Abstract
We functionally characterized the grape (Vitis vinifera) VvPIP2;4N (for Plasma membrane Intrinsic Protein) aquaporin gene. Expression of VvPIP2;4N in Xenopus laevis oocytes increased their swelling rate 54-fold. Northern blot and quantitative reverse transcription-polymerase chain reaction analyses showed that VvPIP2;4N is the most expressed PIP2 gene in root. In situ hybridization confirmed root localization in the cortical parenchyma and close to the endodermis. We then constitutively overexpressed VvPIP2;4N in grape ‘Brachetto’, and in the resulting transgenic plants we analyzed (1) the expression of endogenous and transgenic VvPIP2;4N and of four other aquaporins, (2) whole-plant, root, and leaf ecophysiological parameters, and (3) leaf abscisic acid content. Expression of transgenic VvPIP2;4N inhibited neither the expression of the endogenous gene nor that of other PIP aquaporins in both root and leaf. Under well-watered conditions, transgenic plants showed higher stomatal conductance, gas exchange, and shoot growth. The expression level of VvPIP2;4N (endogenous + transgene) was inversely correlated to root hydraulic resistance. The leaf component of total plant hydraulic resistance was low and unaffected by overexpression of VvPIP2;4N. Upon water stress, the overexpression of VvPIP2;4N induced a surge in leaf abscisic acid content and a decrease in stomatal conductance and leaf gas exchange. Our results show that aquaporin-mediated modifications of root hydraulics play a substantial role in the regulation of water flow in well-watered grapevine plants, while they have a minor role upon drought, probably because other signals, such as abscisic acid, take over the control of water flow.
Plant aquaporins are involved in maintaining water transport from roots to leaves and cell homeostasis under all environmental conditions (Hachez et al., 2006). Reverse genetics has been up to now the most effective strategy to elucidate the physiological functions of specific aquaporin genes and to understand their roles in water transport and drought tolerance mechanisms in plants (Kaldenhoff et al., 1998; Siefritz et al., 2002). In the past decade, pioneering studies of overexpression or silencing of aquaporin genes were carried out in herbaceous model plants (for review, see Kaldenhoff et al., 2008). To date, with the exception of transgenic Eucalyptus species overexpressing a radish (Raphanus sativus) Plasma membrane Intrinsic Protein (PIP) aquaporin (Tsuchihira et al., 2010), reverse genetics studies have been performed in herbaceous species, such as Arabidopsis (Arabidopsis thaliana; Cui et al., 2008; Peng et al., 2008; Postaire et al., 2010), rice (Oryza sativa; Li et al., 2008; Matsumoto et al., 2009), tomato (Solanum lycopersicum; Sade et al., 2009), and tobacco (Nicotiana tabacum; Zhang et al., 2008). These plants, however, are not well suited for the task of assessing the role of aquaporins in complex processes of water transport and homeostasis, such as the transduction of hydraulic and nonhydraulic messages (Lovisolo et al., 2010) and the formation and recovery of embolisms (Secchi and Zwieniecki, 2010), which are typical of woody plants. Understanding the role of aquaporins in these processes requires reverse genetics to be applied to woody plants, despite the greater difficulty generally encountered in efficiently delivering foreign constructs into their DNA.
Many grapevine (Vitis spp.) species are well adapted to drought conditions and are thus expected to have developed efficient mechanisms to transfer water to their growing aerial organs and to limit negative effects of drought. Patterns of aquaporin expression and function are suspected to contribute significantly to this adaptation (Galmés et al., 2007; Vandeleur et al., 2009). Cultivated grapevine (Vitis vinifera) is traditionally a nonirrigated crop, and both yield and berry quality strongly depend on the vine adaptability to drought (for review of the molecular and physiological aspects of grapevine drought responses, see Lovisolo et al., 2010).
For years, protocols for the genetic transformation of grapevine have not been standardized into routinely applicable methods. Nevertheless, significant advances have recently been made in the development of transgenic technology, and transgenic plants from commercially important cultivars have been produced (Reustle and Büchholz, 2009). However, the genetic transformation of grapevine has been mainly focused on enhancing disease resistance against pathogens, and only a few studies aimed at elucidating the functions of endogenous genes have been carried out to date (Tesnière et al., 2006; Cutanda-Perez et al., 2009).
Eighty-three sequences annotated as aquaporins, belonging to different Vitis species, are present in GenBank, and 28 putative aquaporin genes have been identified in the grapevine genome (Fouquet et al., 2008; Shelden et al., 2009). Expression of some of these genes, belonging to the PIP and Tonoplast Intrinsic Protein subfamilies, has been studied in grape berry (Picaud et al., 2003; Fouquet et al., 2008; Choat et al., 2009) and vegetative tissues (Baiges et al., 2001; Vandeleur et al., 2009). The results of these studies suggest that aquaporins have important roles in facilitating water redistribution within the growing berry (Picaud et al., 2003; Fouquet et al., 2008; Choat et al., 2009) and in regulating adaptation to water stress (Vandeleur et al., 2009). However, these results, being based on correlations between the expression of aquaporin genes and morphological and physiological observations, lack reliable in vivo functional evidence.
In this study, we adopted a reverse genetics approach to elucidate the role of a PIP-type aquaporin gene in grapevine. We constitutively overexpressed VvPIP2;4N in grapevine plants. Physiological and molecular parameters of six independent transgenic lines were analyzed in well-watered and drought conditions. The expression level of endogenous VvPIP2;4N and of other aquaporins was assessed in order to investigate possible compensation effects present within the gene family. For each line, we analyzed the single resistances that control water flow across the plant. The behavior of transgenic lines with different levels of transgene expression allowed us to correlate gene expression to physiological parameters and to hypothesize specific functions of this aquaporin.
RESULTS
Cloning and in Silico Analysis of VvPIP2;4N
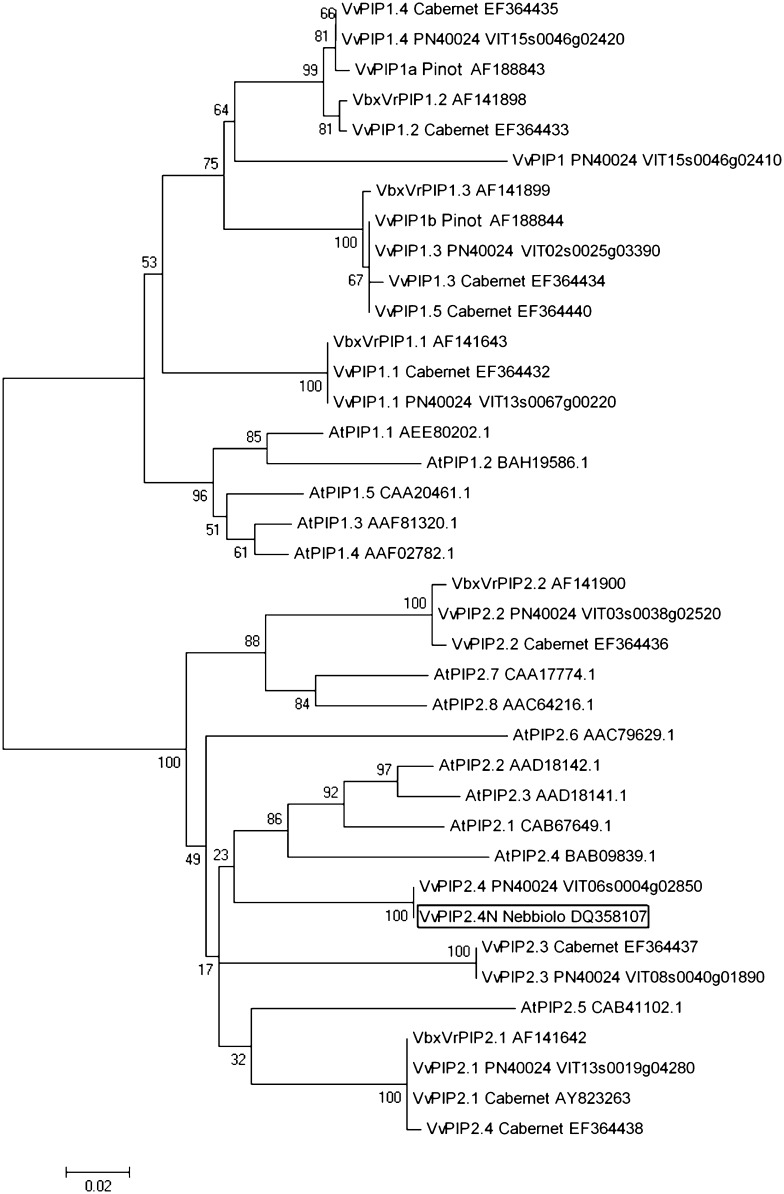
A putative aquaporin gene was isolated from complementary DNA (cDNA) of grapevine ‘Nebbiolo’ using primers designed on an EST contig sequence. The isolated gene (DQ358107) was nearly identical in its coding sequence (single nucleotide mismatch, not leading to an amino acid change) to the gene predicted in the grapevine ‘PN40024’ 12× genome draft (http://genomes.cribi.unipd.it/) at locus VIT06s0004g02850 on chromosome 6 and was called VvPIP2;4 by Shelden et al. (2009). The same authors isolated an aquaporin sequence that they called VvPIP2;4 (EF364438) from cv Cabernet Sauvignon. However, this sequence shares a mere 83% identity at the amino acid level with the gene we isolated, and it is nearly identical (five nucleotide mismatches and one amino acid mismatch) to VvPIP2;1, described by Fouquet et al. (2008; DQ834698) and later by the same authors (Shelden et al., 2009; AY823263; Fig. 1). Given that functional characterization of the VvPIP2;4 gene at the water transport (Shelden et al., 2009) and expression pattern (Vandeleur et al., 2009) levels was performed using GenBank accession EF364438, our gene represents a yet uncharacterized PIP2 from grapevine. To avoid confusion, we have used the name VvPIP2;4N for the gene we isolated from cv Nebbiolo.
