salleo et al., 1996; Zwieniecki and Holbrook, 2009; Secchi and Zwieniecki, 2010; Nardini et al., 2011), the mechanism responsible for embolism refilling under negative pressure is still not well understood.
Various studies have proposed and partially confirmed that the refilling process requires a source of water to fill the empty conduits and a source of energy to overcome existing free-energy gradients acting against it. Both sources, water and energy, have to be provided by the adjacent living parenchyma cells, and their role in embolism refilling is confirmed by studies showing that physical damage to phloem or metabolic inhibition of parenchyma cells in stems prohibited the recovery process (Salleo et al., 2004; Zwieniecki et al., 2004). If xylem parenchyma cells supply water for refilling, or at least for part of it, a role for aquaporins in this process can be expected. Studies on walnut (Juglans regia) showed that higher expression of two Plasma membrane Intrinsic Protein2 (PIP2) genes (JrPIP2.1 and JrPIP2.2) was observed in vessel-associated parenchyma cells at the same time that embolism refilling took place (Sakr et al., 2003). Moreover, expression levels of several PIP1 and PIP2 genes were shown to increase during the refilling process in some other species, including Populus trichocarpa and grapevine (Vitis vinifera; Kaldenhoff et al., 2008; Secchi and Zwieniecki, 2010; Secchi et al., 2011; Perrone et al., 2012). Recently, a detailed full analysis of the transcriptome in response to the presence of embolism in poplar (Populus trichocarpa) stems has revealed the complexity of genetic activity that is associated with the process of refilling. It was shown that different aquaporin subfamilies were strongly up-regulated during refilling. This up-regulation may facilitate the release of water volumes to refill the empty vessels during recovery from embolism (Secchi et al., 2011). While aquaporins allow for water facilitation between parenchyma cells and xylem, they are passive transporters (i.e. water flows through them down the free-energy gradient). Thus, to achieve refilling, a mechanism for driving water potentials in the embolized xylem lumens more negative than in the surrounding vascular parenchyma is required.
As the process of embolism refilling under tension is energy demanding, it consequently requires an adequate supply of carbohydrates to alter the preexisting free-energy gradients. Several studies have demonstrated that the incidence of embolism alters carbohydrate metabolism and carbon partitioning between starch and soluble sugars in the different tissues as well as related enzyme activities and gene expression. Both visualization techniques and enzymatic analysis of nonstructural carbohydrates showed a decrease in starch content and a decreased expression of the related genes (e.g. amylase) in response to embolism formation. Furthermore, the drop in starch content was associated with an increase in the level of Suc in parenchyma cells (Regier et al., 2009; Salleo et al., 2009; Secchi and Zwieniecki, 2010; Nardini et al., 2011). These results are strongly supported by a recent transcriptome analysis that found in response to embolism both down-regulation of genes transcribing for the monosaccharide metabolic pathways and strong up-regulation of those involved in the disaccharide metabolic pathways that include starch metabolism (Secchi et al., 2011). However, the role in refilling of sugars derived from depolymerization of starch stored in xylem parenchyma is unknown, and two hypothesis may be proposed: (1) the sugars contribute indirectly to the generation of an ion efflux into the xylem apoplast via respiration (Glc is converted into pyruvate and the energy released is stored in NADH and ATP that can be used to activate the membrane transporters), thereby producing the necessary osmotic gradient; or (2) the sugars themselves are transported out of the parenchyma cells and loaded into cavitated vessels, where they directly contribute to the generation of the osmotic gradients driving water flow from the parenchyma to the embolized conduits.
The transport of sugars between cells and apoplast is mediated by plasma membrane sugar/proton cotransporters, as energized by membrane H+-ATPase. Proton pumps have also been localized in xylem-associated cells (De Boer and Volkov, 2003), and treatments inducing their inhibition led to inhibition of xylem refilling (Salleo et al., 2004). Besides being involved in driving the import/export of sugars between parenchyma and xylem conduits, the proton pumps are hypothesized to control the apoplastic pH by driving H+ ions into the sap of well-watered plants (Sharp and Davies, 2009). Furthermore, the gradient in pH across the cellular membrane plays an important role in influencing the direction of sugar flow via proton cotransport across plasma membranes. Alteration in pH is one of the first chemical changes measurable in xylem sap from plants exposed to drought (Bahrun et al., 2002; Sobeih et al., 2004), and sap alkalization is often observed in transpiring plants. It is theorized that the increase in sap pH results in an increase in abscisic acid concentration in transpiring tissues, limiting water loss in drying soils via stomatal closure. However, the increase in xylem sap pH in drought conditions is not a universal phenomenon. A recent study demonstrates that in woody plants, xylem sap alkalization is much less common than in the annual species, and of the 22 species studied, only four showed a pH increase in sap collected from the transpiration stream (Sharp and Davies, 2009). As sap pH is an important parameter that can influence sugar transport across cellular membranes, measuring pH along with sap osmotic properties should yield a better understanding of the chemistry that drives refilling.
One of the big stumbling blocks in gaining insight into the process of refilling is our inability to characterize the properties of the liquid derived specifically from the vessels that are being refilled. The volume of this water is very small and, as observed recently, randomly distributed across the stem (Brodersen et al., 2010). Accessing this liquid for analysis presents a major technical challenge. Here, we present a new approach to collecting sap designed to separate water from functional vessels (not embolized) from that from nonfunctional vessels (embolized, that are presumably being refilled). We then analyze the chemical properties of the xylem sap collected from both populations of vessels, embolized and functional, in plants subjected to different levels of water stress, with the goal of determining the major components of the osmotic driving force responsible for embolism refilling.
