Abstract
Transparent Testa16 (TT16), a transcript regulator belonging to the Bsister MADS box proteins, regulates proper endothelial differentiation and proanthocyanidin accumulation in the seed coat. Our understanding of its other physiological roles, however, is limited. In this study, the physiological and developmental roles of TT16 in an important oil crop, canola (Brassica napus), were dissected by a loss-of-function approach. RNA interference (RNAi)-mediated down-regulation of tt16 in canola caused dwarf phenotypes with a decrease in the number of inflorescences, flowers, siliques, and seeds. Fluorescence microscopy revealed that tt16 deficiency affects pollen tube guidance, resulting in reduced fertility and negatively impacting embryo and seed development. Moreover, Bntt16 RNAi plants had reduced oil content and altered fatty acid composition. Transmission electron microscopy showed that the seeds of the RNAi plants had fewer oil bodies than the nontransgenic plants. In addition, tt16 RNAi transgenic lines were more sensitive to auxin. Further analysis by microarray showed that tt16 down-regulation alters the expression of genes involved in gynoecium and embryo development, lipid metabolism, auxin transport, and signal transduction. The broad regulatory function of TT16 at the transcriptional level may explain the altered phenotypes observed in the transgenic lines. Overall, the results uncovered important biological roles of TT16 in plant development, especially in fatty acid synthesis and embryo development.
The plant MADS box family genes, which are named after the characterization of four members in this group, MINICHROMOSOME MAINTENANCE1, AGAMOUS, DEFICIENS and SERUM RESPONSE FACTOR, encode transcription factors that share a common DNA-binding domain (the MADS box) and play multiple roles in flower pattern formation, gametophyte cell division, and fruit wall differentiation (Ng and Yanofsky, 2001; Dinneny and Yanofsky, 2005; Colombo et al., 2008). A number of MADS domain proteins from vascular plants share a conserved structural organization, the so-called MIKC-type domain structure, where the MADS (M) domain is followed by an Intervening (I), a Keratin-like (K) and a C-terminal domain (Theissen et al., 1996). MIKC-type MADS box genes are involved in important aspects of plant reproductive development, such as flower initiation, specification of floral meristem and organ identity, and ovule and fruit development (Becker and Theissen, 2003).
MADS box genes are the major members of plant floral organ identity genes, which have been divided into five classes according to the ABCDE model (Theissen, 2001; Krizek and Fletcher, 2005). The ABCDE model explains flower formation by the interaction of five classes of homeotic genes (A–E), with A controlling sepal, A+B+E controlling petal, B+C+E controlling stamen, C+E controlling carpel, and D controlling ovule development (Theissen, 2001; Krizek and Fletcher, 2005). Therefore, MADS box genes appear to have a central role in flower development. The functions of floral organ identity genes are highly correlated to their phylogenetic relationships in that these genes are members of well-defined gene clades termed SQUAMOSA (class A), DEFICIENS or GLOBOSA (class B), AGAMOUS (classes C and D), and SEPALLATA (class E; Becker and Theissen, 2003; Melzer et al., 2010).
Recently, a group of MADS box proteins, closely related to the B class floral homeotic proteins, were identified as the Bsister subfamily (Becker et al., 2002). It has been suggested that the B and Bsister gene lineages were generated by a duplication of an ancestral gene before the divergence of gymnosperm and angiosperm lineages 300 million years ago but after the separation of the fern lineage 400 million years ago (Becker et al., 2002; Stellari et al., 2004). B genes are predominantly expressed in petal and stamen primordia; however, the expression of Bsister genes is found almost exclusively in female reproductive structures or is even restricted to the ovules (Theissen et al., 1996, 2000; Becker et al., 2002; de Folter et al., 2006). It has been hypothesized that the B class proteins are important for male reproductive organ development, whereas the Bsister proteins are important for female reproductive organ development (Becker et al., 2002).
Transparent Testa16 (TT16) is a Bsister gene that acts as a transcription factor and appears to play a role in seed coat pigmentation and proanthocyanidin (PA) accumulation in the endothelium of developing seeds. A tt16 mutant, in which the TT16/ABS gene encoding a MIKC-type MADS box protein was disrupted, showed altered seed pigmentation and PA accumulation, but TT16/ABS alone does not appear to be crucial for female reproductive development in Arabidopsis (Arabidopsis thaliana; Nesi et al., 2002). Interestingly, the recently characterized Bsister MADS box Floral Binding Protein24 (FB24) from petunia (Petunia hybrida) was unable to complement the Arabidopsis tt16 mutant, despite having similar developmental roles to TT16/ABS (de Folter et al., 2006). The only other Bsister MADS box gene in Arabidopsis is GORDITA (GOA; formerly known as AGL63), which like TT16/ABS arose from duplication during the diversification of the Brassicaceae (Erdmann et al., 2010). Functional studies suggest that GOA not only contributes to integument development but also regulates fruit growth (Erdmann et al., 2010; Prasad et al., 2010); however, up to now, the detailed physiological functions of the Bsister transcription factors have not been extensively studied.
In developing oilseeds, transcription factors not only govern fruit and seed development but also storage lipid metabolism, including fatty acid (FA) and triacylglycerol synthesis, which is of interest for biotechnological applications in seed oil modification. WRINKLED1 (WRI1) is a member of the APETALA2/ETHYLENE-RESPONSIVE ELEMENT BINDING PROTEINS belonging to the A class in the ABCDE model, and overexpression of WRI1 enhanced the transcription of FA biosynthetic genes, leading to increased triacylglycerol accumulation in both seeds and leaves (Cernac and Benning, 2004; Baud and Lepiniec, 2009). LEAFY COTYLEDON1 (LEC1) is an NFY-B-type or CCAAT-binding factor-type transcription factor, and LEC2 belongs to the plant-specific B3 transcription factor family (Lotan et al., 1998; Stone et al., 2001). LEC2 and LEC1 can control the expression of FA biosynthesis genes (Baud et al., 2007; Mu et al., 2008). To date, however, there is no report on the function of Bsister proteins in FA synthesis or triacylglycerol accumulation, despite their importance in other aspects of seed development.
This study aimed to determine the physiological functions of four recently identified canola (Brassica napus) TT16 (BnTT16) homologs in plant development and seed oil synthesis. Using an RNA interference (RNAi) approach, we found that down-regulation of TT16 in canola affects pollen tube guidance, reduces fertility, and influences FA synthesis and embryo development. The results strongly suggest that TT16 plays multiple physiological roles beyond endothelial development and PA accumulation and that TT16 may be a suitable biotechnological target for seed oil modification.