Figure 1.
Neighbor-joining tree of grape and Arabidopsis PIP proteins. Protein sequences of GenBank accessions for grapevine (cv Pinot noir, Cabernet Sauvignon, and Nebbiolo), for Vitis berlandieri × Vitis rupestris, and for Arabidopsis, and genomic loci from the grapevine ‘PN40024’ 12× genome draft (http://genomes.cribi.unipd.it/) were clustered using MEGA4. The significance of each node was tested using 1,000 bootstrap replicates.
The transcript sequence (843 bp) of VvPIP2;4N encodes a protein that is 280 amino acids long, with theoretical pI and molecular mass of 7.00 and 30 kD, respectively, similar to PIP2s from other species. Analysis of the deduced protein sequence of VvPIP2;4N allowed the prediction of all residues characteristic of the PIP family: six transmembrane domains connected by five loops, two NPA (Asn-Pro-Ala) motifs, which are conserved features of all aquaporins (Chrispeels and Maurel, 1994), and the sequences G-G-G-A-N-X-X-X-X-G-Y in loop C and T-G-I/T-N-P-A-R-S-L/F-G-A-A-I/V-I/V-F/Y-N in loop E (Barone et al., 1997). VvPIP2;4N also shows a shorter N-terminal region and a longer C-terminal region when compared with PIP1 proteins, as described in the literature (Chaumont et al., 2000). Residues known to control protein activity are present, such as His-194, located in loop D and involved in pH sensing (Tournaire-Roux et al., 2003), as well as two putative phosphorylation sites, Ser-116 and Ser-275, present in loop B and in the C-terminal region (Johansson et al., 1998), and the diacidic DVE motif (Asp-Val-Glu), which has a putative role in endoplasmic reticulum export (Zelazny et al., 2009; Sorieul et al., 2011; Supplemental Fig. S1).
Functional Characterization of VvPIP2;4N
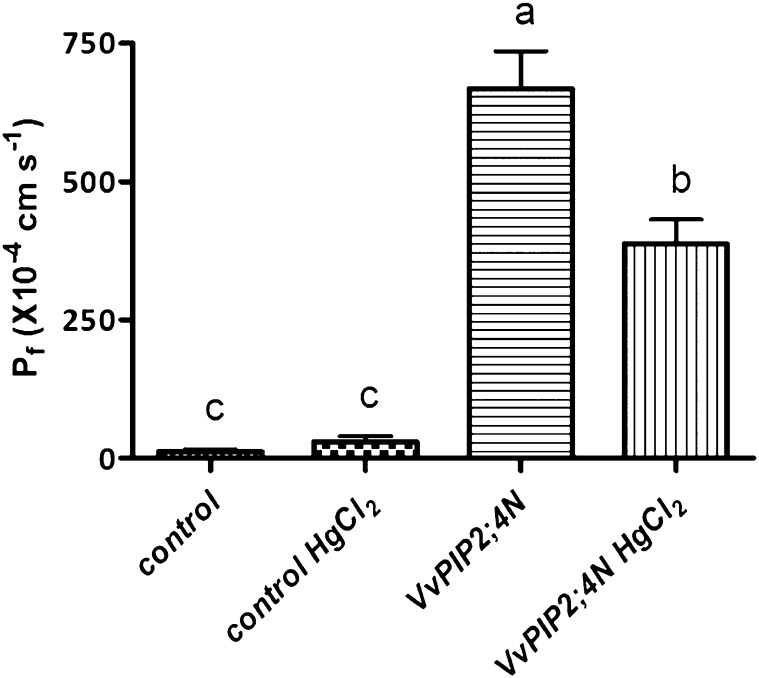
Xenopus laevis oocytes expressing VvPIP2;4N were exposed to osmotic shock. The expression of this aquaporin triggered a rapid increase in oocyte volume, with a water permeability (Pf) value of 0.067 cm s−1, meaning a 54-fold increase in the swelling rate when compared with control oocytes. Addition of HgCl2 to the incubation solution caused an approximately 2-fold decrease in osmotic permeability, showing an inhibition by mercury common to most plant aquaporins (Hukin et al., 2002; Fig. 2) and comparable to what observed for OePIP2;1 in the same experimental conditions (Secchi et al., 2007). Higher reductions of Pf have been obtained with mercury treatment of aquaporin-expressing oocytes, but at higher doses: 1 mm for 30 min for NtPIP2;1 (Biela et al., 1999) and 0.5 mm for Samanea saman SsAQP2 (Moshelion et al., 2002).
Figure 2.
Functional expression of VvPIP2;4N in X. laevis oocytes. Pf of oocytes injected with VvPIP2;4N complementary RNA solution, or with water (control), was measured from swelling kinetics. The assay was performed with a 10-min preincubation in the absence or presence of 0.3 mm HgCl2. Values shown are means ± se (VvPIP2;4N, n = 12; VvPIP2;4N + HgCl2, n = 5; control, n = 20; control + HgCl2, n = 5). Histograms labeled by different letters differ significantly at P < 0.05 according to variance analysis and Tukey’s test.
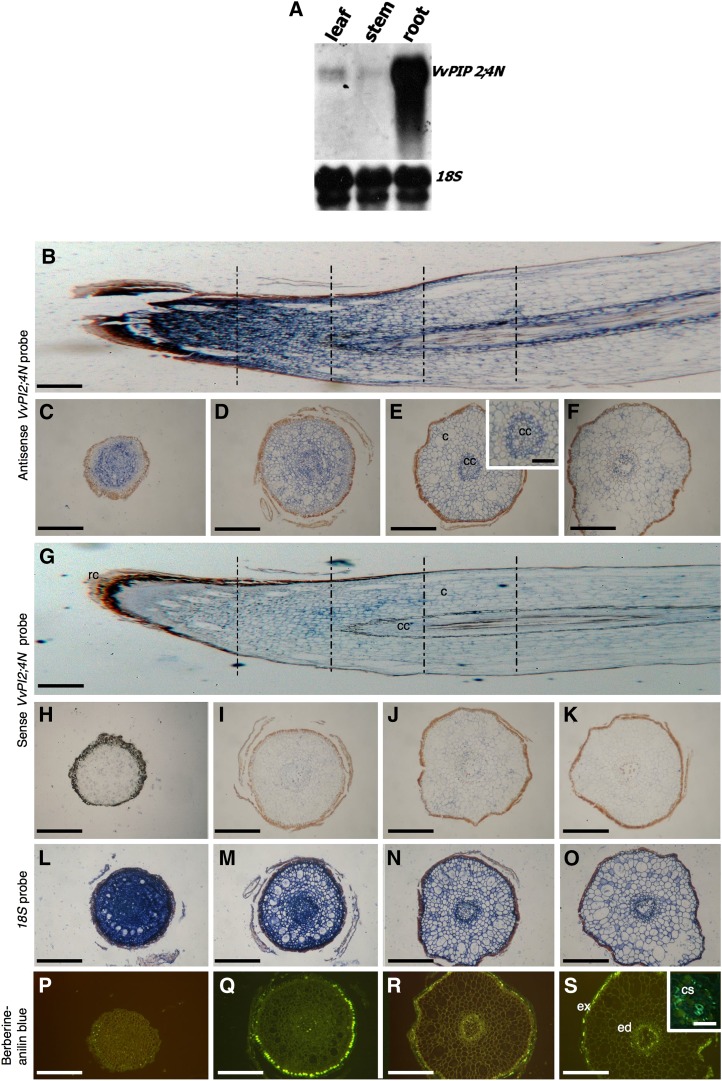
Transcript concentration analysis by northern blot was performed in different cv Nebbiolo organs using the full-length VvPIP2;4N probe. Despite the high homology between PIP2 genes, a single band was detected in a Southern-blot hybridization of cv Nebbiolo genomic DNA with the same probe, indicating that it hybridized to a single locus (Supplemental Fig. S2C) and supporting its specificity within the PIP2 subfamily. Strong expression of VvPIP2;4N was observed in roots, whereas transcripts were barely present in shoots and leaves (Fig. 3A). These results are in contrast with the very low expression levels of VvPIP2;4 in roots reported by Vandeleur et al. (2009); however, no correspondence was observed between the primers used by those authors and those used in this study, confirming that VvPIP2;4N is different from VvPIP2;4 described by Shelden et al. (2009) and Vandeleur et al. (2009). The expression pattern of VvPIP2;4N suggests that this aquaporin could be a specific root isoform, probably involved in the regulation of root hydraulic conductance.
Figure 3.