RESULTS
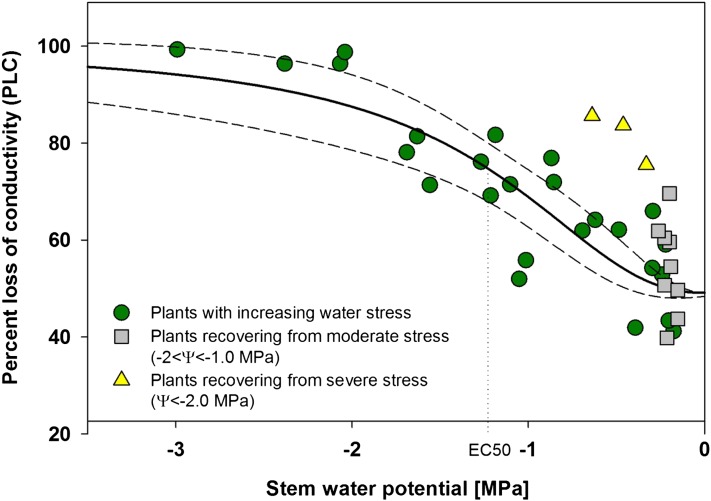
Populus nigra stems were vulnerable to stress-induced embolism. Initial percentage loss of conductivity (PLC) in well-watered plants was relatively high, averaging around 50% (Fig. 1). Further increases in PLC were observed with decreasing stem water potential, reaching approximately 100% loss below −2.5 MPa. The fitted four-parameter logistic curve (“dose response curve”), in the form of PLC = minPLC + (maxPLC − minPLC)/(1 + (Ψ/EC50)slope), was constrained with minimum PLC (minPLC) at 49.1% (average of initial PLC values on well-watered plants) and maximum PLC (maxPLC) at 100%. The resulting response function predicts 50% loss of functional vessels (EC50) at −1.22 MPa (sd = 0.1068, t = 11.42, P < 0.0001) with relatively slow phase of increase in PLC (i.e. slope = −2.27 [MPa−1]; sd = 0.53, t = 4.22, P = 0.0003). The fit was statistically significant (r2 = 0.74, P < 0.0001; Fig. 1). Relief from water stress resulted in a significant increase in stem water potential over 2 h to prestress levels, but recovery of PLC was highly variable, with moderately stressed plants (−2.0 < Ψ < 1.2 MPa) showing a significant drop in PLC over a period of 2.5 h from an average of 75% to 54% (t = 4.52, degrees of freedom [df] = 12, P < 0.001). PLC in recovered plants was not significantly different from that observed in never-stressed plants, 54% and 51%, respectively (t = 0.67, df = 14, P = 0.51). Severely stressed plants (Ψ < −2.0 MPa) also showed significant recovery in PLC, from 97% to 82% (t = 10.8, df = 5, P < 0.001), but these plants did not recover to prestress values.
Figure 1.
P. nigra PLC in relation to stem water potential. Data were fitted with a dose-response curve (solid line) in the form of PLC = minPLC + (maxPLC − minPLC)/(1 + (Ψ/EC50)slope), where minPLC is minimum PLC in nonstressed plants (49.1%), maxPLC is 100%, EC50 represents 50% loss of initial functionality [minPLC + (maxPLC − minPLC)/2], and slope is the rate of PLC increase at EC50. The dashed lines represent the 95% confidence interval for the fit. PLC of plants recovering from stress was not used in the fitting procedure. [See online article for color version of this figure.]
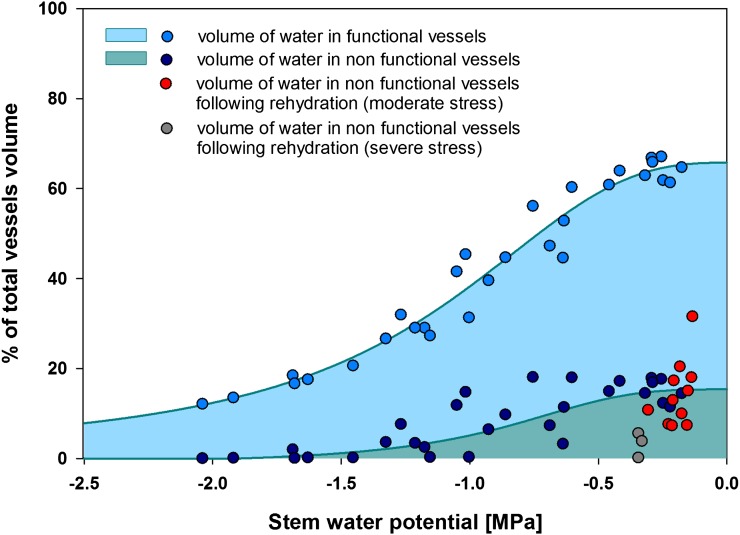
The volume of water in nonfunctional vessels was negatively correlated with stem water potential. Nonfunctional volume was in the range of 5% to 20% of total vessel volume in well-watered and low-stressed plants and dropped dramatically to under 10% in moderately stressed plants. Only very small volumes of water were collected from nonfunctional conduits of plants stressed to below −2.0 MPa (Fig. 2). Estimates of water volume from functional vessels combined with volume from nonfunctional vessels gave the total water content in vessels of transpiring plants across the range of experienced water potentials. The volume estimate is higher than that predicted by the PLC curve, as it also contains water from nonfunctional vessels. Plants recovering from severe stress (Ψ < −2.0 MPa) had very low volumes of water in nonfunctional vessels (below that predicted based on water potential), while plants recovering from moderate stress (−2.0 < Ψ < −1.20 MPa) showed volumes in the range expected for current water potential (Fig. 2).
Figure 2.
Analysis of water volume in functional and nonfunctional vessels of P. nigra in relation to water stress level.
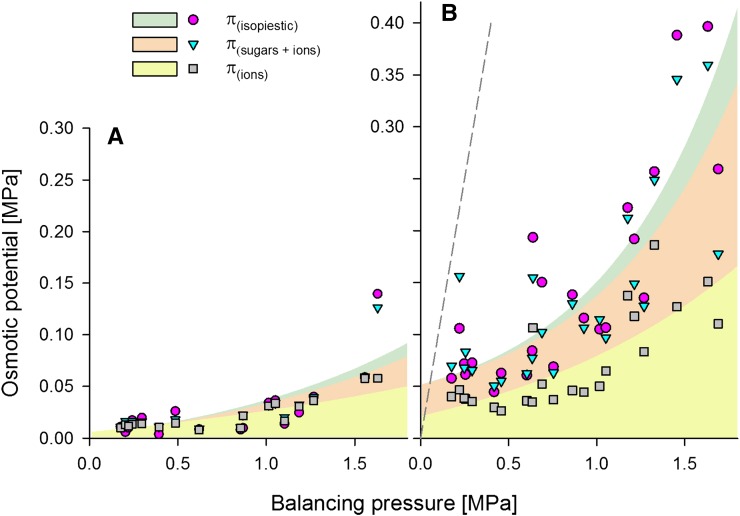
The osmotic potentials of water collected from functional vessels over the range of plant stress from well-watered to moderately stressed plants were very low (in most cases below 0.05 MPa; Fig. 3A). In the case of sap collected from nonfunctional vessels from the same range of the stress, osmotic potential was higher, reaching 0.4 MPa in plants stressed to −1.5 MPa (Fig. 3B). Despite these much higher values, they were not close to a 1:1 relation with stress (i.e. the total osmotic potential of the liquid remaining in nonfunctional vessels could not balance the stress level). In liquid collected from functional vessels, osmotic potential could be accounted for by the presence of cation-based osmotica, as calculated from K+ equivalent concentrations. Sugars were almost not present in samples collected from stems with stress levels above −1.5 MPa, and only one sample, at −1.6 MPa, had an elevated sugar level. This chemical composition pattern dramatically changed in liquid collected from nonfunctional vessels. Here, ion-based osmoticum constituted only 50% of total osmoticum, while the rest came from total soluble carbohydrates (estimated from the Glc concentration equivalent). Interestingly, the 50% level for sugar held over the entire range of water stress tested (Fig. 3B).