RESULTS
Down-Regulation of Bntt16s Alters Canola Development
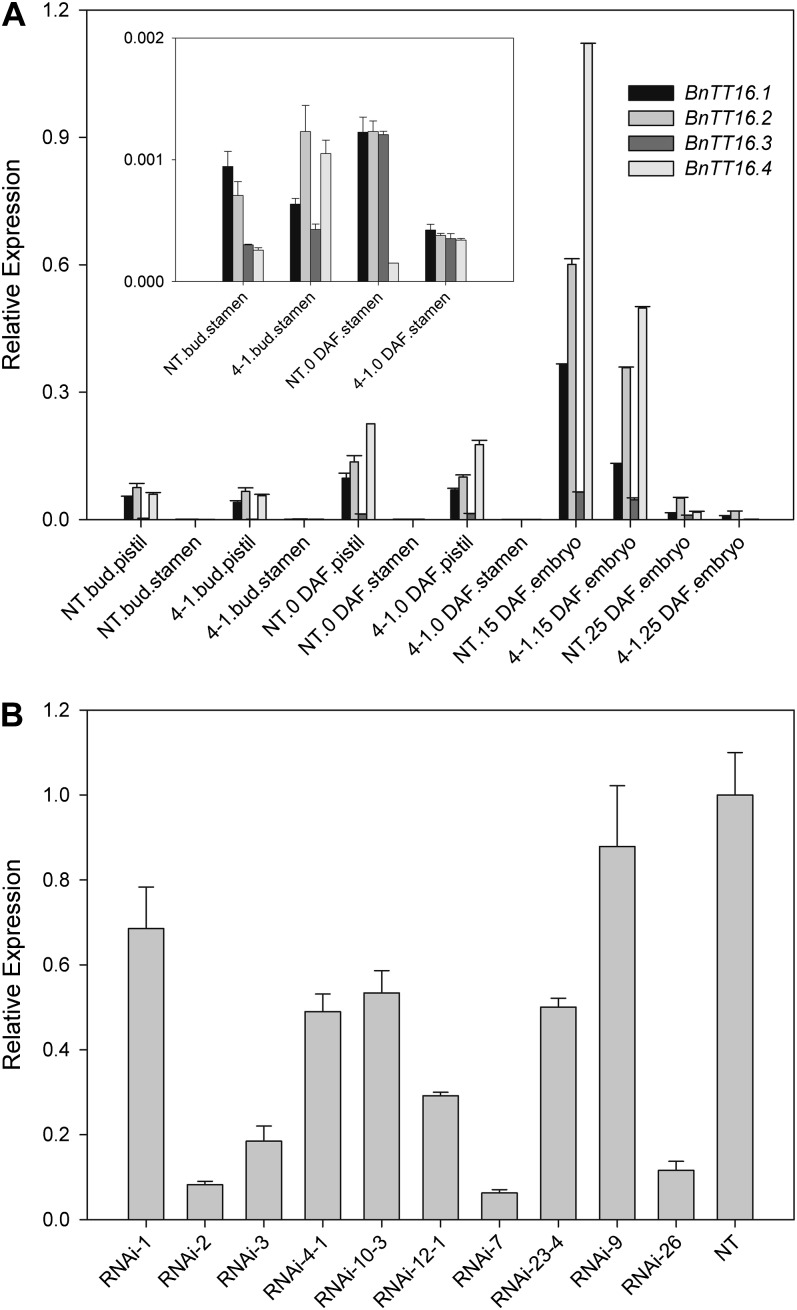
We identified four TT16 homologs from canola based on the analysis of ESTs and genomic DNA sequences, in which two shared the identical sequences of the reported BnTT16.1 (EU192028) and BnTT16.2 (EU192029). The other two were named BnTT16.3 (HM449990) and BnTT16.4 (HM449989). Similar to TT16 in Arabidopsis, the BnTT16s regulate endothelium development and PA accumulation (G. Chen, W. Deng, F. Peng, M. Truksa, S. Singer, C. Snyder, and R. Weselake, unpublished data). As shown in Figure 1A, TT16s have much higher expression levels in female organs, consistent with other plant Bsister genes (Chen et al., 2012). Although the expression levels of the four TT16s varied, they all have the highest expression level in the embryo of early developing seeds (15 d after flowering [DAF]). To assess the effects of the loss of function of four TT16s on canola growth and development, a 146-bp conserved complementary DNA fragment was cloned into pHellsgate 12 under the control of a cauliflower mosaic virus 35S promoter for subsequent generation of canola RNAi transgenic plants. Over 40 transgenic lines (TT16s RNAi) were generated, and 10 were used for further study. All 10 transgenic lines showed lower accumulation of TT16 transcripts (Fig. 1B). In line RNAi-4-1, the overall TT16 expression level decreased about 50% (Fig. 1B) and the expression of each TT16 was down-regulated (Fig. 1A). Therefore, this line was selected as a representative for further study.
Figure 1.
RNAi-mediated silencing of Bntt16 expression. A, Gene expression patterns (2−ΔCT) of BnTT16s in canola tissues. BnTT16 expression levels in the stamen are also shown in the inset. B, Overall expression levels of BnTT16s in samples 2 DAF were down-regulated in all RNAi lines. Bntt16 expression level in NT plants was set as 1 for comparison. 4-1, tt16 RNAi transgenic line 4-1.
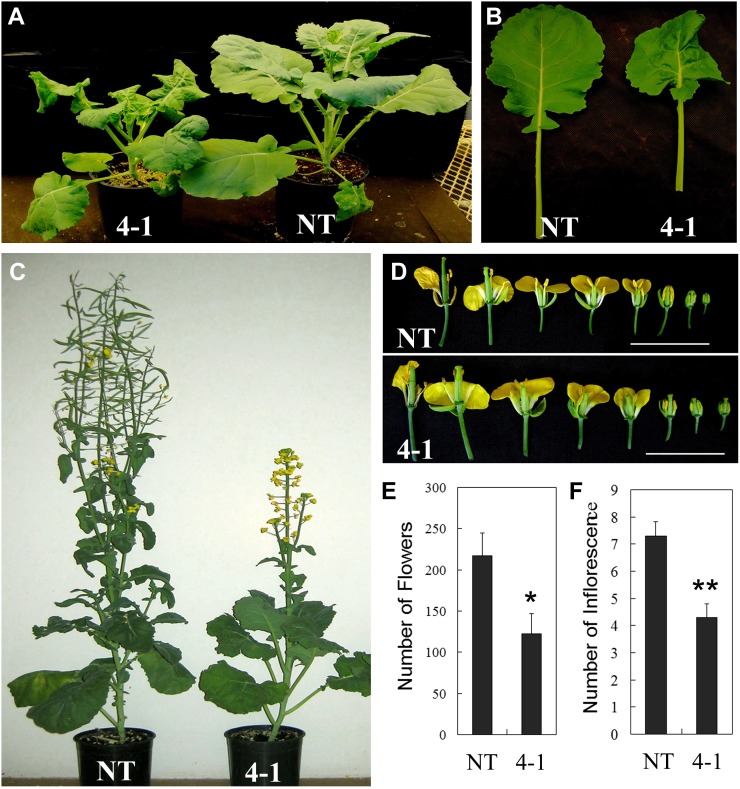
Multiple phenotypes related to vegetative growth and development were identified in transgenic plants. Nontransgenic (NT) plants flowered earlier than the transgenic plants, indicating that down-regulation of TT16s delayed the transition from vegetative growth to reproductive stage in canola. As shown in Figure 2, A and B, the 40-d-old transgenic plants exhibited dwarf stature and smaller, wrinkled leaves compared with the NT plants. Dwarf phenotypes were also identified in 70-d-old TT16 RNAi transgenic plants (Fig. 2C). Regarding the influence of tt16 down-regulation on the reproductive organs, the first phenotype is that the RNAi plants had larger flowers and floral organs with stigmas protruding out of the unopened buds (Fig. 2D; Supplemental Table S2). Moreover, the number of inflorescences and total flowers in transgenic plants significantly decreased compared with the NT plants (Fig. 2, E and F; P < 0.01). In addition, the transgenic plants produced shorter siliques and fewer seeds (Supplemental Fig. S1; P < 0.01).
Figure 2.
Morphological alteration exhibited by the tt16 down-regulated canola line. A, Dwarf phenotypes of 40-d-old tt16 RNAi transgenic lines. B, Leaves of 40-d-old transgenic lines. C, Delayed flowers of 70-d-old tt16 RNAi transgenic lines. D, Flowers of 70-d-old transgenic lines. E, Number of flowers and inflorescences in 70-d-old tt16 RNAi transgenic lines. 4-1, tt16 RNAi transgenic line 4-1. sd (n = 3) is indicated by vertical bars. Single and double asterisks indicate significant differences between transgenic and NT plants with P < 0.05 and P < 0.01, respectively, as determined by t test.