VvPIP2:4N gene expression analyses. A, Northern-blot analysis of VvPIP2;4N aquaporin expression in leaf, stem, and root tissues of cv Nebbiolo. Total RNA was probed with a specific DNA probe corresponding to the full-length VvPIP2;4N gene labeled with DIG. The blots were stripped and reprobed with 18S ribosomal DNA DIG-labeled probe. B to Q, Localization of VvPIP2;4N expression in grape roots. In situ hybridization was performed on longitudinal sections of cv Nebbiolo roots with full-length VvPIP2;4N DIG-labeled antisense (B) and sense (G) RNA probes and on a series of transverse sections (at several distances from the root apex, as indicated by the vertical lines in B and G) with antisense (C–F and E, inset) or sense (H–M) probes. Specific blue signal is mostly evident in meristematic regions and in the regions of vascular differentiation using antisense VvPIP2;4N probe. The sense VvPIP2;4N probe indicates the background level of nonspecific binding in these experiments. In N to Q, a strong signal is evident in the positive controls hybridized with ribosomal Vv18S-DIG-labeled antisense RNA probe. R to U, Fluorescence microscopy of transverse root sections stained with berberine-aniline blue to mark cell wall suberification. The inset in U shows that Casparian bands are evident in the older root region. Bars = 320 μm except in the insets (80 μm). c, Cortical cells; cc, central cylinder; cs, Casparian bands; ed, endodermis; ex, exodermis.
In order to localize VvPIP2;4N transcripts, in situ hybridization experiments were carried out in different tissues of cv Nebbiolo plants: roots, stems, and leaves. The results confirmed that expression was concentrated in roots, while only a faint signal was observed in leaves, and no signal at all was detected in shoots. VvPIP2;4N expression was widespread in the root elongating and absorbing apical zones, and transcripts were observed in the cortex and associated with pericycle and vascular bundles (Fig. 3, B–D). In the older parts of roots (more than 3 mm distant from the apex), VvPIP2;4N mRNA became restricted to the xylem bundles (Fig. 3, E and F). Control experiments with the sense probe did not show a significant signal (Fig. 3, G–M), while a high chromogenic signal became evident when using a ribosomal antisense 18S probe as a positive internal control (Fig. 3, N–Q). By staining with 0.1% berberine, we observed a progressive suberification of tangential cell walls with the formation of an exodermis and an endodermis, showing evident Casparian bands starting at about 2 mm from the apex (Fig. 3, R–U).
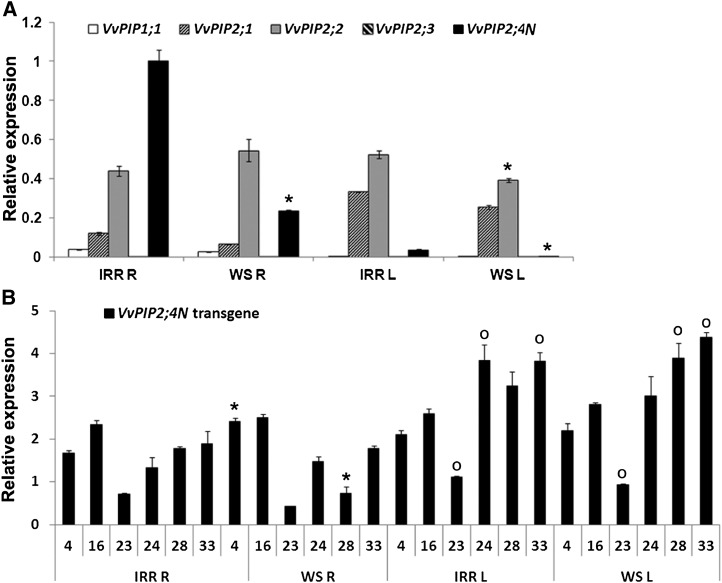
We then analyzed by means of quantitative reverse transcription (qRT)-PCR the expression pattern of VvPIP2;4N, together with other known PIP2 genes and a PIP1-type aquaporin, in cv Brachetto, which was subsequently used for transformation experiments. Results confirmed a root-specific expression pattern of VvPIP2;4N. In roots of well-watered plants, VvPIP2;4N was the most abundant PIP2-type aquaporin transcript, followed by VvPIP2;2 and VvPIP1;1, which were present in roots but virtually absent in leaves. In cv Brachetto, we also tested the effects of water stress, which induced down-regulation of VvPIP2;4N in roots and in leaves. Accordingly, the PIP2-type aquaporin with the highest expression under these conditions was VvPIP2;2 in both organs (Fig. 4A).
Figure 4.
Expression of endogenous and transgenic VvPIP2;4N in cv Brachetto. A, Relative expression of VvPIP1;1, VvPIP2;1, VvPIP2;2, VvPIP2;3, and endogenous VvPIP2;4N was determined by qRT-PCR in roots (R) and leaves (L) of wild-type plants upon irrigated (IRR) and water stress (WS) conditions. For each gene, asterisks mark significant differences (P < 0.05) between stressed and irrigated conditions. B, Relative expression of transgenic VvPIP2;4N determined by qRT-PCR in roots and leaves belonging to six different transgenic lines (4, 16, 23, 24, 28, and 33) upon well-watered (IRR) and water stress (WS) conditions. For each line, asterisks mark significant (P < 0.05) differences between well-watered and water-stressed plants, while circles mark those between shoots and roots. The expression ratio of each target gene to the geometric mean of the housekeeping genes was further divided by the expression ratio of endogenous VvPIP2;4N in irrigated roots (IRR R). Significant differences were detected by means of Student’s t test. Data are means ± se (n = 3).
Transgenic Grapevine Plants
To investigate the role of VvPIP2;4N in vivo, we ectopically expressed VvPIP2;4N in cv Brachetto plants using a Cauliflower mosaic virus 35S promoter. Thirty-three putatively transgenic grapevine lines transformed with pJam1469-35s::VvPIP2;4N binary vector, showing transgene products of the expected size for both nptII and the adjacent VvPIP2;4N genes, were obtained by regeneration and micropropagation. About 85% of transgenic lines displayed only one or two T-DNA insertions. Several lines showed the same hybridization pattern (Supplemental Fig. S2, B and C). The 33 regenerated transgenic lines of cv Brachetto arose from 11 independent transformation events, as grouped according to Southern-blot results. Six lines with different T-DNA configurations were chosen for further molecular and physiological analyses (Supplemental Table S1).
Within the selected lines, transgenic VvPIP2;4N expression was lowest in line 23 in both roots and leaves and highest in line 16 in roots and in lines 24 and 33 in leaves. The expression of the transgene was higher than in roots in four lines (23, 24, 28, and 33); we observed an expression decrease in line 28, and an increase in line 4, compared with well-watered conditions (Fig. 4B).
In order to check for modifications in the transcription of endogenous genes (Heinen et al., 2009), we assessed the expression levels of endogenous VvPIP2;4N and of four other PIP genes in the transgenic lines. The concentration of endogenous VvPIP2;4N transcripts was mostly unaffected by the presence of the transgene in the roots of well-watered plants, while, under water stress, transgenic plants showed an increase in the expression of endogenous VvPIP2;4N. Expression of VvPIP1;1, VvPIP2;1, and VvPIP2;2 in transgenic plants was in some cases higher than in wild-type plants (e.g. VvPIP2;1 in lines 4 and 28; Supplemental Fig. S3).
Effects of VvPIP2;4N Overexpression on Hydraulic Conductivity and Leaf Gas Exchange
The availability of six different transgenic grapevine lines with different VvPIP2;4N transcript levels allowed us to study the correlation between the expression of this aquaporin and water transport processes at the whole-plant level.
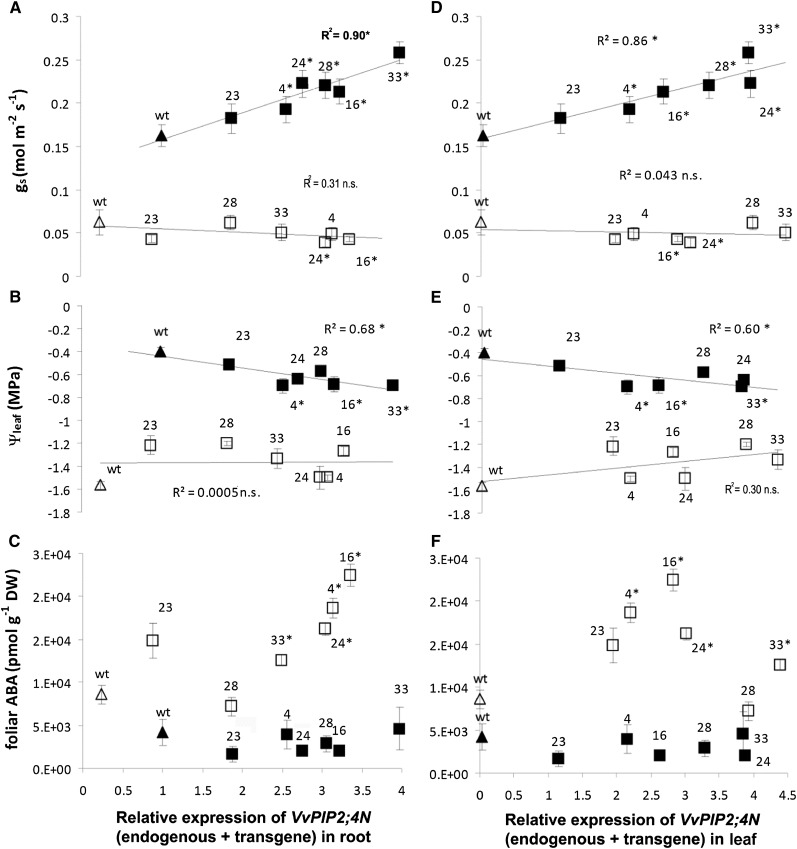
Under well-watered conditions, most transgenic lines showed higher stomatal conductance (Fig. 5A) than wild-type plants, and stomatal conductance was positively correlated to root VvPIP2;4N transcript levels. Similarly, leaf transpiration and net photosynthesis were higher in transgenic plants and were correlated to the level of transgene expression (Supplemental Fig. S4, A and B). Leaf water potential was lower in some transgenic lines (4, 16, and 33) than in the wild type (Fig. 5B).