Figure 3.
Changes in the osmotic potential of liquid collected from functional (A) and nonfunctional (B) vessels in relation to stem water potential (balancing pressure at the time of sample collection). Total osmotic potential (π) was determined using isopiestic psychrometric measurements. Estimation of π from sugar was calculated as the equivalent of Glc content and that of π from ions as the equivalent of K+ ion concentration. The dashed line represents a 1:1 relation between balancing pressure and osmotic potential of liquid.
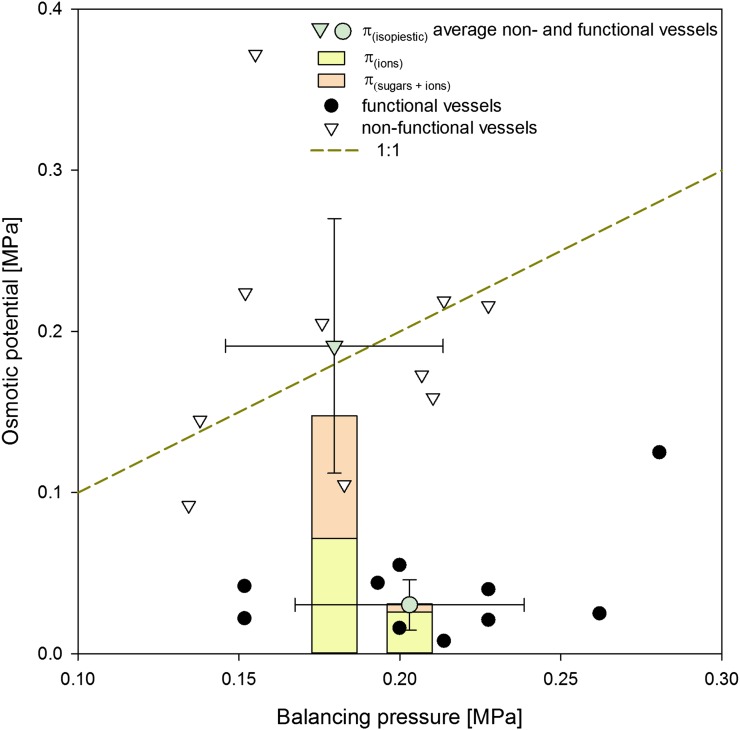
The osmotic potential of liquid collected from functional vessels in plants recovering from stress was very similar to that of nonstressed plants (Fig. 4). Average osmotic potential was approximately 0.03 MPa and could be accounted for by ions with very little contribution from sugars. Liquid collected from nonfunctional vessels had significantly higher osmotic potential (on average approximately 0.2 MPa) than the liquid collected from functional vessels (P < 0.001, df = 22, t = 6.19) as well as a higher contribution from sugar-based osmotica (approximately 50%). It is also important to note that the osmotic potential of sap from nonfunctional vessels in plants recovering from stress fell on a 1:1 ratio to balancing pressure (i.e. plant water potential).
Figure 4.
Osmotic potential of liquid collected from functional and nonfunctional vessels of plants recovering from moderate stress (−2.0 < Ψ < 1.0 MPa). Total osmotic potential (π) of liquid collected from nonfunctional vessels was significantly higher than that collected from functional vessels (Student’s t test, P < 0.001). The composition of the osmoticum also differed between two water sources, with ions being a major component in functional vessels and an equal importance of sugars and ions in nonfunctional vessels. [See online article for color version of this figure.]
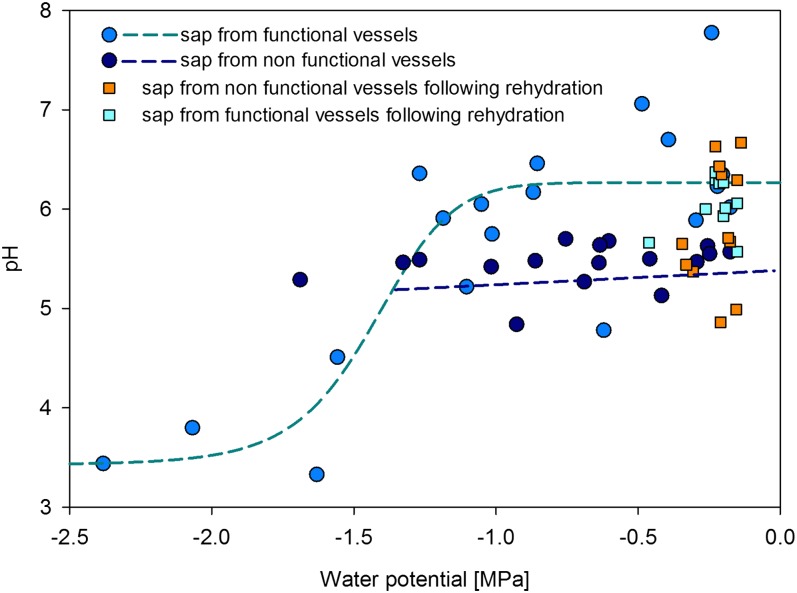
Xylem sap pH collected from functional vessels of well-watered to moderately stressed plants (−1.2 < Ψ < −0.3 MPa) was not correlated with the stress level and ranged between 5.5 and 7.5. Severe water stress resulted in a sudden drop of xylem sap pH to approximately 3.5. Such a response was well described by a dose-response curve (Fig. 5). Values of pH in nonfunctional vessels were significantly lower (pH = 5.44) than in functional vessels (pH = 6.18) for the same interval of stem water potential (well-watered and moderately stressed plant; −1.2 < Ψ < −0.2 MPa; P < 0.001, df = 30, t = 4.08). However, there were no significant differences in the pH values of sap collected from the nonfunctional (pH = 5.82) and functional (pH = 6.04) vessels of plants recovering from stress (Fig. 5).
Figure 5.
Relationship between xylem sap pH and stem water potential. Sap from functional vessels was fitted with a dose-response curve: pH = minpH + (maxpH − minpH)/(1 + (Ψ/EC50)slope), where maxpH = 6.26, minpH = 3.42, EC50 = −1.43, and slope = 10 (r2 = 0.71). The volume of liquid collected from nonfunctional vessels of severely stressed plants was not enough to measure pH. No obvious relationship between pH and plant water stress was found for liquid from nonfunctional vessels from a linear fit (r2 = 0.01).