Down-Regulation of Bntt16s Affects Pollen Tube Guidance
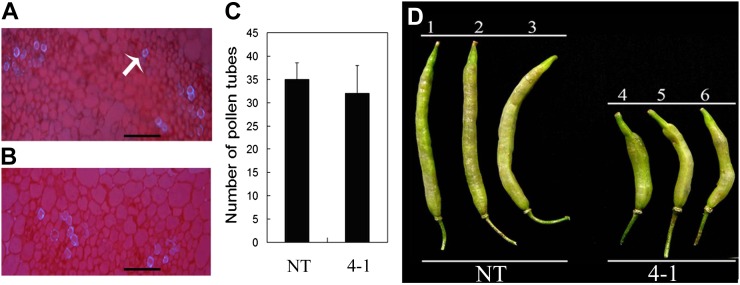
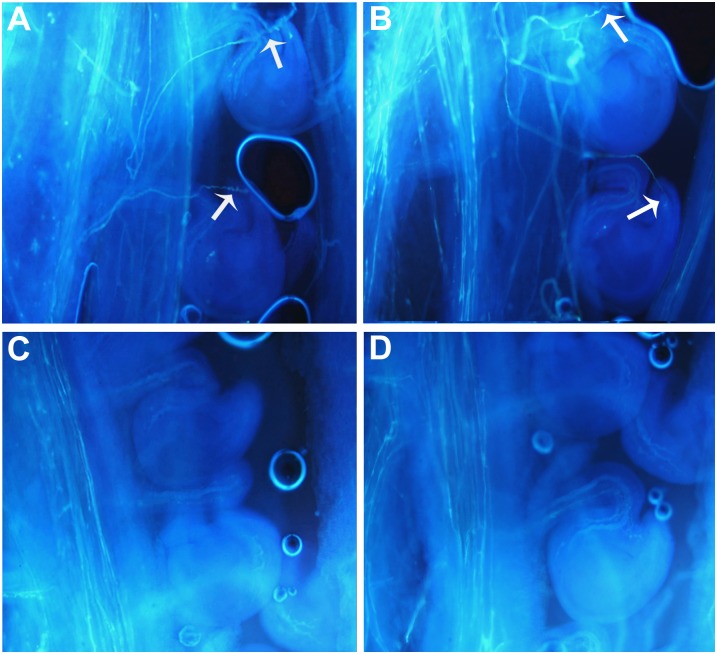
To investigate the cause(s) for the reduced seed set of the canola tt16 lines, pollen viability was first tested using iodine-potassium iodide staining. Pollen in both tt16 RNAi and NT lines stained as normal dark brown color, indicating that pollen viability was not affected (data not shown). Second, to determine if pollen tube development in style was affected by tt16 down-regulation, styles were stained with aniline blue and pollen tubes were counted by fluorescence microscopy. The pollen tube numbers in RNAi and NT plants showed no statistical difference, indicating that the pollen tube developed normally in RNAi lines (Fig. 3, A–C). RNAi and NT lines were manually cross-pollinated to examine pollen development. As shown in Figure 3D, transgenic lines pollinated with pollen from NT lines produced shorter siliques and fewer seeds, whereas NT lines pollinated with pollen from RNAi plants produced normal siliques and seeds. Therefore, the reduced fertility in RNAi plants was not due to pollen development. Finally, we examined pollen tube extension by fluorescence microscopy. Most of the pollen tubes can extend into the ovules in 2-DAF siliques of self-pollinated NT plants and NT plants pollinated with line 4-1 (Fig. 4, A and B), whereas only a few pollen tubes can extend into the ovules in siliques (2 DAF) of self-pollinated line 4-1 and line 4-1 pollinated with NT plants (Fig. 4, C and D). Overall, these results revealed that tt16 down-regulation affected pollen tube guidance, thus resulting in reduced fertility.
Figure 3.
Pollen tubes development in a tt16 RNAi transgenic line and artificial pollination. A, Style section of a NT plants. The arrow indicates a pollen tube. B, Style section of the tt16 RNAi transgenic line 4-1. Bars = 8 μm. C, Number of pollen tubes in style sections. se values (n = 3) are indicated by vertical bars. D, Artificial pollination. 1, NT plants without artificial pollination. 2, NT plants with artificial pollination (self-pollination). 3, NT plants pollinated with line 4-1 (cross-pollination). 4, Line 4-1 without artificial pollination. 5, Line 4-1 with artificial pollination (self-pollination). 6, Line 4-1 pollinated with NT plants (cross-pollination).
Figure 4.
Guiding of pollen tubes to ovules in self- and cross-pollinated plants. A, Self-pollinated NT plants. B, NT plants pollinated with tt16 RNAi transgenic line 4-1. C, Self-pollinated line 4-1. D, Line 4-1 pollinated with NT plants. Arrows indicate pollen targeted into ovules.
Down-Regulation of Bntt16s Alters Seed Development and Lipid Synthesis
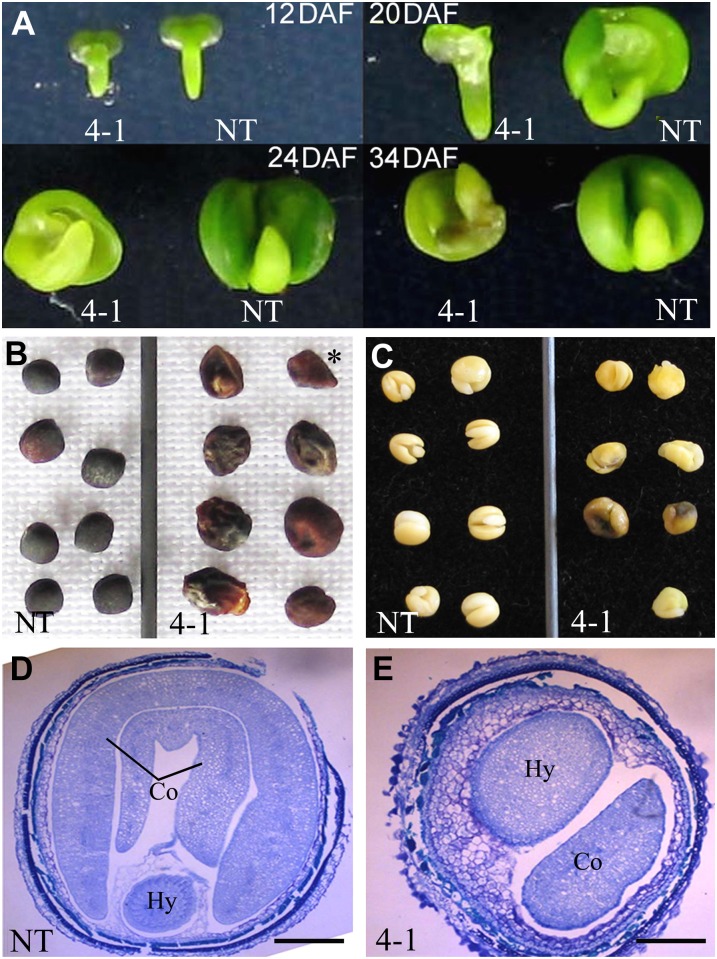
The Bntt16 RNAi plants also produced abnormal mature seeds in terms of seed morphology. In order to characterize this phenotype in detail, we first examined embryos at 8, 12, 20, 24, and 34 DAF. As shown in Figure 5A, the embryos of tt16 RNAi transgenic plants developed normally at 8 and 12 DAF but abnormally at 20, 24, and 34 DAF. We compared seed morphology between RNAi and NT lines. The seeds of the tt16 RNAi lines can be classified into two types. Type 1 seeds were deflated and flattened, whereas type 2 seeds were wrinkled and irregular in shape (Fig. 5B). Type 1 seeds had only seed coats with no embryo. Type 2 seeds contained defective embryos with irregular single cotyledons. Some embryos of type 2 seeds turned dark brown at maturity (Fig. 5C). Finally, we compared the seeds by microscopy. As shown in Figure 5, D and E, the transverse sections of mature type 2 seeds illustrate the defective embryo with single cotyledon and remaining endosperm. The transverse sections of mature type 1 seeds had seed coats but no embryo, demonstrating that embryo abortion occurred in type 1 seeds. Collectively, these results strongly indicate that down-regulation of tt16s affects the development of embryos and seeds.
Figure 5.
Embryo and seed development in tt16s RNAi transgenic lines. A, Embryo development in line 4-1. B, Mature seeds (43 DAF) of transgenic lines. The single asterisk indicates a type 1 seed with no embryo. The rest are type 2 seeds. C, Embryos in mature seeds (43 DAF). D, Seed transverse sections (34 DAF) of NT plants. E, Seed transverse sections (34 DAF) of line 4-1. Co, Cotyledon; Hy, hypocotyl. Bar = 40 mm.
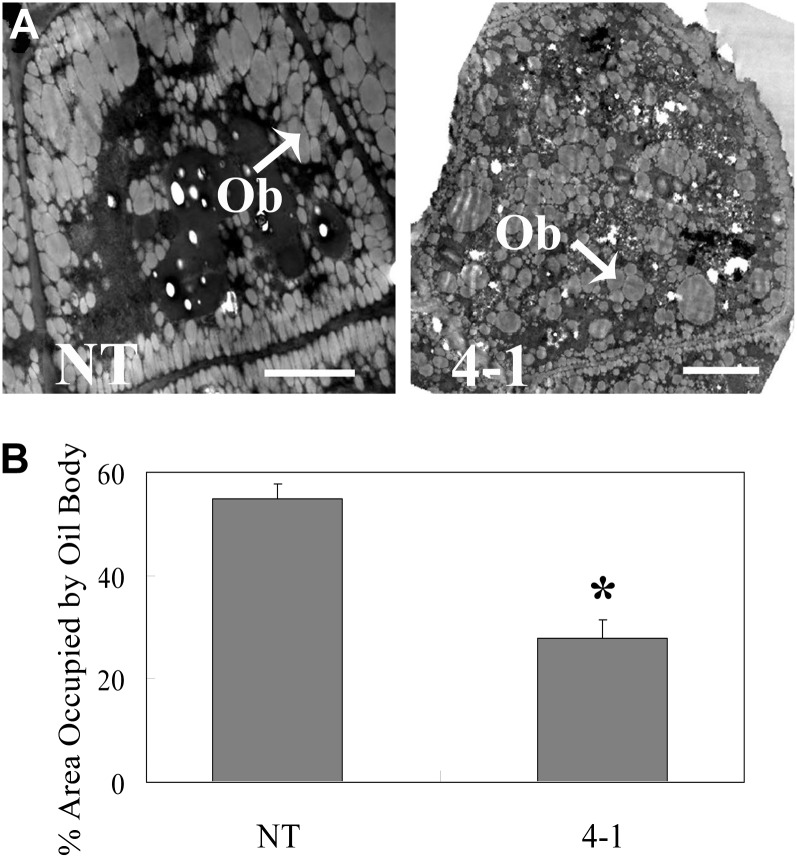
The FA content and composition in mature seeds were analyzed using gas chromatography-mass spectrometry. The FA content was dramatically decreased, which was consistent with transmission electron microscopy observations showing fewer oil bodies in RNAi seeds compared with the NT (Fig. 6). The FA composition of RNAi seeds was also significantly different from the NT (Table I). The percentage of 16-carbon FA and very-long-chain FA (C20-22) was higher in the RNAi lines. The transgenic seeds also showed an increase in linoleic acid (18:2cisΔ9,12) at the expense of oleic acid (18:1cisΔ9) compared with NT seeds.