Figure 5.
Stomatal regulation in cv Brachetto plants. Stomatal conductance (gs [n = 12]; A and D), leaf water potential (Ψleaf [n = 8]; B and E), and leaf ABA content (n = 4; C and F) in wild-type (wt; triangles) and transgenic (squares) plants belonging to six lines (4, 16, 23, 24, 28, and 33) upon well-watered (black symbols) and water stress (white symbols) conditions (means ± se). Data are plotted for VvPIP2;4N relative expression in root (A–C) and in leaves (D–F). Asterisks mark significant differences from the wild type, calculated by Student's t test, and, when applied to the squared correlation coefficient (R2), the significance of regression to root expression levels of VvPIP2;4N (endogenous + transgene): *P < 0.05.
Upon water stress, transgenic plants showed a slight decrease in stomatal conductance and leaf gas exchange compared with wild-type plants, while water potential was not affected (Fig. 5, A and B; Supplemental Fig. S4, A and B). Similar observations were made for both irrigated and water-stressed plants when VvPIP2;4N expression in leaves was addressed (Fig. 5, D and E).
Although net photosynthesis increased in irrigated transgenic plants, water use efficiency and concentration of CO2 in intercellular spaces did not significantly change between transgenic and wild-type grapevine. This suggests that VvPIP2;4N overexpression did not affect the photosynthetic machinery, while the increase in photosynthesis was rather due to increased stomatal conductance. Water stress impaired water use efficiency and enhanced intercellular CO2 concentration but showed no divergent effects on wild-type and transformed plants (Supplemental Fig. S4, C and D).
In irrigated plants, abscisic acid (ABA) concentration was low and was not affected by transgene expression. Leaf ABA concentration was significantly higher in water-stressed plants than in irrigated plants of all lines, and in water-stressed plants it increased significantly in transgenic plants, alongside the increase in VvPIP2;4N expression (Fig. 5, C and F).
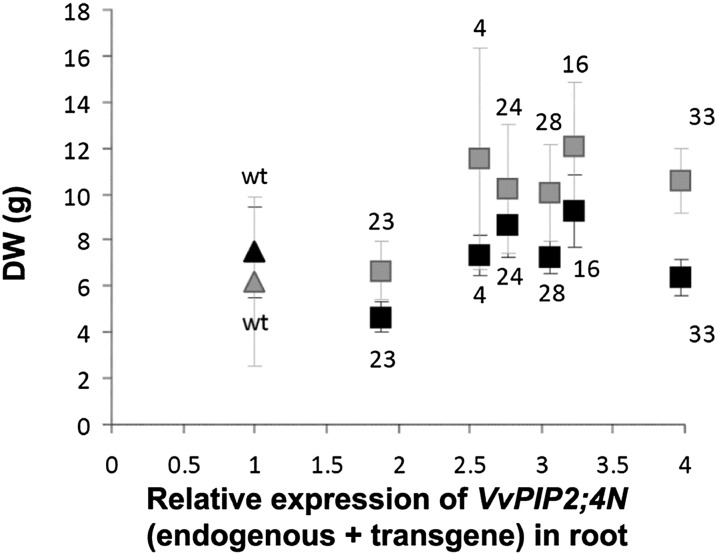
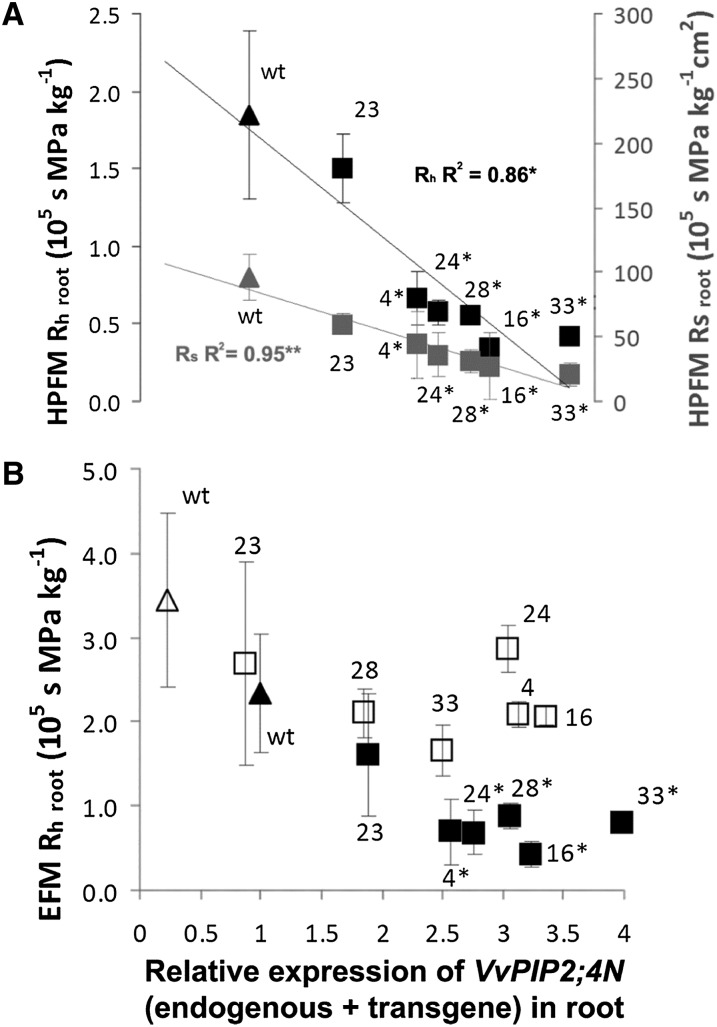
Shoot weight of well-watered plants showed a tendency to increase (although not significantly) with increasing VvPIP2;4N expression, while root weight remained stable (Fig. 6). Whole-root hydraulic resistance (Rh root) and surface area-specific root hydraulic resistance, measured with the high-pressure flux method (HPFM), were lower in most transgenic plants than in wild-type ones and were negatively correlated to the expression of VvPIP2;4N (endogenous + transgene) in roots (Fig. 7A). By directly measuring soil and shoot water potential, we also assessed Rh root by using the evaporative flux method (EFM). The results were in agreement with those obtained with the HPFM, although EFM values were slightly higher than HPFM values. The EFM, different from the HPFM, could be used to assess Rh root upon conditions of water stress; again, the results showed a negative dependency on the level of transgene expression (Fig. 7B).
Figure 6.
Root and shoot growth in cv Brachetto plants. Dry weight (DW) of shoots (gray symbols) and of roots (black symbols; n = 4) in wild-type (wt; triangles) and transgenic (squares) well-watered plants belonging to six lines (4, 16, 23, 24, 28, 33; means ± se) was plotted for VvPIP2;4N relative expression in root.
Figure 7.
Root hydraulic resistance in cv Brachetto plants. A, Root hydraulic resistance (HPFM Rh root; black symbols) and surface area-specific root hydraulic resistance (HPFM Rs root; gray symbols) measured with the HPFM method (n = 4) upon well-watered conditions. B, Root hydraulic resistance measured with the evaporative method (EFM Rh root,) upon well-watered (black symbols) and water stress (white symbols) conditions (n = 4) in wild-type (wt; triangles) and transgenic (squares) plants belonging to six lines (4, 16, 23, 24, 28, and 33; means ± se). Data are plotted for VvPIP2;4N relative expression in root. Asterisks mark significant differences from the wild type, calculated by Student's t test, and, when applied to the squared correlation coefficient (R2), the significance of regression to root expression levels of VvPIP2;4N (endogenous + transgene): *P < 0.05, **P < 0.01.
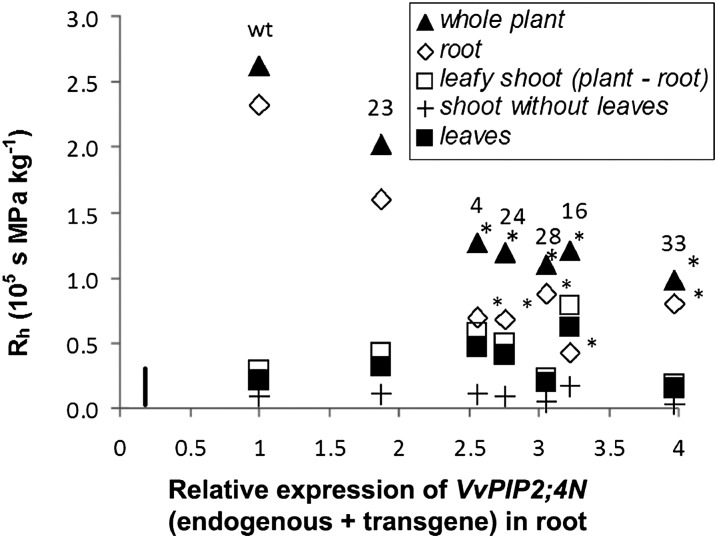
Using the EFM, we partitioned, in well-watered conditions, the hydraulic resistance of the whole plant (Rh plant), obtaining leaf hydraulic resistance. Rh plant was significantly lower in all transgenic lines. Leaf hydraulic resistance did not display any changes between wild-type and transgenic plants, showing that changes in Rh plant were substantially due to modifications of the Rh root induced by transgene expression (Fig. 8).