DISCUSSION
The research presented in this report provides a “first look” into the basic chemistry of refilling. Direct observations of water droplets in embolized vessels using cryoscanning electron microscopy (Facette et al., 2001; Melcher et al., 2001) or observations of the dynamics of the refilling process using x-ray microscopy (Lee and Kim, 2008; Brodersen et al., 2010), together with the analysis of xylem carbohydrate metabolism dynamics (Améglio et al., 2004; Gupta and Kaur, 2005; Salleo et al., 2009; Secchi and Zwieniecki, 2011) and transcriptome activity of parenchyma cells (Secchi et al., 2011), provide indirect evidence that xylem parenchyma cells supply both the energy and water required to drive the refilling process (Zwieniecki and Holbrook, 2009). Further progress in our understanding of refilling could come from linking observations of droplet dynamics with cellular activity, work that requires the ability to study the properties of water collected from embolized vessels, as demonstrated by a novel method used in this work. The results presented here are focused on the determination of the major components of the driving force to achieve refilling and on the corresponding changes in the volume of water in nonfunctional vessels during the onset of stress. The pattern of drought-induced embolism, and the ability to refill embolized vessels in P. nigra, was not qualitatively different from observations made on other species showing this behavior (Zwieniecki and Holbrook, 1998; Stiller and Sperry, 2002; Stiller et al., 2005; Lovisolo et al., 2008), even if this particular poplar species showed a relatively high native level of embolism. It is possible that fast-growing P. nigra utilizes only outer layers of vessels in a similar way to Acer saccharum (Melcher et al., 2003), while the determination of PLC on short sections includes a pool of permanently nonfunctional early wood vessels. Increased stress resulted in increased levels of embolism, expressed here as PLC, while at any given stress level these plants maintained a particular PLC within a dynamic range, and upon rehydration of the plants, recovery of xylem transport capacity was observed. Thus, the results for the sap compositions for P. nigra are likely to be generalizable to other species showing refilling under tension.
This analysis revealed several novel and important aspects of xylem refilling. The osmotic potential of liquid collected from nonfunctional vessels increased with the increase of water stress in plants. However, it could not account for the total level of stress, leaving a significant gap in the free-energy gradient required to move water from parenchyma cells into adjacent vessels, nor could this energy gap be explained by the less than 5% dilution of water from nonfunctional vessels with water from functional vessels (see contamination analysis in “Materials and Methods”). Yet, despite the observed energy gap, there was a significant volume of water present in nonfunctional vessels, up to 20% of total vessel volume. This relative volume was relatively constant until water stress levels became more negative than −1.2 MPa, when it suddenly dropped. The presence of water that is not under tension in stems of stressed plants, with osmotic water potential lower than that required to balance the water stress as estimated from the balancing pressure method, calls for future tests of the general assumption of free-energy equilibrium across the plant stem. Theoretical considerations suggesting that hydraulic isolation of xylem vessels is required for refilling (Vesala et al., 2003; Choat et al., 2009) may need to be further expanded to include temporal and spatial water potential disequilibria. Requirements for such hydraulic/energy-isolated domains would underline the importance of stem transport sectoriality (Ellmore et al., 2006; Zanne et al., 2006), the persistence of leaf traces, and the role of phyllotaxy in protecting plants from embolism formation and allowing embolism repair against apparent energy gradients (Holbrook and Zwieniecki, 1999; Tyree et al., 1999; Zwieniecki and Holbrook, 2009). Temporal/spatial disequilibria of water potential in stems can also result from the hydraulic properties of xylem parenchyma cells if ratio of water volumes moving across them to resistance is relatively low.
The osmotic potential of liquid derived from nonfunctional vessels was more negative than that derived from functional vessels (approximately five times lower for any given stress level). In addition, the osmotic potential of sap from functional vessels could be entirely accounted for by the measured concentrations of inorganic ions. In contrast, the osmotic potential of liquid from nonfunctional vessels came from an approximately 50:50 split between inorganic ions and sugar molecules. This increased concentration of sugars was already present in well-watered plants, and the split remained similar through the entire range of plant water stress (from −0.2 to −1.5 MPa). The increased level of sugars in nonfunctional vessels persisted in plants that were recovering from moderate stress. In these plants, sugars also accounted for approximately 50% of osmotic potential. These observations of increased concentration of sugars suggest that, indeed, parenchyma cells are involved in the sugar release to embolized vessels (Améglio et al., 2004; Zwieniecki and Holbrook, 2009; Secchi et al., 2011; Secchi and Zwieniecki, 2011). These findings are also consistent with previous studies showing changes in starch content (Bucci et al., 2003; Salleo et al., 2004, 2009; Nardini et al., 2011; Secchi and Zwieniecki, 2011) and a transcriptome response to embolism in changes in sugar metabolism pathways (including starch degradation; Secchi et al., 2011).
The increased sugar efflux to embolized vessels coincides with the efflux of inorganic ions, as in all samples from nonfunctional vessels ion concentrations were also elevated relative to sap from functional vessels. A previous study of transcriptome analysis has shown an increase in the expression level of metal ion transporters in response to embolism but no comparable increase in the expression of sugar transporters (Secchi and Zwieniecki, 2011). However, membrane Suc transporters are often bidirectional proton cotransporters, with the direction of transport depending on proton (pH) and Suc concentration gradients across the plasma membrane. If, indeed, embolism presence triggers starch degradation, it will lead to increased symplastic Suc concentration and stimulation of the Suc efflux. Coefflux of the protons would then lead to a decrease of the apoplastic pH. Indeed, our analysis revealed lower pH in the liquid collected from nonfunctional vessels (approximately 5.4 pH) than in functional vessels (approximately 6.2 pH). This drop in pH would eventually slow down the Suc release, but it could be countered by the activity of metal ion antiporters that would then generate an efflux of ions and an influx of protons, resulting in the maintenance of new pH homeostasis at a more acid apoplastic level. In addition, efflux of protons related to Suc outward transport could be counterbalanced with ATP-proton transporters. Under severe stress conditions, apoplastic pH was very low (3.5). Such low apoplastic pH should strongly reduce the potential for efflux of Suc from cells, preventing the buildup of sugar-related osmoticum necessary to sustain the presence of water in nonfunctional vessels. Indeed, there was very little or no water collected from nonfunctional vessels at severe stress levels. The levels of pH, sugar, and ion concentrations observed here in liquid collected from functional and nonfunctional vessels across the plant water stress levels are consistent with known Suc transporter properties and transcriptome activity.
In plants recovering from stress, the level of osmotica in nonfunctional vessels was on average adequate to account for the driving force required for refilling (i.e. to counterbalance the existing tension estimate; Fig. 4), although the volume of water was not dramatically different from that in plants never experiencing stress. Again, the osmotica present differ between functional and nonfunctional vessels just as in plants under stress, with a large (approximately 50%) contribution of sugars to sap in nonfunctional vessels. Sap pH in recovering plants was only slightly lower in nonfunctional vessels (pH = 5.82) than in functional vessels (pH = 6.04), but it was still consistent with the transport of sugars and ions. The slightly higher pH in nonfunctional vessels might reflect a reduction in refilling activity upon return to nonstress conditions. The fact that the osmotic potential of the liquid in nonfunctional vessels of recovering plants accounts for the free energy required to refill is consistent with the notion that, in some plants, successful refilling requires reduction in the level of water stress (Hacke and Sperry, 2003). However, the fact that this concentration of osmotica is similar to that found in nonfunctional vessels currently at the stress they were recovering from underscores the possibility that hydraulic isolation allows for prolonged persistence of energy disequilibria between different vessels and parts of the stem.