Figure 6.
Transmission electron microscopy observation of hypocotyl cells (34 DAF) in tt16 RNAi transgenic lines. A, Transmission electron microscopy observation of hypocotyl cells. Ob, Oil bodies. Bars = 2.5 μm. B, Quantitation of the area occupied by oil bodies. The percentage area occupied by oil bodies was determined using ImageJ software (http://rsbweb.nih.gov/ij/). se values (n = 3) are indicated by vertical bars. The single asterisk indicates a significant difference between transgenic and NT plants with P < 0.05 as determined by t test. 4-1, tt16 RNAi transgenic line 4-1.
Table I. Total FA and FA compositions in NT and tt16 RNAi plants.
Asterisks denote significant differences (*P < 0.05 and **P < 0.01) between transgenic and NT plants by t test. The data are means ± se corresponding to three independent experiments.
| FA | Composition of Fatty Acids |
|
|---|---|---|
| NT Plant | RNAi Plant | |
| % | ||
| 16:0 | 4.4 ± 0.0 | 7.6 ± 0.0** |
| 16:1 | 0.1 ± 0.0 | 0.4 ± 0.0** |
| 18:0 | 2.8 ± 0.1 | 4.9 ± 0.2** |
| 18:1 | 73.4 ± 0.2 | 60.1 ± 0.1** |
| 18:2 | 11.8 ± 0.0 | 19.4 ± 0.3** |
| 18:3 | 4.9 ± 0.0 | 4.6 ± 0.1* |
| 20:0 | 0.9 ± 0.1 | 1.3 ± 0.0* |
| 20:1 | 0.8 ± 0.0 | 0.3 ± 0.1** |
| 22:0 | 0.9 ± 0.2 | 1.3 ± 0.1** |
| Total FA (mg g−1) | 413.0 ± 4.4 | 261.7 ± 6.2** |
| Total FA (mg seed−1) | 1.9 ± 0.0 | 0.9 ± 0.0** |
Down-Regulation of Bntt16s Alters the Expression of Genes Involved in Gynoecium and Embryo Development, Lipid Metabolism, and Auxin Signaling
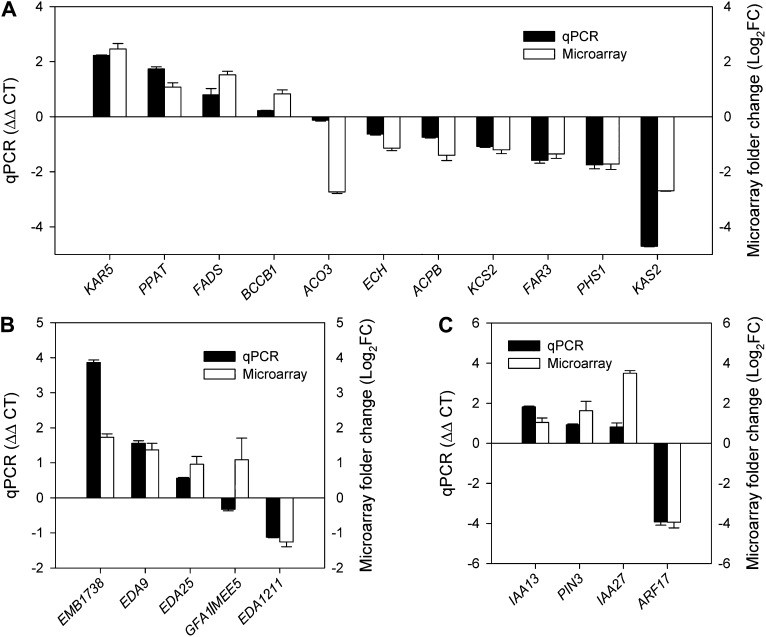
To further understand why the down-regulation of transcription factor tt16s caused the above phenotypes, we compared the gene expression profiles of RNAi plants (2-DAF siliques) with the NT line by microarray. In order to validate the results from the microarray analyses, 20 genes were selected and further studied by real-time PCR. Nineteen out of 20 genes showed similar expression patterns by microarray assay (Fig. 7). The results indicated that the data from the microarray were reproducible and reliable.
Figure 7.
Verification of microarray data using real-time PCR. A, Genes involved in lipid metabolism. B, Genes involved in gynoecium and embryo development. C, Auxin-related genes.
Analysis of the microarray data revealed that genes involved in lipid metabolism, gynoecium and embryo development, and auxin transport and signal transduction were affected by tt16 down-regulation (Tables II–IV). For lipid metabolism, nine genes were down-regulated and 18 genes were up-regulated. For example, 3-Oxoacyl-ACP reductase5 (KAR5) and Chloroplastic acetyl-coenzyme A carboxylase1 (BCCP1), both involved in FA synthesis, were down-regulated in RNAi plants (Table II; Harwood, 1988; O’Hara et al., 2007). For gynoecium and embryo development, 50 genes were down-regulated and 15 genes were up-regulated. GAMETOPHYTE FACTOR1 (GFA1)/MATERNAL EFFECT EMBRYO ARREST5 (MEE5), which regulates egg cell differentiation in embryo sacs, was down-regulated in transgenic plants (Table III; Liu et al., 2009). Interestingly, microarray analysis showed that the expression of many auxin-related genes was altered as well (Table IV).
Table II. Microarray analysis of tt16 RNAi plants: genes involved in lipid metabolism in siliques (2 DAF) of tt16 RNAi plants.
Microarray data were analyzed using the open-source R statistical programming language and Bioconductor packages with P < 0.01 (Gentleman et al., 2004; R Development Core Team 2010). GO, Gene Ontology categories.