Figure 8.
Components of plant hydraulic resistance in cv Brachetto plants. Plant hydraulic resistance (Rh), assessed through the evaporative method in well-watered conditions, was partitioned into resistance of the whole plant (black triangles), of the entire root system (white diamonds), of the leafy shoot (white squares), of the shoot without leaves (crosses), and of the leaves (black squares) in wild-type (wt) and transgenic cv Brachetto grapevine belonging to six lines (4, 16, 23, 24, 28, 33). Data are plotted for VvPIP2;4N relative expression in root. The bar in the bottom left corner represents the se (n = 4). Asterisks mark significant differences from the wild type, calculated with Student’s t test (P < 0.05).
DISCUSSION
Isolation and Functional Characterization of VvPIP2;4N
PIP2s are considered the major facilitators of transmembrane water transport in plants (Kaldenhoff and Fischer, 2006). They increase swelling of X. laevis oocytes in hypotonic solutions (Chaumont et al., 2000) and enhance cell membrane water permeability and whole-tissue water conductivity in Arabidopsis (Martre et al., 2002) and rice (Katsuhara et al., 2003). In grape, partly because of the existence of different genotypes, the composition of the PIP2 subfamily has not yet been fully defined. Fouquet et al. (2008) proposed four members for this subfamily in the cv PN40024 homozygous, fully sequenced genome, naming them PIP2;1 to PIP2;4. Later on, four PIP2s, named PIP2;1 to PIP2;4, were isolated from the heterozygous cv Cabernet Sauvignon (Shelden et al., 2009); however, while PIP2;1 to PIP2;3 correspond to the cv PN40024 genes, VvPIP2;4 of cv Cabernet Sauvignon appears to be an allele of VvPIP2;1 from cv PN40024. The size of the PIP2 subfamily is reduced in grape when compared with other plants: eight and 11 members are present in Arabidopsis (Johanson et al., 2001) and rice (Sakurai et al., 2005), respectively.
In this study, we have cloned, from cv Nebbiolo, the gene homologous (100% amino acid identity) to VvPIP2;4 from cv PN40024. The sequence of this gene (which we refer to as VvPIP2;4N) only shares 83% amino acid identity with VvPIP2;4 isolated by Shelden et al. (2009) in cv Cabernet Sauvignon, thus appearing to be an uncharacterized grape PIP2 gene. Functional assays in oocytes showed that VvPIP2;4N expression induced a 54-fold increase in membrane Pf, a value comparable to those detected when using this experimental system for plant aquaporins (Marjanović et al., 2005; Secchi et al., 2007) and to those reported for the four grapevine PIP2 aquaporins analyzed by Shelden et al. (2009). Taken together, these results suggest that VvPIP2;4N is a water-transporting aquaporin that may potentially offer a remarkable contribution to water transcellular transport in grape tissues.
VvPIP2;4N Is a Root-Specific Grapevine Aquaporin
Several pieces of evidence support the idea that specific aquaporins may have specific functional roles different from those played by their relatives belonging to the MIP family. For example, in Arabidopsis, facilitation of CO2 transport has been demonstrated for AtPIP1;2 but not for AtPIP2;3 (Heckwolf et al., 2011). Tissue localization patterns can be used as indicators of functional specificity, since physiological roles can be restricted to specific tissues and organs. Our results in cv Brachetto show that VvPIP2;1 and VvPIP2;2 are expressed in both roots and leaves, while VvPIP2;3 is almost absent in both organs, thereby confirming previous reports (Baiges et al., 2001; Vandeleur et al., 2009). However, among the PIP2 genes, VvPIP2;4N shows a characteristic root-specific expression profile, resembling other root-specific aquaporins, such as rice OsPIP2;5 (Sakurai et al., 2005). In situ hybridization shows that VvPIP2;4N expression is localized in the cortex and associated with the vascular bundle of young roots, where an endodermis is developing, thus confirming the root tissue localization pattern of other PIP transcripts, such as grapevine VvPIP2;2 (Vandeleur et al., 2009) and tobacco NtAQP1 (Otto and Kaldenhoff, 2000).
The root localization of VvPIP2;4N agrees with its putative function in the control of transmembrane water flow in the root. Expression of this aquaporin is higher in most transgenic lines than in wild-type plants and is correlated to root hydraulic conductance. In wild-type plants, VvPIP2;4N is the only PIP2 down-regulated by water stress, and root hydraulic conductance is affected accordingly. Correlations between the expression of specific aquaporins in roots and root hydraulic conductance have been reported several times, especially in plants where suberified exodermis and endodermis develop (Barrowclough et al., 2000; North et al., 2004), as is the case for grapevine. Subsequently, our results suggest that VvPIP2;4N may act as a major controller of root hydraulic conductance in grapevine plants.
Transgenic Grapevine Plants Overexpressing VvPIP2;4N
Transgenic grapevines containing the VvPIP2;4N transgene were obtained from embryogenic cultures of cv Brachetto. Although transgenic plants of cv Nebbiolo have previously been obtained (Gambino et al., 2005), we chose the cv Brachetto for its high capacity to induce somatic embryogenesis and plant regeneration after genetic transformation (G. Gambino and I. Gribaudo, unpublished data). Furthermore, both genotypes show anisohydric behavior when subjected to water stress (Supplemental Fig. S5). The hybridization patterns observed for the different plants in Southern analyses followed a few common motifs, which allowed the clustering of the transgenic lines into groups whose members presumably issued from the same transformation event. A similar situation has previously been reported in other grape transformation experiments (Iocco et al., 2001; Gambino et al., 2005; Maghuly et al., 2006). The transformation protocol we used requires the maintenance of embryogenic cultures, after Agrobacterium tumefaciens coculture, for several months under kanamycin selection pressure before embryo germination. Although this protocol favors the recovery of genetically uniform transgenic plants without escapes, it has the tendency to induce the proliferation of numerous embryos from the same transformed cellular clone, thus originating several plants deriving from a single transformation event.
The insertion of foreign DNA into a plant genome can lead to alterations in its structure, which can affect transgene expression (Gelvin, 2003). In agreement with this, analysis of transgene expression by qRT-PCR revealed important differences among transformed lines and a lack of correlation between T-DNA copy numbers and transgene-derived mRNA accumulation.
Although VvPIP2;4N was under the control of the constitutive 35S promoter, its expression varied from organ to organ and from well-watered conditions to drought. Such results have rarely been reported in herbaceous transgenic plants, where transgenic lines are stabilized after several cycles of self-crossing. However, these results are not surprising in woody plants, where hemizygous T0 transgenic plants are commonly employed (Kumar and Fladung, 2001) and where developmental stages and environmental conditions can affect the expression of transgenes introduced under the control of a constitutive promoter. D’Angeli and Altamura (2007), for instance, showed that a transgenic protein was detected in olive (Olea europaea) stems but not in leaves and meristems. In grapevine, transgene expression decreases following transfer from in vitro culture to the greenhouse, concomitant with an increase in symmetric cytosine (CpG) methylation in the transgene sequence (Gambino et al., 2010). Epigenetic phenomena such as DNA methylation or histone modification could be the causes of the observed variations in transgene expression. Furthermore, it must be noted that aquaporin activity is also controlled by posttranscriptional regulation processes, such as phosphorylation, interaction with protons, or with inhibitors such as hydrogen peroxide (Maurel et al., 2008). However, several reports have confirmed that significant relationships between aquaporin expression and water transport processes can be observed in plants, independently of possible posttranscriptional regulation effects (Hachez et al., 2006; Kaldenhoff et al., 2008).
VvPIP2;4N Overexpression Enhances Water Transport at the Whole-Plant Level Under Well-Watered Conditions But Not upon Drought
Transgenic grapevine overexpressing VvPIP2;4N showed increased stomatal conductance, leaf gas exchange, and shoot growth rates in well-watered conditions compared with wild-type controls. These results are in agreement with the conclusions drawn by several studies concerning the effects of PIP2 overexpression in tobacco (Aharon et al., 2003), rice (Katsuhara et al., 2003), and Eucalyptus spp. (Tsuchihira et al., 2010). These effects seemed to be guided by the increase in stomatal conductance, although none of the major known regulators of stomatal conductance in grape (ABA, subcellular CO2 concentration, and leaf water potential) were substantially modified by transgene expression under favorable conditions (leaf water potential in some transgenic lines actually decreased, which would involve stomatal closure). VvPIP2;4N transcripts are absent in guard cells (as in the whole leaf) in wild-type plants, while the gene is expressed in the leaves of transgenic plants. It has been shown that the 35S promoter efficiently drives expression in Arabidopsis guard cells, although at a lower level than in other tissues (Yang et al., 2008). Thus, we hypothesize that in transgenic plants, stomatal conductance may directly and positively be affected by the increased turgor of guard cells brought by the constitutive expression of VvPIP2;4N in their plasma membrane. Under well-watered conditions, with high leaf water potential and low ABA levels, guard cells actively build up turgor, and overexpression of an aquaporin could facilitate water uptake from the intercellular spaces, thereby enhancing stomatal opening.