CONCLUSION
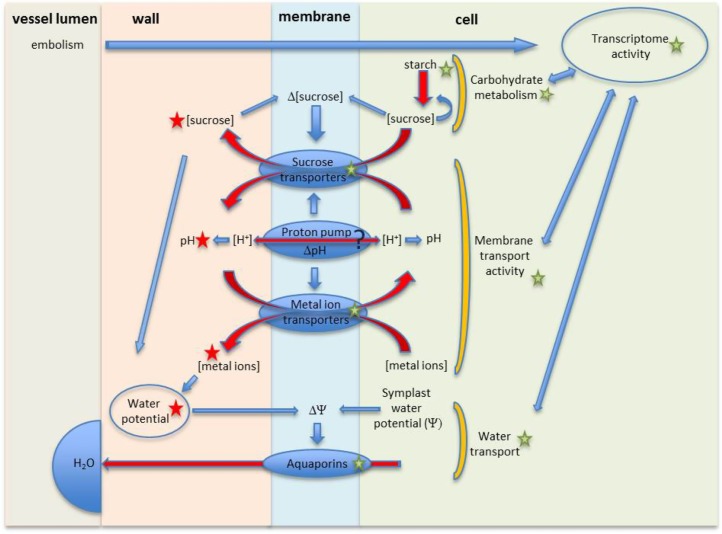
This first look at the basic chemistry of liquid collected from nonfunctional vessels in combination with previously published studies provides new insight into the physiology of refilling (Fig. 6). Embolism formation, or its presence, triggers a large set of transcriptome and physiological responses (Melcher et al., 2003; Arango-Velez et al., 2011; Nardini et al., 2011; Secchi et al., 2011; Perrone et al., 2012).
Figure 6.
Schematic illustration of the membrane-level physiology of refilling. See text for explanation. Red arrows represent fluxes. Blue arrows represent action/influence. Green stars represent information available from previous studies. Red stars represent new information from our analysis. [See online article for color version of this figure.]
Transcription responses include the up-regulation of aquaporins, metal ion transporters, and carbohydrate metabolism but not sugar transporters (Secchi et al., 2011; Secchi and Zwieniecki, 2011). Analyses of carbohydrate metabolism in multiple studies suggest the degradation of starch to be associated with embolism formation (Salleo et al., 2009; Secchi and Zwieniecki, 2011). This presumably leads to Suc accumulation in the cell that would trigger an efflux of sugar via Suc proton cotransporters (Carpaneto et al., 2005; Sauer, 2007; Ayre, 2011; Geiger, 2011). The activity of Suc cotransporters leads to an accumulation of Suc and protons in the vessel walls/lumen. This study confirms the presence of sugars in nonfunctional vessels and their contribution to an osmotic driving force as well as the presence of lower pH (suggesting the efflux of protons). Lower apoplastic pH might trigger the activation of proton pumps and metal ion antiporters (Secchi et al., 2011). The activity of the proton pumps and metal antiporters can counteract the drop of pH due to Suc efflux and stabilize it at a desirable level. The generated ion efflux provides additional osmotica, as shown in this report. Together, sugars and ions can account for the driving force that could generate the refilling process under low-water stress (Fig. 4; during rehydration) or if embolized vessels are hydraulically isolated from functional xylem even during active transpiration under moderate stress (Fig. 3). The interaction of sugar and metal ion channels that recirculate protons to accumulate osmoticum in the apoplast represents a physiological activity that would promote refilling and that is strongly supported by the chemical properties of liquid from nonfunctional vessels found in this study (Fig. 6).
MATERIALS AND METHODS
Plant Materials and Experimental Design
Populus nigra cuttings were rooted into moist potting mix in a 5.7- × 8.3-cm Rose Pot. Plants were then transferred into 1-gallon pots filled with potting mix and were grown in a greenhouse for 10 months (July–April). Ambient conditions in the greenhouse were characterized by a temperature maintained in the range of 17°C to 29°C, and the natural daylight was supplemented with light from metal halogen lamps (500–600 µmol photons m−2 s−1) to maintain a 12/12-h light/dark cycle. Plants were approximately 1.2 to 1.5 m tall at the onset of the experiments.
A total of 53 P. nigra plants were used in this study, and of these, 10 plants were kept as controls. Control plants were watered to field capacity twice during the day (around 8 am and 2 pm). Water stress was imposed in succession on the remaining 43 plants by a reduction of irrigation. The level of water stress depended on drought duration. Approximately one-half of the control and stressed plants were used to collect xylem sap from functional vessels and to determine the PLC. The remaining plants (both control and stressed) were used to collect xylem sap from nonfunctional vessels (details of the applied technique are described below). All physiological measurements were performed in the morning from 9 am to 12 pm.
An additional 26 plants were first stressed and then rewatered in the morning (9 am), and the dynamics of refilling were followed. Measurements on plants recovering from stress were performed 2 h after irrigation, with one final measurement implemented the following day at 9 am.
Measurements of Stem Water Potential and Stem Hydraulic Conductivity
Stem water potential was measured for each plant using equilibrated nontranspiring (bagged) leaves. Mature leaves were covered with aluminum foil and placed in a humidified plastic bag for at least 15 min prior to excision and measurement. Fifteen minutes was shown to be an adequate time for hydraulic equilibration of nontranspiring leaves with stem water potential in some woody species (Fulton et al., 2001). In addition, we tested the validity of the 15-min equilibration time for our P. nigra plants (Supplemental Fig. S1). After excision, leaves were allowed to equilibrate for a few minutes and water potential was measured using a Scholander-type pressure chamber (Soil Moisture Equipment).
Following the determination of stem water potential, stem hydraulic conductivity was measured for one-half of the plants using a standard approach described previously (Secchi and Zwieniecki, 2010). Briefly, small sections of stems (approximately 4 cm long) were cut under water to prevent embolisms caused by air entering into the cut vessels. The initial hydraulic conductance (ki) of each stem segment was measured by determination of the flow rate of a filtered 10 mm KCl solution through the stem section from a water source located on a balance (Sartorius ± 0.01 mg) and connected to the stem by a plastic tube. The stem was submerged in a water bath with the water level being approximately 10 cm below the level of water on the balance. After a steady flow rate was reached (within just a few minutes), the tube connecting the stem to the balance was closed, and a bypass was used to push water across the segment under approximately 2 bars of pressure for approximately 20 s to remove embolism. Stem conductance was then remeasured to find maximum conductance (kmax). The PLC was calculated as PLC = 100 × (kmax − ki)/kmax.