| Gene Name | Gene Identifier | Arabidopsis Genome Initiative Locus Identifier | Ratio | P Value | Description |
|---|---|---|---|---|---|
| LTPG1 | EV178367 | AT1G27950 | 2.73 | 0.0004 | Glycosylphosphatidylinositol-anchored lipid protein transfer1 (LTPG1); GO: lipid transport |
| KAR5 | CN830460 | AT1G24360 | 2.46 | 0.0004 | 3-Oxoacyl-acyl-reductase5 (KAR5); GO: fatty acid biosynthesis process |
| ACOX3 | TA34635_3708 | AT1G06290 | 2.16 | 0.0014 | Acyl-CoA oxidase3 (ACOX3); GO: fatty acid β-oxidation, medium-chain fatty acid metabolic process |
| FADS | ES267177 | AT1G06090.1 | 1.52 | 0.0023 | Fatty acid desaturase family protein (FADS); GO: lipid metabolic process, oxidation-reduction process |
| FAR4 | TA27656_3708 | AT3G11980 | 1.22 | 0.0012 | Fatty acid reductase2 (FAR4); GO: oxidation-reduction process, pollen exine formation |
| PPAT | TA25260_3708 | AT2G18250 | 1.08 | 0.0024 | 4-Phosphopantetheine adenylyltransferase (PPAT); GO: lipid metabolic process, lipid storage |
| LTP12 | TA20970_3708 | AT3G51590 | 0.91 | 0.0029 | Lipid transfer protein12 (LTP12); GO: lipid transport, bind fatty acids and acyl-CoA esters and can transfer several different phospholipids |
| ACO2 | DY023994 | AT5G65110 | 0.85 | 0.0021 | Acyl-CoA oxidase2 (ACO2); GO: fatty acid β-oxidation, long-chain fatty acid metabolic process, long-chain fatty acid biosynthesis |
| BCCP1 | CB331865 | AT5G16390 | 0.83 | 0.0027 | Chloroplastic acetyl-CoA carboxylase1 (BCCP1); GO: fatty acid biosynthetic process, encodes for the biotin carboxyl-carrier subunit of the multienzyme plastidial acetyl-CoA carboxylase complex |
| PLC | EE464144 | AT1G49740 | −3.41 | 0.0001 | PLC-like phosphodiesterase superfamily protein (PLC); GO: intracellular signal transduction, lipid metabolic process |
| SGNH | EV135949 | AT2G38180 | −2.95 | 0.0001 | Hydrolase-type esterase superfamily protein (SGNH); GO: lipid metabolic process, metabolic process |
| ACO3 | ES911417 | AT1G06290 | −2.73 | 0.0004 | Acyl-CoA oxidase3 (ACO3); GO: fatty acid β-oxidation, medium-chain fatty acid metabolic process |
| KAS2 | TA34448_3708 | AT1G74960 | −2.69 | 0.0005 | β-Ketoacyl-ACP synthetase2 (KAS2); GO: unsaturated fatty acid biosynthetic process, involved in fatty acid elongation from 16:0-ACP to 18:0-ACP |
| PHS1 | TC105857 | AT4G14440 | −1.72 | 0.0018 | 3-Hydroxyacyl-CoA dehydratase1 (PHS1); GO: fatty acid catabolic process, involved in unsaturated fatty acid degradation |
| ACPB | TA21354_3708 | AT1G31812 | −1.4 | 0.0016 | Acyl-CoA-binding protein6 (ACBP); GO: lipid transport, response to absence of light, response to cold, response to freezing |
| PLC | BQ704400 | AT3G08510 | −1.4 | 0.0013 | Phospholipase C (PLC); GO: lipid metabolic process, metabolic process, signal transduction |
| GLIP1 | DY024637 | AT3G48460 | −1.39 | 0.0031 | GDSL-like lipase (GLIP1); GO: lipid metabolic process, metabolic process |
| ECH | TA26530_3708 | AT4G29010 | −1.36 | 0.001 | Enoyl-CoA hydratase/isomerase family (ECH); GO: fatty acid β-oxidation, flower development, jasmonic acid biosynthetic process |
| FAR3 | CB686175 | AT4G33790 | −1.35 | 0.0007 | Fatty acid reductase3 (FAR3); GO: microsporogenesis, oxidation-reduction process, wax biosynthetic process |
| FADS2 | TA21960_3708 | AT3G12120 | −1.34 | 0.0011 | Fatty acid desaturase2 (FADS2); GO: lipid metabolic process, oxidation-reduction process, responsible for the synthesis of 18:2 fatty acids |
| ECHIb | CX278875 | AT4G14430 | −1.28 | 0.0026 | Enoyl-CoA hydratase/isomerase b (ECHIb); GO: fatty acid catabolic process, involved in unsaturated fatty acid degradation |
| LTP | CD843685 | AT5G64080 | −1.23 | 0.0025 | Lipid-transfer protein (LTP); GO: lipid transport |
| KCS2 | TC95262 | AT1G04220 | −1.2 | 0.0035 | 3-Ketoacyl-CoA synthase2 (KCS2); GO: biosynthesis of very-long-chain fatty acids |
| ACPD | TA19551_3708 | AT3G02630 | −1.06 | 0.001 | Stearoyl-acyl-carrier-protein desaturase family protein (ACPD); GO: fatty acid biosynthetic process, oxidation-reduction process |
| HSP | CD822549 | AT3G49050 | −1.06 | 0.0019 | α/β-Hydrolase superfamily protein (HSP); GO: lipid catabolic process, lipid metabolic process |
| LTP | EV148422 | AT1G36150 | −1.02 | 0.0016 | Lipid-transfer protein (LTP); GO: lipid transport |
| LTP | CX192351 | AT1G18280 | −0.83 | 0.0025 | Lipid-transfer protein (LTP); GO: lipid transport |
Table IV. Microarray analysis of tt16 RNAi plants: genes involved in auxin signal transduction and transport in 2-DAF siliques of tt16 RNAi plants.
Microarray data were analyzed using the open-source R statistical programming language and Bioconductor packages with P < 0.01 (Gentleman et al., 2004; R Development Core Team 2010). GO, Gene Ontology categories.
| Gene Name | Gene Identifier | Arabidopsis Genome Initiative Locus Identifier | Ratio | P Value | Description |
|---|---|---|---|---|---|
| IAA27 | EV128512 | AT4G29080 | 3.49 | 0.0005 | IAA inducible27 (IAA27); GO: regulation of translation, response to auxin stimulus |
| WXR1 | TA34132_3708 | AT2G31190 | 2.95 | 0.0002 | Weak auxin response1 (WXR1); GO: auxin polar transport, response to UV-B |
| PIN3 | TC103218 | AT1G70940 | 1.62 | 0.0031 | Pin-formed3 (PIN3); GO: auxin polar transport |
| TIR3 | EV025801 | AT3G02260 | 1.57 | 0.0015 | Transport inhibitor response3 (TIR3); GO: auxin polar transport, response to auxin stimulus |
| ILR1 | TC102010 | AT3G02875 | 1.34 | 0.0026 | IAA-Leu resistant1 (ILR1); GO: auxin metabolic process, metabolic process, proteolysis |
| ARF8 | TC64476 | AT5G37020 | 1.14 | 0.0029 | Auxin response factor8 (ARF8); GO: regulation of transcription, response to auxin stimulus |
| IAA13 | CX187822 | AT2G33310.3 | 1.04 | 0.0007 | Auxin-induced protein13 (IAA13); GO: regulation of transcription, response to auxin stimulus |
| MYB15 | TC91892 | AT3G23250 | 0.84 | 0.0025 | Myb domain protein15 (MYB15); GO: regulation of transcription, response to auxin stimulus |
| ARF17 | EE455835 | AT1G77850 | −3.94 | 0.0003 | Auxin response factor17 (ARF17); GO: auxin-mediated signaling pathway, regulation of transcription |
| RBX1 | TC92162 | AT5G20570 | −2.57 | 0.0002 | RING box1 (RBX1); GO: protein ubiquitination, response to auxin stimulus |
| Auxin homeostasis gene | CD818157 | AT2G32410 | −2.07 | 0.0026 | Auxin homeostasis; GO: auxin homeostasis, auxin-mediated signaling pathway |
| BRX | EE465134 | AT1G31880 | −1.76 | 0.0018 | Brevis radix (BRX); GO: auxin-mediated signaling pathway |
| ETA3 | DY002982 | AT4G11260 | −1.26 | 0.0016 | Enhancer of tir1-1 auxin resistance3 (ETA3); GO: auxin-mediated signaling pathway |
| AXR1 | TC75767 | AT1G05180 | −1.21 | 0.0007 | Auxin resistant1 (AXR1); GO: auxin homeostasis, auxin-mediated signaling pathway |
| CNX1 | CD836602 | AT5G20990 | −1.16 | 0.0022 | Molybdopterin biosynthesis protein (CNX1); GO: auxin-mediated signaling pathway |
| GH3 | EE455254 | AT4G03400 | −1.1 | 0.0015 | Auxin-responsive GH3 family protein (GH3); GO: response to auxin stimulus, response to light stimulus |
| TIR1 | ES900600 | AT3G62980 | −0.94 | 0.0018 | Transport inhibitor response1 (TIR1); GO: auxin-mediated signaling pathway, response to auxin stimulus |
Table III. Microarray analysis of tt16 RNAi plants: genes involved in gynoecium and embryo development in siliques (2 DAF) of tt16 RNAi plants.