Although the benefits brought by VvPIP2;4N overexpression to leaf gas exchange and shoot growth in well-watered conditions have been proven here, the situation concerning water-stressed grapevine was quite different. Herbaceous species overexpressing aquaporins showed reduced resistance to drought stress compared with wild-type plants, paired with the acceleration of wilting (Aharon et al., 2003; Katsuhara et al., 2003). By contrast, in our transgenic grapevine, VvPIP2;4N overexpression did not induce any signs of wilting. In addition, water potential measurement indicated that water stress conditions were not more severe than those applied to wild-type plants, similar to what has been reported for Eucalyptus spp. overexpressing a PIP2 (Tsuchihira et al., 2010). These results suggest that in grape (and possibly other plants) under water stress, mechanisms other than the hydraulic conductance of cell membranes are responsible for water flow across the plant and into the environment.
We provide evidence that one such mechanism is leaf ABA concentration, which was unaffected by transgene expression when plants were irrigated but was positively correlated to VvPIP2;4N expression in water-stressed grapevine. In grapevine, the control of ABA on drought response is crucial, and this aspect has been well characterized in the literature (Loveys, 1984; Lovisolo et al., 2008a). In our plants, under well-watered conditions, leaf ABA concentration was not correlated to transpiration, while upon water stress, a higher ABA concentration in transgenic plants corresponded to a lower stomatal conductance compared with the (already low) values observed in wild-type plants. Upon the sensing of water stress in roots in grapevine, ABA is transported from the roots to the leaves via the xylem (Stoll et al., 2000). Although we have no experimental supporting data, we hypothesize that VvPIP2;4N overexpression in roots, by increasing root hydraulic conductance, could induce water loss to the soil and, as a consequence, a more severe stress than in roots of wild-type plants and a more intense mobilization of ABA toward the leaves. This mobilization could, in turn, effectively safeguard tissues from wilting and maintain the control of water flow at the stomatal level.
Leaf Hydraulic Resistance Is Not Affected by VvPIP2;4N Overexpression
Although VvPIP2;4N overexpression effectively modified root hydraulic conductance, partitioning of plant hydraulic resistance at the organ level showed that leaf resistance was not affected, despite the fact that the transgene was expressed in both roots and shoots. Leaf hydraulic resistance is controlled by cell-to-cell water movement at different degrees in different species (Tyree et al., 2005). Increases in leaf conductance induced by light exposure coincide with the enhancement of aquaporin expression in walnut (Juglans regia; Cochard et al., 2007). Accordingly, overexpression of aquaporin genes increased rosette conductivity in Arabidopsis (Postaire et al., 2010) and leaf conductivity in rice (Li et al., 2008). Nardini et al. (2005) showed that in grapevine, as in other sun-adapted species, the contribution of mesophyll hydraulic resistance to total leaf resistance is relatively high and that overexpression of an aquaporin in the leaf should lead to a reduction of leaf resistance. However, the technique of minor vein cutting used by those authors includes within the concept of mesophyll resistance only the resistance opposed to flow after water exits the apical part of minor (fifth-order) veins, while water can also exit the vasculature by directly crossing the bundle sheath cells surrounding the proximal part of minor veins and lower order veins. Grapevine has heterobaric leaves showing bundle sheath extensions that create transparent, nonphotosynthetic regions on the leaf lamina (Liakoura et al., 2009). Recently, Buckley et al. (2011) have shown that, in heterobaric leaves, bundle sheath extensions reduce hydraulic resistance between bundle sheath and epidermis cells. A further reduction of the resistance along this pathway, which could be obtained by overexpression of an aquaporin, would not be expected to significantly affect leaf resistance, in opposition to what could happen in a homobaric leaf (a leaf without bundle sheath extensions), where hydraulic resistance between bundle sheath and epidermis cells is 1 order of magnitude higher than in heterobaric ones (Buckley et al., 2011).
Another possible explanation of the apparent inactivity of transgenic VvPIP2;4N in leaves is based on the possibility that, in vivo, its activity might require interaction with other proteins. According to the observations made by Vandeleur et al. (2009), grapevine VvPIP2;2 requires coexpression of VvPIP1;1 to be fully active as a water channel. A similar interaction of PIP1s with PIP2s has previously been reported (Fetter et al., 2004) and can be due to heteromerization of the aquaporin tetramer (Otto et al., 2010). We observed that VvPIP1;1 was not expressed in leaves, confirming the results of Baiges et al. (2001), while it was expressed in roots, in particular in some transgenic lines (4, 28, and 33) characterized by low Rh root. Thus, although VvPIP2;4N efficiently facilitates transmembrane water transport when expressed alone in oocytes, the possibility exists that it may require interaction with a PIP1, such as VvPIP1;1, to be fully active in planta as a water transport facilitator.
CONCLUSION
In this study, we have characterized a root-specific aquaporin of grapevine and have overexpressed it in order to provide information about its functional properties. VvPIP2;4N acts as an effective aquaporin in oocytes, and its overexpression affects leaf gas exchange and root hydraulic conductance, but not leaf hydraulic resistance, under irrigated conditions. Under water stress conditions, however, the potentially negative effects of overexpression of this aquaporin are avoided by ABA control of stomatal opening. As grapevines worldwide are grafted, the existence of this aquaporin in commercial grapevine rootstocks could potentially represent a marker of vegetative growth in vineyards where adequate irrigation or rainfall is available.
MATERIALS AND METHODS
Bioinformatic Analyses and cDNA Cloning of VvPIP2;4N
Total RNA was isolated from roots of cultivated grapevine (Vitis vinifera ‘Nebbiolo’). First-strand cDNA was synthesized from total RNA treated with DNaseI (Invitrogen; http//www.invitrogen.com/) using oligo(dT)12–18 as primers and SuperScript II Reverse Transcriptase (Invitrogen). Gene-specific primers (Supplemental Table S1) were designed based on a TC sequence (TC38138) deposited at the Vitis vinifera Gene Index (release 4.0) database (http://compbio.dfci.harvard.edu/cgi-bin/tgi/gimain.pl?gudb=grape/). PCR was performed using Platinum Pfx DNA Polymerase (Invitrogen), according to the manufacturer’s instructions. The expected product length was gel purified, inserted into the pGEM-T Easy vector (Promega; www.promega.com), and sequenced using M13 forward and reverse primers. The isolated sequence was registered in GenBank with accession number DQ358107 and was given the name VvPIP2;4N.
The protein sequence was deduced by applying the ExPASy Translate tool (http://expasy.org/tools/dna.html), and the theoretical pI and molecular mass were calculated using the ExPASy compute pI/molecular mass tool (http://web.expasy.org/compute_pi/). Prediction of transmembrane domains of the deduced protein sequence was performed by the TMHMM software (http://www.cbs.dtu.dk/services/TMHMM/).
Functional Analysis in Xenopus laevis Oocytes
The function of the VvPIP2;4N gene was analyzed in Xenopus laevis oocytes following the method described by Biela et al. (1999). Fifty nanograms of complementary RNA (or an equivalent volume of water) was injected per oocyte, and 3 d after complementary RNA injection, the osmotic Pf coefficient was determined by measuring the rate of oocyte swelling induced by a hypo-osmotic shock of 160 mOsm kg−1. The Pf was calculated using the formula Pf = V0[d(V/V0)/dt]/[S×Vw(Osmin − Osmout)], where the initial oocyte volume (V0) and the initial oocyte surface area (S) were calculated from every single oocyte 5 s after transferring them into hypotonic medium. The molar volume of water (Vw) is given as 18 cm3 mol−1.
Expression Profile of VvPIP2;4N
Samples for expression analysis were collected at 11:00 am to avoid interference from diurnal patterns of aquaporin expression. Total RNA was isolated from roots, stems, and leaves of 2-year-old cv Nebbiolo plants grown under greenhouse conditions. Northern hybridization was carried out using a specific DNA probe corresponding to the full-length VvPIP2;4N sequence. The probe was amplified by PCR using the primers reported in Supplemental Table S2 and digoxigenin (DIG) labeled using the PCR DIG Probe Synthesis Kit (Roche; http//www.roche.com/) according to the manufacturer’s instructions. Blots were stripped and reprobed with 18S ribosomal DNA probes.
In situ hybridization was performed using cultivated grapevine root apices and young leaves. Tissue was fixed in 4% paraformaldehyde overnight at 4°C, washed with saline solution (150 mm NaCl), dehydrated in a graded ethanol series, cleared in Bio-Clear (Bio-Optica; http//www.bio-optica.it/), and infiltrated with Paraplast plus (Sigma; http//www.sigmaaldrich.com/). Longitudinal and transverse sections of 8 μm were transferred to poly-l-Lys (Sigma)-pretreated slides and dried overnight at 40°C. RNA hybridization and detection experiments were carried out on dewaxed sections as described (Balestrini et al., 2000) using DIG-labeled RNA probes. Sense and antisense probes were generated by in vitro transcription of linearized template DNAs as described by the manufacturer (Roche). After hybridization and detection, the sections were dehydrated through an ethanol-Bio-Clear series and mounted in Bio-Mount (Bio-Optica). The berberine-aniline blue fluorescent staining method (Brundrett et al., 1988) was applied to root sections to detect the presence of suberin. After treatment, sections were observed under UV light with a Leitz Ortholux (http//www.leitz.com/) microscope.