Xylem Sap Collection from Functional Vessels
Xylem sap of functional vessels was collected from the same plants that were used to determine PLC values and stem water potential. A few seconds after cutting the stem under water for PLC determination (described above), leaves were removed in order to prevent evaporation and to avoid any loss of water in functional vessels due to evaporation. A new cut 20 cm above the first one was made (the 20-cm piece of stem, divided in three different sections, was used to determine PLC). The remaining whole stem was then attached through a plastic tube to a syringe needle. The needle was threaded through a rubber cork to a small vacuum chamber with the needle tip placed in the 1.5-mL plastic tube. After generation of a vacuum (0.027 MPa absolute pressure), small pieces of stem were consecutively cut from the top, allowing liquid from open vessels to be sucked out of the stem and collected in the tube. The collected liquid was then frozen and kept until further analysis. This method only allows for collecting liquid that a near vacuum can remove from the stem (i.e. no liquid could be removed if it was separated from the applied suction by a bordered pit membrane).
Xylem Sap Collection from Nonfunctional Vessels
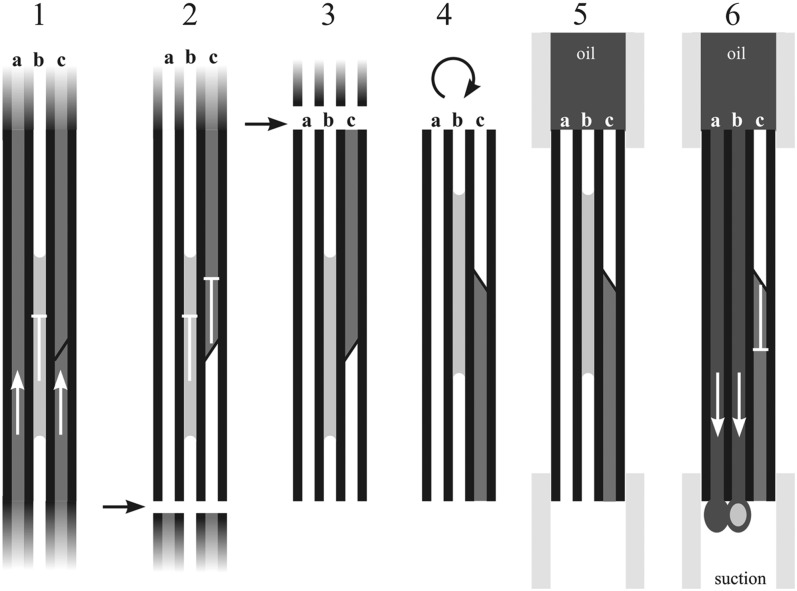
A new approach was developed to collect liquid from nonfunctional vessels. Stems were cut in the air approximately 20 cm above the soil, allowing for the removal of water from functional conduits to the first border pit membrane field by apical plant suction (Fig. 7, step 1). Plants were then placed in a plastic bag to prevent the development of further water stress. After this initial preparation, xylem sap was collected following these steps. Stems were cut in air, allowing all nonembolized vessels to empty themselves due to preexisting suction within the remaining apical part of the plant. Water in functional vessels was presumably only sucked to the nearest (most basal) bordered pit membrane (Fig. 7, step 2). The first cut was then followed by the second cut, producing an approximately 4-cm-long stem section that could contain water in nonfunctional vessels (i.e. that was not under suction) and water from functional vessels that was held at the border pit field (Fig. 7, step 3). The approximately 4-cm stem segment was then rotated, and the distal end was placed into a tube connected to a small vacuum chamber (as described above), while the proximal end was placed into a tube filled with low-viscosity silicon oil (Fig. 7, steps 4 and 5). Application of the vacuum forced oil to pass through all vessels that were open at both ends, removing any liquid from them, while vessels occluded by bordered pit fields would have remained impassible, as suction could not be translated through them, thus allowing any remaining water from functional vessels to stay in the stem section (Fig. 7, step 6). Since the volumes of water collected with this method were very small and could potentially evaporate in the vacuum environment, silicon oil was used to prevent that. Approximately 60 to 80 cm of stem was consecutively cut to collect liquid from nonfunctional vessels. The oil-filled tubes with suspended water droplets were then spun in a centrifuge to collect the water at the bottom, and its volume was measured. In order to estimate the total vessel volume (see below), the length and diameter of each stem section were determined.
Figure 7.
Schematics present the technical steps involved in collecting sap from nonfunctional vessels. 1, An intact plant with functional vessels under tension (a and c) and a nonfunctional vessel partially filled with water (b). 2, Collection starts with the first cut made in the air. This would allow water under tension to be sucked toward the leaves in vessels a and c but not the water present in vessel b. In vessel c, water would only be sucked to the nearest border pit field. 3, Within several seconds following the first cut, a second cut is made and a portion of stem (3–4 cm) long is removed. It presumably contains some water under tension stuck on the bordered pit field (c) and water in nonfunctional vessels. 4, The section is then inverted and both ends are fitted to flexible tubes. 5, The upper tube is then filled with low-viscosity silicon oil and the lower end is fitted to a vacuum system. 6, A vacuum is generated that sucks oil through the empty vessels (a) and vessels that are open across the stem but filled with water droplets (b). However, the vacuum is not adequate to break water away from the border pit field (c). Oil containing small volumes of water from nonfunctional vessels is collected in centrifuge tubes and protects small droplets from evaporation in vacuum conditions. After several collection cycles, centrifuge tubes are spun and water is separated from oil at the bottom of the tube. Arrows and flat-ended lines represent the movement of water in vessels during the procedure of water collection.
Test of the Technique to Collect Water from Nonfunctional Vessels
The above technique for the collection of water from nonfunctional vessels was tested on a separate set of plants. A low concentration of sulforhodamine 101 dye was prepared such that it did not saturate the reading of light absorbance in 540-nm wavelength (Multiscan; Thermo Scientific). Then, a calibration curve was made with a series of subsequent dilutions of the dye. This low-concentration dye was then used to perfuse stems (1 m long) of well-watered plants (approximately −0.3 MPa water potential) using suction. Suction was applied as long as it was needed for dye to perfuse the whole stem, such that the collected dye was at the same level of absorption as the dye applied to the stem. Perfused stems were then cut in half, and one section was used to collect water from functional vessels (see above) and the other section was used to collect water from nonfunctional vessels (see above).
Relative absorption of 100% was assigned to the initial dye. Liquid collected using the functional vessel collection technique showed no dilution (101.9% with sd = 4.67 and n = 3). Liquid collected using the nonfunctional vessel technique showed a low-level presence of the dye, suggesting small contamination from functional vessels (3.8% with sd = 7.05 and n = 3). This small dilution was not significantly different from zero, and thus it was not used to adjust the determination of sap chemistry.