Microarray data were analyzed using the open-source R statistical programming language and Bioconductor packages with P < 0.01 (Gentleman et al., 2004; R Development Core Team 2010). GO, Gene Ontology categories.
| Gene Name | Gene Identifier | Arabidopsis Genome Initiative Locus Identifier | Ratio | P Value | Description |
|---|---|---|---|---|---|
| Pepper | ES902629 | AT4G26000 | 3.26 | 0.0018 | Pepper; GO: gynoecium development, shoot development |
| EMB1865 | EE453093 | AT3G18390 | 2.46 | 0.0015 | Embryo defective1865 (EMB1865); GO: embryo development ending in seed dormancy |
| LEA | TC104467 | AT2G44060 | 2.32 | 0.0004 | Late embryogenesis abundant protein (LEA); GO: embryo development ending in seed dormancy |
| EMB1745 | EV057385 | AT1G13120 | 1.78 | 0.0005 | Embryo defective1745 (EMB1745); GO: embryo development ending in seed dormancy |
| EMB1738 | TA24074_3708 | AT1G11680.1 | 1.73 | 0.0012 | Embryo defective1738 (EMB1738); GO: embryo development ending in seed dormancy |
| L19e | TC101398 | AT1G02780 | 1.7 | 0.0027 | Ribosomal protein L19e family protein (L19e); GO: embryo development ending in seed dormancy |
| EDA9 | TA29939_3708 | AT4G34200.1 | 1.37 | 0.0004 | Embryo sac development arrest9 (EDA9); GO: megagametogenesis |
| EMB161 | EG019088 | AT5G27740 | 1.23 | 0.0009 | Embryo defective161 (EMB161); GO: embryo development ending in seed dormancy |
| GFA1/MEE5 | TA30692_3708 | AT1G06220.1 | 1.09 | 0.0035 | Gametophyte factor1 (GFA1)/maternal effect embryo arrest5 (MEE5); GO: embryo development ending in seed dormancy, regulation of embryo sac egg cell differentiation |
| LEA4-1 | EE407756 | AT1G32560 | 1.07 | 0.0016 | Late embryogenesis abundant4-1 (LEA4-1); GO: embryo development ending in seed dormancy, seed development |
| EDA2474 | TA33458_3708 | AT3G46560 | 0.98 | 0.0019 | Embryo defective2474 (EDA2474); GO: embryo development ending in seed dormancy |
| EDA25 | EE414397 | AT1G72440 | 0.96 | 0.0019 | Embryo sac development arrest 25 (EDA25); GO: embryo sac development, polar nucleus fusion |
| OVA6 | TC98067 | AT5G52520 | 0.96 | 0.0027 | Ovule abortion6 (OVA6); GO: embryo sac development, metabolic process, ovule development |
| MEE49 | DY017410 | AT4G01560 | 0.82 | 0.0033 | Maternal effect embryo arrest49 (MEE49); GO: embryo development ending in seed dormancy |
| EDA3004 | TC98127 | AT3G06350 | 0.77 | 0.0032 | Embryo defective3004 (EDA3004); GO: embryo development ending in seed dormancy |
| EDA1637 | TA21841_3708 | AT3G57870 | −4.52 | 0.0001 | Embryo defective1637 (EDA1637); GO: embryo development ending in seed dormancy |
| EDA2759 | EV176859 | AT5G63050 | −3.25 | 0.0004 | Embryo defective2759 (EDA2759); GO: embryo development ending in seed dormancy |
| PRD3 | EE505648 | AT1G01690 | −2.8 | 0.0003 | Putative recombination initiation defects 3 (PRD3); GO: embryo sac development |
| EDA1624 | TA23462_3708 | AT3G55620 | −2 | 0.0007 | Embryo defective1624 (EDA1624); GO: embryo development ending in seed dormancy |
| EDA1144 | EE467621 | AT1G48850 | −1.78 | 0.0005 | Embryo defective1144 (EDA1144); GO: embryo development ending in seed dormancy |
| LEA | TA23013_3708 | AT3G17520 | −1.75 | 0.0012 | Late embryogenesis abundant protein family protein (LEA); GO: embryo development ending in seed dormancy |
| EDA1303 | CD832555 | AT1G56200 | −1.73 | 0.0030 | Embryo defective1303 (EDA1303); GO: embryo development ending in seed dormancy |
| EDA60 | TC82547 | AT1G01040 | −1.63 | 0.0026 | Embryo defective60 (EDA60); GO: regulation of seed maturation |
| EDA140 | TC100011 | AT4G24270 | −1.32 | 0.0007 | Embryo defective140 (EDA140); GO: embryo development ending in seed dormancy |
| EDA1401 | EV093340 | AT5G20920 | −1.26 | 0.0017 | Embryo defective1401 (EDA1401); GO: embryo development ending in seed dormancy |
| EDA1211 | EE474572 | AT5G22640 | −1.26 | 0.0022 | Embryo defective1211 (EDA1211); GO: embryo development, embryo development ending in seed dormancy |
| EDA1138 | TC108258 | AT5G26742 | −1.11 | 0.0013 | Embryo defective1138 (EDA1138); GO: embryo development ending in seed dormancy |
| LEA | TA24711_3708 | AT3G53040 | −1.07 | 0.0013 | Late embryogenesis abundant protein (LEA); GO: embryo development ending in seed dormancy |
| EDA1401 | BQ704950 | AT5G20920 | −1.01 | 0.0022 | Embryo defective1401 (EDA1401); GO: embryo development ending in seed dormancy |
| LEA | DY002842 | AT4G21020 | −0.89 | 0.0030 | Late embryogenesis abundant protein family protein (LEA); GO: embryo development ending in seed dormancy |
Down-Regulation of tt16s Alters the Response to Auxin
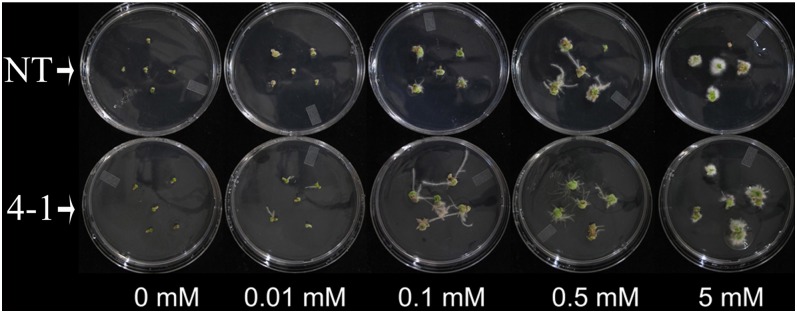
As shown in Table IV, eight genes related to auxin transport and signal transduction were down-regulated and nine were up-regulated in the microarray data. We hypothesized that down-regulation of TT16s may have changed canola’s response to auxin. To further investigate this, we examined the auxin dose response on adventitious root formation of hypocotyl segments. The results showed that the promotion of root organ regeneration from hypocotyl explants was auxin dose dependent in both NT and tt16 RNAi plants (Fig. 8). In NT seedlings, however, the synthetic auxin, naphthaleneacetic acid, promoted adventitious root regeneration at concentrations above 0.1 mm, while in tt16 RNAi seedlings, the critical concentration was 10-fold lower (0.01 mm). At 0.1 and 0.5 mm, there were more adventitious roots in tt16s RNAi lines than in NT plants. These results indicated that down-regulation of tt16s conferred increased sensitivity to auxin.
Figure 8.
Auxin dose response of tt16 RNAi transgenic lines. Hypocotyl explants of 9-d-old canola seedlings were incubated on one-half-strength Murashige and Skoog medium containing the indicated naphthaleneacetic acid concentrations for 20 d. 4-1, tt16 RNAi transgenic line 4-1.
DISCUSSION
The function of transcription factor TT16 in regulating endothelial differentiation and PA accumulation has been reported in Arabidopsis and petunia (Nesi et al., 2002; de Folter et al., 2006). Our knowledge of its other physiological functions, however, is very limited. Here, we generated Bntt16 RNAi plants and comprehensively studied the physiological function of canola TT16s, including possible mechanisms at the transcription level. Currently, the RNAi method is broadly applied to study gene functions. However, several reports have shown that off-target gene silencing can occur during RNAi and result in misleading conclusions in RNAi experiments (Jackson et al., 2003; Xu et al., 2006). In order to minimize the potential off-target gene silencing, we selected the 146-bp TT16-specific fragment by a BLAST search against the Brassica genome database (http://brassicadb.org). In addition, a publicly Web-based computational tool called siRNA Scan was developed to identify potential off-targets (Xu et al., 2006). Using this tool, we further analyzed the designed fragment to check the risk of potential nonspecific effects (off-target effects), and no potential off-target candidates against the canola mRNA database were detected in our designed fragment.