Grapevine Transformation and Selection
The VvPIP2;4N gene was cloned into pJam1469 binary vector, obtained by insertion of a Cauliflower mosaic virus 35S promoter cassette (http://www.pgreen.ac.uk/a_cst_fr.htm) into the pBIN19 plasmid (Bevan, 1984). The vector harboring the VvPIP2;4N gene (pJam1469-35s::VvPIP2;4N) was inserted into Agrobacterium tumefaciens strain LBA4404 through heat-shock transformation.
Embryogenic calli of cv Brachetto were transformed following the protocol by Gambino et al. (2005). Single embryo-derived plantlets were micropropagated by repeated subcultural apical cuttings on Murashige and Skoog medium without plant growth regulators and with kanamycin. The plants were acclimatized and transferred to a greenhouse. Expression and physiological analyses of the plants were carried out during the following summer.
PCR and Southern blotting confirmed transformation. DNA was extracted from grapevine plantlets grown in vitro using the method of Thomas et al. (1993). PCR amplification was performed using specific primer pairs for transgenic VvPIP2;4N (Supplemental Table S2) and nptII (Gambino et al., 2010) genes. For Southern hybridization, genomic DNA was digested by means of restriction endonucleases HindIII and EcoRI (50 units each; Promega), and the nptII gene was used as a DIG-labeled probe. To exclude cross-hybridization between PIP genes, we performed Southern analysis on cv Nebbiolo genomic DNA digested by XbaI restriction endonuclease (Promega) and hybridized with the full-length VvPIP2;4N DIG-labeled probe used for northern and in situ hybridization.
Expression Analyses of Aquaporin in Transgenic Grapevine
Primers for the endogenous and transgenic VvPIP2;4N sequences were designed using Primer Express 3.0 software (Applied Biosystems; http//www.appliedbiosystems.com/), while primers previously reported by Choat et al. (2009) were used to amplify VvPIP2;1, VvPIP2;2, VvPIP2;3, and VvPIP1;1 (Supplemental Table S2). First-strand cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems), and PCR was performed using PowerSYBR Green master mix (Applied Biosystems). Cycling conditions for all primer pairs consisted of an initial denaturation at 95°C for 10 min followed by 40 cycles at 95°C for 15 s, 56°C for 15 s, and 60°C for 1 min. PCR was performed in triplicate, and specific annealing of the primers was controlled by monitoring dissociation kinetics at the end of each PCR run. The geometric mean of the expression ratios of two endogenous housekeeping genes (actin and ubiquitin; ACT1 and UBI primers are described in Supplemental Table S2) was used as a normalization factor for all samples. The efficiency of each primer pair was measured on a serial dilution of a calibrator sample. The qRT-PCR data of all tissues and genes were expressed as abundance relative to endogenous VvPIP2;4N in wild-type irrigated roots.
Measurements of Physiological Parameters and Leaf ABA Concentrations
Six transgenic lines with different T-DNA configurations were chosen for analysis: lines 4, 16, 23, 24, 28, and 33. Eight plants for each line, together with eight wild-type plants, were acclimated in soil in greenhouse conditions (Lovisolo et al., 2008a), and four plants for each line were subjected to a water-stress treatment that consisted of withholding water for a 14-d period. Four further cv Nebbiolo plants were water stressed in the same conditions in order to compare their stomatal response to stress with wild-type cv Brachetto.
CO2 assimilation and leaf transpiration were recorded using the infrared gas analyzer ADC-LCPro+ system (Analytical Development Co.; www.adc-service.co.uk/) in the central hours of the day (11:00 am–1:00 pm) without photosynthetically active radiation limitations by measuring three leaves per plant, originating from the central region of the shoot, and averaging measurements every 10 min. During measurements, air temperature was 32.9°C ± 0.45°C, vapor pressure deficit was 24.8 ± 1.06 Pa kPa−1, and incident photon flux density was 1,210 ± 32.9 µmol m−2 s−1. Water use efficiency was calculated from CO2 assimilation and transpiration rates. For each plant, at the end of gas-exchange measurements, leaf water potential was measured on two transpiring leaves inserted in the central region of the shoot, using a Scholander-type pressure chamber (Soil Moisture Equipment; www.soilmoisture.com). Soil and shoot water potential measurements, and foliar ABA content measurement (one leaf per replicate plant collected at midday), were performed as described previously (Lovisolo et al., 2008a).
Root hydraulic conductance was measured on well-watered plants by means of an HCFM-XP Hydraulic Conductance Flowmeter (Dynamax; http//www.dynamax.com/). Rh plant was partitioned as described by Lovisolo et al. (2007), by considering root, shoot, and leaf resistances as additive. Shoot and root weight was measured. Surface area-specific root hydraulic conductance was calculated using an average root surface area-dry weight ratio measured on eight 1-g root samples. To this aim, root surface was determined based on measurements of average root diameter (assessed in 100 random points at 10× magnification) and of volume (assessed gravimetrically; Lovisolo et al., 2008b), considering the root shape as cylindrical.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison of VvPIP2;4N with grapevine PIPs.
Supplemental Figure S2. Southern analysis of DNA from cv Nebbiolo and Brachetto.
Supplemental Figure S3. Expression of endogenous PIP genes in transgenic cv Brachetto plants.
Supplemental Figure S4. Leaf gas exchange in transgenic cv Brachetto plants.
Supplemental Figure S5. Stomatal regulation in response to stress in cv Nebbiolo and Brachetto.
Supplemental Table S1. Transgenic cv Brachetto lines showing the same hybridization pattern in Southern blots.
Supplemental Table S2. Oligonucleotides used in this study.
Acknowledgments
We thank Danila Cuozzo and Tiziano Strano for plant micropropagation and greenhouse management and Marco Incarbone for editing of the manuscript.
Glossary
- qRT
quantitative reverse transcription
- Pf
water permeability
- ABA
abscisic acid
- HPFM
high-pressure flux method
- EFM
evaporative flux method
- Rh root
whole-root hydraulic resistance
- Rh plant
whole-plant hydraulic resistance
- DIG
digoxigenin
- cDNA
complementary DNA
References
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G. (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiges I, Schäffner AR, Mas A. (2001) Eight cDNA encoding putative aquaporins in Vitis hybrid Richter-110 and their differential expression. J Exp Bot 52: 1949–1951 [DOI] [PubMed] [Google Scholar]
- Balestrini R, Mainieri D, Soragni E, Garnero L, Rollino S, Viotti A, Ottonello S, Bonfante P. (2000) Differential expression of chitin synthase III and IV mRNAs in ascomata of Tuber borchii Vittad. Fungal Genet Biol 31: 219–232 [DOI] [PubMed] [Google Scholar]
- Barone LM, Shih C, Wasserman BP. (1997) Mercury-induced conformational changes and identification of conserved surface loops in plasma membrane aquaporins from higher plants: topology of PMIP31 from Beta vulgaris L. J Biol Chem 272: 30672–30677 [DOI] [PubMed] [Google Scholar]
- Barrowclough DE, Peterson CA, Steudle E. (2000) Radial hydraulic conductivity along developing onion roots. J Exp Bot 51: 547–557 [DOI] [PubMed] [Google Scholar]
- Bevan M. (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12: 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R. (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18: 565–570 [DOI] [PubMed] [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. (1988) A berberine-aniline blue fluorescent staining procedure for suberin, lignin and callose in plant tissue. Protoplasma 146: 133–142 [Google Scholar]
- Buckley TN, Sack L, Gilbert ME. (2011) The role of bundle sheath extensions and life form in stomatal responses to leaf water status. Plant Physiol 156: 962–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ. (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122: 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Gambetta GA, Shackel KA, Matthews MA. (2009) Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiol 151: 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C. (1994) Aquaporins: the molecular basis of facilitated water movement through living plant cells? Plant Physiol 105: 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. (2007) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol 143: 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XH, Hao FS, Chen H, Chen J, Wang XC. (2008) Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves their drought resistance. J Plant Res 121: 207–214 [DOI] [PubMed] [Google Scholar]
- Cutanda-Perez MC, Ageorges A, Gomez C, Vialet S, Terrier N, Romieu C, Torregrosa L. (2009) Ectopic expression of VlmybA1 in grapevine activates a narrow set of genes involved in anthocyanin synthesis and transport. Plant Mol Biol 69: 633–648 [DOI] [PubMed] [Google Scholar]
- D’Angeli S, Altamura MM. (2007) Osmotin induces cold protection in olive trees by affecting programmed cell death and cytoskeleton organization. Planta 225: 1147–1163 [DOI] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet R, Léon C, Ollat N, Barrieu F. (2008) Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep 27: 1541–1550 [DOI] [PubMed] [Google Scholar]
- Galmés J, Pou A, Alsina MM, Tomàs M, Medrano H, Flexas J. (2007) Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): relationship with ecophysiological status. Planta 226: 671–681 [DOI] [PubMed] [Google Scholar]