Determination of Vessel Volume
The PLC technique provides information about the percentage of nonfunctional vessels, while liquid collected from nonfunctional vessels informed us about the volume of the water in stem that was not under tension at the time of collection. To allow for the comparative analysis of these two sets of data, we conducted an analysis of stem segment vessel volume. Short pieces of stem segments (without bark) were perfused (2 bars of pressure) with water. Then, water was sucked from stem segments into a 1.5-mL plastic tube using the system described above. Water volume was then determined, and the stem’s length and diameter were measured. These measurements allowed for determination of the relationship between stem size and vessel volume and for subsequent assessment by nonfunctional water volume as a fraction of total vessel volume. An exponential function in the form of Vv = 28.049 × e(0.001049 × Sv) was fitted to the experimental data and later used to estimate total vessel volume (r2 = 0.78, P < 0.0001), where Vv is vessel volume and Sv is stem volume.
Isopiestic Determination of Sap Osmotic Potential
Xylem sap osmotic potentials were measured using an isopiestic psychrometer (Isopiestics). The psychrometer vapor chambers were prepared with filter paper discs saturated with distilled water ultrafiltered to 18.02 MΩ (EMD Millipore). The osmotic potential of the sap was determined by placing a 5- to 10-µL sample on a thermocouple suspended in the vapor chamber, followed by measurement of the resulting voltage output. The osmotic potential corresponding to the measured output was found by linear interpolation between two voltages induced by known solutions that bracketed the voltage induced by the sample. The sequence of measurements was as follows. First, the “high” known potential output reading was established, using 5 to 10 µL of distilled ultrafiltered water (Ψ = 0) on the thermocouple. Next, the unknown sample output was recorded, followed by a known solution expected to have a lower (more negative) osmotic potential than the unknown, with that expectation based on the observation of the difference in output between the unknown sample and the first known solution. All three droplet measurements were made with the same thermocouple and vapor chamber. After the voltage output for the second known potential solution was recorded, the final estimate of the osmotic potential of the unknown was calculated as: Ψsap = Ψh − (Vh − Vsap) × (Ψh − Ψl)/(Vh −Vl), where Ψsap = osmotic potential of the unknown sample, Ψh = osmotic potential of the low-output solution (here 0), Ψl = osmotic potential of the known solution more negative than the unknown, Vsap = thermocouple voltage output generated by the unknown sample, Vh = thermocouple voltage output generated by Ψh, and Vl = thermocouple voltage output generated by Ψl.
Carbohydrate Content in Xylem Sap
The anthrone-sulfuric acid assay described by Leyva et al. (2008) was used to quantify the carbohydrate content in xylem sap samples. The anthrone reagent was prepared right before analysis by dissolving 0.1 g of anthrone (0.1%, w/v) in a concentrated sulfuric acid (98%) solution. Standard solutions were prepared by diluting Glc Standard Solution (1.0 mg mL−1; Sigma).
Briefly, 150 µL of anthrone reagent was added to each well of the microplate containing 50 µL of standard solutions, positive control (water), xylem sap solutions, and blank. Plates were then kept for 10 min at 4°C. Then, the plates were incubated for 20 min at 100°C. After heating, plates were cooled for 20 min at room temperature, and A620 was read with a microplate multiscan reader (Thermo Scientific Multiskan). The colorimetric response was compared with the Glc standard curve (5, 1.5, 0.5, 0.15, and 0.05 mm), and total carbohydrate content was calculated as mg mL−1 Glc. From the deduced molal concentration of each xylem sap solution, the relative osmotic potential was calculated based on the law for perfect gases: Π = miRT, where m = molality of the solution (mol solutes per 1,000 g of water), i = a constant that accounts for ionization of the solute (for Glc, i = 1), R = the gas constant (0.00831 L MPa−1 mol−1 K−1), and T = temperature, 293.16 K.
Measurement of Ion Concentration and pH
Electrical conductivity measurements of liquid samples were performed with a custom-made system. A 5-µL capillary was fitted with gold electrodes at both ends and connected to a digital multimeter (True RMS digital multimeter 289; Fluka Europe). Liquid samples were sucked into the capillary using a pipettor. After each measurement, the pipette was washed with deionized water and air dried. Before and after each set of measurements, a series of potassium chloride solutions with different concentrations was used to establish a new calibration curve. Thus, electrical conductivity reflects the equivalent concentration of potassium ions.
The xylem sap from functional and nonfunctional vessels was collected in 100-mm3 tubes and kept on ice before the pH of each sample was measured using a micro pH electrode (MicroElectrodes).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Validation of hydraulic equilibration time for nontranspiring leaves.
Acknowledgments
We thank Fulton Rockwell for help with the isopiestic psychrometric measurements and comments on the manuscript.