The tt16 RNAi transgenic plants displayed reduced fertility, and fluorescence microscopy observation indicated impaired guidance of pollen tubes to the ovule in transgenic plants (Fig. 4). The fertilization process begins when the pollen grain germinates on the female stigmatic cells. Then, pollen tubes emerge at the base of the stigmatic papillae and grow down to the style and through the transmitting tissue of the style and septum (Hill and Lord, 1987; Lennon et al., 1998). When the pollen tube emerges from the septum, it grows over the surface of the septum to a funiculus and then grows along the funicular surface to the micropylar opening of the ovule, where it enters to release the sperm cells (Yadegari and Drews, 2004). Pollen tubes are guided through female tissues until they turn toward an available ovule. The two synergid cells in the female gametophyte have important roles in pollen tube guidance (Shimizu and Okada, 2000; Higashiyama et al., 2001; Okuda et al., 2009). The egg cells and central cells also play a role in pollen tube guidance (Chen et al., 2007; Alandete-Saez et al., 2008). GFA1/MEE5, which regulates egg cell differentiation in the embryo sac, was down-regulated in transgenic plants (Table III; Pagnussat et al., 2005; Liu et al., 2009). Based on our study, the down-regulation of GFA1/MEE5 in tt16 RNAi transgenic plants may have affected the development of egg cells, resulting in impaired guidance of pollen tubes to the ovule.
Lipids are essential for the growth and development of plants. Although many of the reactions in storage lipid metabolism have been well studied in plants, less is known about their regulatory mechanisms, especially at the transcriptional level. A few transcription factors have been identified as potential regulators of storage lipid biosynthesis in plants. WRI1, for example, can enhance the transcription of FA biosynthetic genes, including BCCP2 (Baud et al., 2009). LEC2 directly regulates WRI1, which, in turn, controls the expression of a subset of genes involved in late glycolysis and FA biosynthesis as well as the biosynthesis of biotin and lipoic acids (Baud et al., 2007). Overexpression of the Arabidopsis LEC1 gene causes globally increased expression of FA biosynthetic genes, encoding products involved in key reactions of condensation, chain elongation, and desaturation of FA biosynthesis (Mu et al., 2008). In our study, we found that down-regulation of four TT16 genes resulted in decreased FA content (Table I) and oil body abundance (Fig. 6). Consistent with this observation, tt16 RNAi altered the expression of some genes encoding enzymes and/or proteins involved in the lipid metabolism pathway.
Microarray analysis showed that transcripts of two FA synthesis genes, KAR5 and BCCP1, accumulated at lower rates than in NT plants (Table II). The KAR enzyme catalyzes the first reduction step in FA biosynthesis (Harwood, 1988). O’Hara et al. (2007) reported that antisense expression of the canola KAR gene results in reduced seed oil content and seed yield. BCCP1 is an essential subunit for a heteromeric acetyl-CoA carboxylase, which catalyzes the ATP-dependent formation of malonyl-CoA in the first committed step of the FA biosynthetic pathway (Ohlrogge and Browse, 1995). Down-regulation of BCCP1 decreases FA accumulation in seeds and severely affects normal vegetative plant growth (Li et al., 2011). The down-regulation of KAR5 and BCCP1 expression in siliques (2 DAF) may explain the decreased FA accumulation in tt16 RNAi transgenic plants. We speculate that TT16s may directly or indirectly interact with the cis-regulatory elements of KAR5 and BCCP1 genes, thereby regulating FA synthesis in canola seeds. Down-regulation of these genes in the tt16 RNAi lines is consistent with the reduced seed oil content in mature seeds.
The changes observed in seed FA composition in tt16 RNAi plants (Table I) may also be explained by our microarray data. The down-regulation of β-Ketoacyl-ACP Synthetase2 (KAS2) in transgenic seeds is consistent with the observed increase in 16-carbon FA in the RNAi lines, which occurred primarily at the expense of the total 18-carbon FA. KAS2 is involved in the elongation of FA from palmitic acid (16:0) to stearic acid (18:0). The up-regulation of Fatty Acid Desaturase2 (FADS2), encoding a Δ12 desaturase activity, is in agreement with the observed increase in 18:2cisΔ9,12 in the transgenic lines. Moreover, the content of very-long-chain FA (20- and 22-carbon FA) in transgenic plants is higher than that in NT plants (Table I). This result may be explained by the up-regulation of 3-Ketoacyl-CoA Synthase2 (KCS2), which is required for the biosynthesis of these longer FA (Lee et al., 2009).
We also found that the embryos of tt16 RNAi transgenic lines develop abnormally beyond 20 DAF (Fig. 5). In canola, the synthesis and accumulation of storage lipids in the embryo occurs between about 3 and 6 weeks after flowering (Weselake et al., 2009). The abnormal embryo development may be due, at least partly, to the decreased synthesis of storage oil, although we could not rule out other possibilities. Indeed, other studies have shown that some genes encoding components of FA synthesis play important roles in embryo and seed development in Arabidopsis and canola (O’Hara et al., 2007; Wu and Xue, 2010; Li et al., 2011).
The phytohormone auxin regulates essential aspects of plant growth and developmental processes (Friml, 2003). Auxin regulates gene expression through a ubiquitin-dependent proteolytic signal transduction system (Dharmasiri and Estelle, 2004). The auxin/indole-3-acetic acid (Aux/IAA) proteins are able to repress the activity of Auxin Response Factor (ARF) proteins (Tiwari et al., 2001, 2004). Increased auxin reduces the levels of Aux/IAA proteins by accelerating their degradation (Zenser et al., 2001), such that ARF activity is derepressed and numerous auxin-mediated transcriptional changes occur (Tiwari et al., 2001, 2004). Aux/IAA proteins act as negative regulators of the auxin response (Wang et al., 2005). ARF proteins can be either activators or repressors of auxin-related gene transcription (Vanneste and Friml, 2009). In this study, it is interesting that down-regulation of tt16s confers increased sensitivity to auxin (Fig. 8). Microarray data showed that two AUX/IAA genes, IAA27 and IAA13, are down-regulated in transgenic plants, which may explain why the transgenic plants show more sensitivity to auxin. Also, the fact that tt16 RNAi transgenic lines had fewer inflorescences and flowers suggests that the plants are more sensitive to auxin (Mockaitis and Estelle, 2008; Deng et al., 2012), which further confirms the conclusion that down-regulation of tt16s confers increased sensitivity to auxin. However, analysis of the root growth of transgenic plants showed that there was no obvious difference between the tt16 RNAi and NT plants. This result indicates a complex mechanism of tt16 and auxin signal transduction in the regulation of root development in canola.
In summary, our data demonstrate that TT16 plays roles in pollen tube guidance, FA synthesis, and embryo development in addition to its known role in endothelium development in the seed coat. These results expand our knowledge about the physiological functions of Bsister MADS proteins in plant development and provide valuable information for improving canola quality by using modern breeding biotechnologies.
MATERIALS AND METHODS
Plant Material and Growing Conditions
Canola (Brassica napus double haploid line DH12075) plants were grown in the greenhouse or growth chamber. The growth conditions were set as follows: 16-h-day/8-h-night cycle, 25°C/20°C day/night temperature, 60% relative humidity, 250 μmol m−2 s−1 light intensity. Canola plants were transformed as described by Bondaruk et al. (2007).
Light Microscopy
Mature seeds were fixed in formaldehyde:acetic acid:ethanol (5:5:90) for 24 h. Fixed tissues were dehydrated in a series of ethanol-toluene concentrations and embedded in paraffin wax at 56°C. Sections of 8 μm thickness were cut with a rotary microtome and fixed on glass slides. Sections were dewaxed with toluene and then stained with 0.01% toluidine blue. Observations were made with a light microscope.
Pollination Assay and Fluorescence Observation of Pollen Tube Development
For hand pollination, large flower buds (1 d before anthesis) were emasculated and covered with pollination bags. Tagged pistils were hand pollinated 1 d later, covered for 5 to 7 d for seed production, and then left uncovered to maturity. The pistils (2 DAF) were embedded in paraffin, and sections were made with a rotary microtome. Style sections were stained with aniline blue and observed with a Leica DMRXA epifluorescence microscope. For fluorescence observation of pollen tube extension to ovules, pistils (2 DAF) were fixed with 3:1 95% ethanol:glacial acetic acid overnight and softened with 8 m sodium hydroxide for 2 d. Then, the pistils were stained for 3 h with 0.05% aniline blue and mounted in a drop of 50% glycerin. Samples were observed with a Leica DMRXA epifluorescence microscope.
Electron Microscopy
Mature embryos were fixed in 2% glutaraldehyde in 75 mm phosphate buffer, pH 7.2, and were postfixed in 2% osmium in the same buffer. An ethanol dehydration series was performed, and the samples were embedded in Spurr resin. Ultrathin sections were stained with uranyl acetate followed by lead citrate. Micrographs were taken using a Philips/FEI Morgagni transmission electron microscope.