- Gambino G, Gribaudo I, Leopold S, Schartl A, Laimer M. (2005) Molecular characterization of grapevine plants transformed with GFLV resistance genes. I. Plant Cell Rep 24: 655–662 [DOI] [PubMed] [Google Scholar]
- Gambino G, Perrone I, Carra A, Chitarra W, Boccacci P, Torello Marinoni D, Barberis M, Maghuly F, Laimer M, Gribaudo I. (2010) Transgene silencing in grapevines transformed with GFLV resistance genes: analysis of variable expression of transgene, siRNAs production and cytosine methylation. Transgenic Res 19: 17–27 [DOI] [PubMed] [Google Scholar]
- Gelvin SB. (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67: 16–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Zelazny E, Chaumont F. (2006) Modulating the expression of aquaporin genes in planta: a key to understand their physiological functions? Biochim Biophys Acta 1758: 1142–1156 [DOI] [PubMed] [Google Scholar]
- Heckwolf M, Pater D, Hanson DT, Kaldenhoff R. (2011) The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO₂ transport facilitator. Plant J 67: 795–804 [DOI] [PubMed] [Google Scholar]
- Heinen RB, Ye Q, Chaumont F. (2009) Role of aquaporins in leaf physiology. J Exp Bot 60: 2971–2985 [DOI] [PubMed] [Google Scholar]
- Hukin D, Doering-Saad C, Thomas CR, Pritchard J. (2002) Sensitivity of cell hydraulic conductivity to mercury is coincident with symplasmic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta 215: 1047–1056 [DOI] [PubMed] [Google Scholar]
- Iocco P, Franks T, Thomas MR. (2001) Genetic transformation of major wine grape cultivars of Vitis vinifera L. Transgenic Res 10: 105–112 [DOI] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P. (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126: 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P. (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Fischer M. (2006) Aquaporins in plants. Acta Physiol (Oxf) 187: 169–176 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U. (1998) Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. Plant J 14: 121–128 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Ribas-Carbo M, Sans JF, Lovisolo C, Heckwolf M, Uehlein N. (2008) Aquaporins and plant water balance. Plant Cell Environ 31: 658–666 [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Koshio K, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K. (2003) Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant Cell Physiol 44: 1378–1383 [DOI] [PubMed] [Google Scholar]
- Kumar S, Fladung M. (2001) Gene stability in transgenic aspen (Populus). II. Molecular characterization of variable expression of transgene in wild and hybrid aspen. Planta 213: 731–740 [DOI] [PubMed] [Google Scholar]
- Li GW, Zhang MH, Cai WM, Sun WN, Su WA. (2008) Characterization of OsPIP2;7, a water channel protein in rice. Plant Cell Physiol 49: 1851–1858 [DOI] [PubMed] [Google Scholar]
- Liakoura V, Fotelli MN, Rennenberg H, Karabourniotis G. (2009) Should structure-function relations be considered separately for homobaric vs. heterobaric leaves? Am J Bot 96: 612–619 [DOI] [PubMed] [Google Scholar]
- Loveys B. (1984) Abscisic acid transport and metabolism in grapevine (Vitis vinifera L.). New Phytol 98: 575–582 [Google Scholar]
- Lovisolo C, Perrone I, Carra A, Ferrandino A, Flexas J, Medrano H, Schubert A. (2010) Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Funct Plant Biol 37: 98–116 [Google Scholar]
- Lovisolo C, Perrone I, Hartung W, Schubert A. (2008a) An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytol 180: 642–651 [DOI] [PubMed] [Google Scholar]
- Lovisolo C, Secchi F, Nardini A, Salleo S, Buffa R, Schubert A. (2007) Expression of PIP1 and PIP2 aquaporins is enhanced in olive dwarf genotypes and is related to root and leaf hydraulic conductance. Physiol Plant 130: 543–551 [Google Scholar]
- Lovisolo C, Tramontini S, Flexas J, Schubert A. (2008b) Mercurial inhibition of root hydraulic conductance in Vitis spp. rootstocks under water stress. Environ Exp Bot 63: 178–182 [Google Scholar]
- Maghuly F, Leopold S, da Câmara Machado A, Borroto Fernandez E, Ali Khan M, Gambino G, Gribaudo I, Schartl A, Laimer M. (2006) Molecular characterization of grapevine plants transformed with GFLV resistance genes. II. Plant Cell Rep 25: 546–553 [DOI] [PubMed] [Google Scholar]
- Marjanović Z, Uehlein N, Kaldenhoff R, Zwiazek JJ, Weiss M, Hampp R, Nehls U. (2005) Aquaporins in poplar: what a difference a symbiont makes! Planta 222: 258–268 [DOI] [PubMed] [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130: 2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Lian HL, Su WA, Tanaka D, Liu C, Iwasaki I, Kitagawa Y. (2009) Role of the aquaporin PIP1 subfamily in the chilling tolerance of rice. Plant Cell Physiol 50: 216–229 [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59: 595–624 [DOI] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R. (2002) Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14: 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Gortan E, Salleo S. (2005) Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. Funct Plant Biol 32: 953–961 [DOI] [PubMed] [Google Scholar]
- North GB, Martre P, Nobel PS. (2004) Aquaporins account for variations in hydraulic conductance for metabolically active root regions of Agave deserti in wet, dry, and rewetted soil. Plant Cell Environ 27: 219–228 [Google Scholar]
- Otto B, Kaldenhoff R. (2000) Cell-specific expression of the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 211: 167–172 [DOI] [PubMed] [Google Scholar]
- Otto B, Uehlein R, Sdorra S, Fischer M, Ayaz M, Belastegui-Macadam X, Heckwolf M, Lichnit M, Pede N, Priem N, et al. (2010) Aquaporin tetramer composition modifies the function of tobacco aquaporins. J Biol Chem 285: 31253–31260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Arora R, Li G, Wang X, Fessehaie A. (2008) Rhododendron catawbiense plasma membrane intrinsic proteins are aquaporins, and their over-expression compromises constitutive freezing tolerance and cold acclimation ability of transgenic Arabidopsis plants. Plant Cell Environ 31: 1275–1289 [DOI] [PubMed] [Google Scholar]
- Picaud S, Becq F, Dédaldécheamp F, Ageorges A, Delrot S. (2003) Cloning and expression of two plasma membrane aquaporins expressed during the ripening of grape berry. Funct Plant Biol 30: 621–630 [DOI] [PubMed] [Google Scholar]
- Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, Schäffner AR, Maurel C. (2010) A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol 152: 1418–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reustle GM, Büchholz G. (2009) Recent trends in grapevine genetic engineering. In KA Roubelakis-Angelakis, ed, Grapevine Molecular Physiology and Biotechnology. Springer, Dordrecht, The Netherlands, pp 495–508
- Sade N, Vinocur BJ, Diber A, Shatil A, Ronen G, Nissan H, Wallach R, Karchi H, Moshelion M. (2009) Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol 181: 651–661 [DOI] [PubMed] [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol 46: 1568–1577 [DOI] [PubMed] [Google Scholar]
- Secchi F, Lovisolo C, Uehlein N, Kaldenhoff R, Schubert A. (2007) Isolation and functional characterization of three aquaporins from olive (Olea europaea L.). Planta 225: 381–392 [DOI] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. (2010) Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant Cell Environ 33: 1285–1297 [DOI] [PubMed] [Google Scholar]
- Shelden MC, Howitt SM, Kaiser BN, Tyerman SD. (2009) Identification and functional characterisation of aquaporins in the grapevine, Vitis vinifera. Funct Plant Biol 36: 1065–1078 [DOI] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorieul M, Santoni V, Maurel C, Luu DT. (2011) Mechanisms and effects of retention of over-expressed aquaporin AtPIP2;1 in the endoplasmic reticulum. Traffic 12: 473–482 [DOI] [PubMed] [Google Scholar]
- Stoll M, Loveys B, Dry P. (2000) Hormonal changes induced by partial rootzone drying of irrigated grapevine. J Exp Bot 51: 1627–1634 [DOI] [PubMed] [Google Scholar]
- Tesnière C, Torregrosa L, Pradal M, Souquet JM, Gilles C, Dos Santos K, Chatelet P, Gunata Z. (2006) Effects of genetic manipulation of alcohol dehydrogenase levels on the response to stress and the synthesis of secondary metabolites in grapevine leaves. J Exp Bot 57: 91–99 [DOI] [PubMed] [Google Scholar]
- Thomas MR, Matsumoto S, Cain P, Scott NS. (1993) Repetitive DNA of grapevine: classes present and sequences suitable for cultivar identification. Theor Appl Genet 86: 173–180 [DOI] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C. (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397 [DOI] [PubMed] [Google Scholar]
- Tsuchihira A, Hanba YT, Kato N, Doi T, Kawazu T, Maeshima M. (2010) Effect of overexpression of radish plasma membrane aquaporins on water-use efficiency, photosynthesis and growth of Eucalyptus trees. Tree Physiol 30: 417–430 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Nardini A, Salleo S, Sack L, El Omari B. (2005) The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: any role for stomatal response? J Exp Bot 56: 737–744 [DOI] [PubMed] [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD. (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149: 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YZ, Costa A, Leonhardt N, Siegel RS, Schroeder JI. (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazny E, Miecielica U, Borst JW, Hemminga MA, Chaumont F. (2009) An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J 57: 346–355 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Chai T, Wen Z, Zhang H. (2008) Indian mustard aquaporin improves drought and heavy-metal resistance in tobacco. Mol Biotechnol 40: 280–292 [DOI] [PubMed] [Google Scholar]