Glossary
- PLC
percentage loss of conductivity
- df
degrees of freedom
References
- Améglio T, Decourteix M, Alves G, Valentin V, Sakr S, Julien JL, Petel G, Guilliot A, Lacointe A. (2004) Temperature effects on xylem sap osmolarity in walnut trees: evidence for a vitalistic model of winter embolism repair. Tree Physiol 24: 785–793 [DOI] [PubMed] [Google Scholar]
- Arango-Velez A, Zwiazek JJ, Thomas BR, Tyree MT. (2011) Stomatal factors and vulnerability of stem xylem to cavitation in poplars. Physiol Plant 143: 154–165 [DOI] [PubMed] [Google Scholar]
- Ayre BG. (2011) Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol Plant 4: 377–394 [DOI] [PubMed] [Google Scholar]
- Bahrun A, Jensen CR, Asch F, Mogensen VO. (2002) Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). J Exp Bot 53: 251–263 [DOI] [PubMed] [Google Scholar]
- Brodersen CR, McElrone AJ, Choat B, Matthews MA, Shackel KA. (2010) The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography. Plant Physiol 154: 1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Da L, Sternberg SL. (2003) Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ 26: 1633–1645 [Google Scholar]
- Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R. (2005) Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem 280: 21437–21443 [DOI] [PubMed] [Google Scholar]
- Choat B, Gambetta GA, Shackel KA, Matthews MA. (2009) Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiol 151: 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearwater M, Goldstein G. (2005) Embolism repair and long distance transport. In NM Holbrook, MA Zwieniecki, eds, Vascular Transport in Plants. Elsevier, pp 201–220
- De Boer AH, Volkov V. (2003) Logistics of water and salt transport through the plant: structure and functioning of the xylem. Plant Cell Environ 26: 87–101 [Google Scholar]
- Ellmore GS, Zanne AE, Orians CM. (2006) Comparative sectoriality in temperate hardwoods: hydraulics and xylem anatomy. Bot J Linn Soc 150: 61–71 [Google Scholar]
- Facette MR, McCully ME, Shane MW, Canny MJ. (2001) Measurements of the time to refill embolized vessels. Plant Physiol Biochem 39: 59–66 [Google Scholar]
- Fulton A, Buchner R, Olson B, Schwankl L, Gilles C, Bertagna N, Walton J, Shackel K. (2001) Rapid equilibration of leaf and stem water potential under field conditions in almonds, walnuts, and prunes. Horttechnology 11: 609–615 [Google Scholar]
- Geiger D. (2011) Plant sucrose transporters from a biophysical point of view. Mol Plant 4: 395–406 [DOI] [PubMed] [Google Scholar]
- Gupta AK, Kaur N. (2005) Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci 30: 761–776 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS. (2003) Limits to xylem refilling under negative pressure in Laurus nobilis and Acer negundo. Plant Cell Environ 26: 303–311 [Google Scholar]
- Holbrook NM, Ahrens ET, Burns MJ, Zwieniecki MA. (2001) In vivo observation of cavitation and embolism repair using magnetic resonance imaging. Plant Physiol 126: 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook NM, Zwieniecki MA. (1999) Embolism repair and xylem tension: do we need a miracle? Plant Physiol 120: 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä T, Cochard H, Nikinmaa E, Mencuccini M. (2009) Capacitive effect of cavitation in xylem conduits: results from a dynamic model. Plant Cell Environ 32: 10–21 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Ribas-Carbo M, Sans JF, Lovisolo C, Heckwolf M, Uehlein N. (2008) Aquaporins and plant water balance. Plant Cell Environ 31: 658–666 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kim Y. (2008) In vivo visualization of the water-refilling process in xylem vessels using x-ray micro-imaging. Ann Bot (Lond) 101: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva A, Quintana A, Sánchez M, Rodríguez EN, Cremata J, Sánchez JC. (2008) Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: method development and validation. Biologicals 36: 134–141 [DOI] [PubMed] [Google Scholar]
- Lovisolo C, Perrone I, Hartung W, Schubert A. (2008) An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytol 180: 642–651 [DOI] [PubMed] [Google Scholar]
- Melcher PJ, Goldstein G, Meinzer FC, Yount DE, Jones TJ, Holbrook NM, Huang CX. (2001) Water relations of coastal and estuarine Rhizophora mangle: xylem pressure potential and dynamics of embolism formation and repair. Oecologia 126: 182–192 [DOI] [PubMed] [Google Scholar]
- Melcher PJ, Zwieniecki MA, Holbrook NM. (2003) Vulnerability of xylem vessels to cavitation in sugar maple: scaling from individual vessels to whole branches. Plant Physiol 131: 1775–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Lo Gullo MA, Salleo S. (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Sci 180: 604–611 [DOI] [PubMed] [Google Scholar]
- Perrone I, Pagliarani C, Lovisolo C, Chitarra W, Roman F, Schubert A. (2012) Recovery from water stress affects grape leaf petiole transcriptome. Planta 235: 1383–1396 [DOI] [PubMed] [Google Scholar]
- Regier N, Streb S, Cocozza C, Schaub M, Cherubini P, Zeeman SC, Frey B. (2009) Drought tolerance of two black poplar (Populus nigra L.) clones: contribution of carbohydrates and oxidative stress defence. Plant Cell Environ 32: 1724–1736 [DOI] [PubMed] [Google Scholar]
- Sakr S, Alves G, Morillon RL, Maurel K, Decourteix M, Guilliot A, Fleurat-Lessard P, Julien JL, Chrispeels MJ. (2003) Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiol 133: 630–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, De Paoli D, Zippo M. (1996) Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytol 132: 47–56 [DOI] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Trifilò P, Nardini A. (2004) New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant Cell Environ 27: 1065–1076 [Google Scholar]
- Salleo S, Trifilò P, Esposito S, Nardini A, Lo Gullo MA. (2009) Starch-to-sugar conversion in wood parenchyma of field-growing Laurus nobilis plants: a component of the signal pathway for embolism repair? Funct Plant Biol 36: 815–825 [DOI] [PubMed] [Google Scholar]
- Sauer N. (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581: 2309–2317 [DOI] [PubMed] [Google Scholar]
- Secchi F, Gilbert ME, Zwieniecki MA. (2011) Transcriptome response to embolism formation in stems of Populus trichocarpa provides insight into signaling and the biology of refilling. Plant Physiol 157: 1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. (2010) Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant Cell Environ 33: 1285–1297 [DOI] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. (2011) Sensing embolism in xylem vessels: the role of sucrose as a trigger for refilling. Plant Cell Environ 34: 514–524 [DOI] [PubMed] [Google Scholar]
- Sharp RG, Davies WJ. (2009) Variability among species in the apoplastic pH signalling response to drying soils. J Exp Bot 60: 4363–4370 [DOI] [PubMed] [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. (2004) Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J Exp Bot 55: 2353–2363 [DOI] [PubMed] [Google Scholar]
- Sperry JS. (2003) Evolution of water transport and xylem structure. Int J Plant Sci 164: S115–S127 [Google Scholar]
- Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT. (1987) Spring filling of xylem vessels in wild grapevine. Plant Physiol 83: 414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller V, Sperry JS. (2002) Cavitation fatigue and its reversal in sunflower (Helianthus annuus L.). J Exp Bot 53: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Stiller V, Sperry JS, Lafitte R. (2005) Embolized conduits of rice (Oryza sativa, Poaceae) refill despite negative xylem pressure. Am J Bot 92: 1970–1974 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Mosca R, Lo Gullo MA, Mosca R. (1999) Refilling of embolized vessels in young stems of laurel: do we need a new paradigm? Plant Physiol 120: 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. (1989) Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol 40: 19–38 [Google Scholar]
- Vesala T, Hölttä T, Perämäki M, Nikinmaa E. (2003) Refilling of a hydraulically isolated embolized xylem vessel: model calculations. Ann Bot (Lond) 91: 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanne AE, Sweeney K, Sharma M, Orians CM. (2006) Patterns and consequences of differential vascular sectoriality in 18 temperate tree and shrub species. Funct Ecol 20: 200–206 [Google Scholar]
- Zwieniecki MA, Holbrook NM. (1998) Diurnal variation in xylem hydraulic conductivity in white ash (Fraxinus americana L.), red maple (Acer rubrum L.) and red spruce (Picea rubens Sarg.). Plant Cell Environ 21: 1173–1180 [Google Scholar]
- Zwieniecki MA, Holbrook NM. (2009) Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci 14: 530–534 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Feild TS, Holbrook NM. (2004) A potential role for xylem-phloem interactions in the hydraulic architecture of trees: effects of phloem girdling on xylem hydraulic conductance. Tree Physiol 24: 911–917 [DOI] [PubMed] [Google Scholar]