Microarray Analysis
Total RNA was isolated from the siliques (2 DAF) using an RNeasy plant mini kit (Qiagen) according to the manufacturer’s instructions. Quantity and purity of RNA were measured with a NanoDrop 1000 (PEQLAB Biotechnologie). Microarray analysis was performed using the Agilent 4x44k Brassica Gene Expression Microarray (Agilent Technologies). Cy3-labeled complementary RNA was produced with the Quick Amp Labeling Kit, one-color (Agilent Technologies), and hybridized to the microarrays according to the manufacturer’s instructions. Hybridized and washed slides were scanned at 5-μm resolution with a GenePix 4000B scanner (Molecular Devices). Image processing was performed with Feature Extraction Software 10.5.1.1 (Agilent Technologies). Microarray data were analyzed using the open-source R statistical programming language and Bioconductor packages (Gentleman et al., 2004, R Development Core Team 2010).
Quantitative Reverse Transcription-PCR
RNA was extracted using the RNeasy plant mini kit (Qiagen). DNase-treated RNA was then reverse transcribed using the QuantiTect Rev Transcription kit (Qiagen). Quantitative reverse transcription-PCR was performed to confirm microarray results according to the method reported previously by Chen et al. (2010). The primers used in this assay are listed in Supplemental Table S1.
Auxin Dose-Response Experiments
Hypocotyl explants of 9-d-old seedlings from NT and tt16 RNAi were incubated on one-half-strength Murashige and Skoog medium containing the indicated naphthaleneacetic acid concentrations in growth chamber conditions described as above for 20 d.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EU192028, EU192029, HM449989, and HM449990.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Short siliques of the tt16 RNAi transgenic line.
Supplemental Table S1. Primers used in this study.
Supplemental Table S2. The lengths of sepals, stamens, and pistils of open flowers and buds in RNAi plants (line 4-1) and NT plants.
Acknowledgments
We thank Lihua Jin and Arlene Oatway for their help in microcopy observations, Troy Locke for his help in microarray data analysis, and Joe Hammerlindl for his help in plant transformation.
Glossary
- PA
proanthocyanidin
- FA
fatty acid
- RNAi
RNA interference
- DAF
days after flowering
- NT
nontransgenic
- Aux/IAA
auxin/indole-3-acetic acid
References
- Alandete-Saez M, Ron M, McCormick S. (2008) GEX3, expressed in the male gametophyte and in the egg cell of Arabidopsis thaliana, is essential for micropylar pollen tube guidance and plays a role during early embryogenesis. Mol Plant 1: 586–598 [DOI] [PubMed] [Google Scholar]
- Baud S, Lepiniec L. (2009) Regulation of de novo fatty acid synthesis in maturing oilseeds of Arabidopsis. Plant Physiol Biochem 47: 448–455 [DOI] [PubMed] [Google Scholar]
- Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B. (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50: 825–838 [DOI] [PubMed] [Google Scholar]
- Baud S, Wuillème S, To A, Rochat C, Lepiniec L. (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60: 933–947 [DOI] [PubMed] [Google Scholar]
- Becker A, Kaufmann K, Freialdenhoven A, Vincent C, Li M, Saedler H, Theissen G. (2002) A novel MADS-box gene subfamily with a sister-group relationship to class B floral homeotic genes. Mol Genet Genomics 266: 942–950 [DOI] [PubMed] [Google Scholar]
- Becker A, Theissen G. (2003) The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol 29: 464–489 [DOI] [PubMed] [Google Scholar]
- Bondaruk M, Johnson S, Degafu A, Boora P, Bilodeau P, Morris J, Wiehler W, Foroud N, Weselake R, Shah S. (2007) Expression of a cDNA encoding palmitoyl-acyl carrier protein desaturase from cat’s claw (Doxantha unguis-cati L.) in Arabidopsis thaliana and Brassica napus leads to accumulation of unusual unsaturated fatty acids and increased stearic acid content in the seed oil. Plant Breed 126: 186–194 [Google Scholar]
- Cernac A, Benning C. (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40: 575–585 [DOI] [PubMed] [Google Scholar]
- Chen G, Deng W, Truksa M, Peng FY, Weselake RJ. (2012) The Bsister MADS-box proteins have multiple regulatory functions in plant development. Biocatalysis and Agricultural Biotechnology 1: 203–206 [Google Scholar]
- Chen X, Truksa M, Shah S, Weselake RJ. (2010) A survey of quantitative real-time polymerase chain reaction internal reference genes for expression studies in Brassica napus. Anal Biochem 405: 138–140 [DOI] [PubMed] [Google Scholar]
- Chen YH, Li HJ, Shi DQ, Yuan L, Liu J, Sreenivasan R, Baskar R, Grossniklaus U, Yang WC. (2007) The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19: 3563–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Masiero S, Vanzulli S, Lardelli P, Kater MM, Colombo L. (2008) AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. Plant J 54: 1037–1048 [DOI] [PubMed] [Google Scholar]
- de Folter S, Shchennikova AV, Franken J, Busscher M, Baskar R, Grossniklaus U, Angenent GC, Immink RGH. (2006) A Bsister MADS-box gene involved in ovule and seed development in petunia and Arabidopsis. Plant J 47: 934–946 [DOI] [PubMed] [Google Scholar]
- Deng W, Yang YW, Ren ZX, Audran-Delalande C, Mila I, Wang XY, Song HL, Hu YH, Bouzayen M, Li ZG. (2012) The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol 194: 379–390 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Estelle M. (2004) Auxin signaling and regulated protein degradation. Trends Plant Sci 9: 302–308 [DOI] [PubMed] [Google Scholar]
- Dinneny JR, Yanofsky MF. (2005) Drawing lines and borders: how the dehiscent fruit of Arabidopsis is patterned. Bioessays 27: 42–49 [DOI] [PubMed] [Google Scholar]
- Erdmann R, Gramzow L, Melzer R, Theißen G, Becker A. (2010) GORDITA (AGL63) is a young paralog of the Arabidopsis thaliana Bsister MADS box gene ABS (TT16) that has undergone neofunctionalization. Plant J 63: 914–924 [DOI] [PubMed] [Google Scholar]
- Friml J. (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge YC, Gentry J, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood JL. (1988) Fatty acid metabolism. Annu Rev Plant Physiol Plant Mol Biol 39: 101–138 [Google Scholar]
- Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T. (2001) Pollen tube attraction by the synergid cell. Science 293: 1480–1483 [DOI] [PubMed] [Google Scholar]
- Hill JP, Lord EM. (1987) Dynamics of pollen tube growth in the wild radish, Raphanus raphanistrum (Brassicaceae). 2. Morphology, cytochemistry, and ultrastructure of transmitting tissues, and path of pollen tube growth. Am J Bot 74: 988–997 [Google Scholar]
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. (2003) Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 21: 635–637 [DOI] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC. (2005) Molecular mechanisms of flower development: an armchair guide. Nat Rev Genet 6: 688–698 [DOI] [PubMed] [Google Scholar]
- Lee SB, Jung SJ, Go YS, Kim HU, Kim JK, Cho HJ, Park OK, Suh MC. (2009) Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. Plant J 60: 462–475 [DOI] [PubMed] [Google Scholar]
- Lennon KA, Roy S, Hepler PK, Lord EM. (1998) The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sex Plant Reprod 11: 49–59 [Google Scholar]
- Li X, Ilarslan H, Brachova L, Qian HR, Li L, Che P, Wurtele ES, Nikolau BJ. (2011) Reverse-genetic analysis of the two biotin-containing subunit genes of the heteromeric acetyl-coenzyme A carboxylase in Arabidopsis indicates a unidirectional functional redundancy. Plant Physiol 155: 293–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yuan L, Liu NY, Shi DQ, Liu J, Yang WC. (2009) GAMETOPHYTIC FACTOR 1, involved in pre-mRNA splicing, is essential for megagametogenesis and embryogenesis in Arabidopsis. J Integr Plant Biol 51: 261–271 [DOI] [PubMed] [Google Scholar]
- Lotan T, Ohto M, Yee KM, West MA, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ. (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93: 1195–1205 [DOI] [PubMed] [Google Scholar]
- Melzer R, Wang YQ, Theissen G. (2010) The naked and the dead: the ABCs of gymnosperm reproduction and the origin of the angiosperm flower. Semin Cell Dev Biol 21: 118–128 [DOI] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M. (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24: 55–80 [DOI] [PubMed] [Google Scholar]
- Mu JY, Tan HL, Zheng Q, Fu FY, Liang Y, Zhang J, Yang XH, Wang T, Chong K, Wang XJ, et al. (2008) LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol 148: 1042–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi N, Debeaujon I, Jond C, Stewart AJ, Jenkins GI, Caboche M, Lepiniec L. (2002) The TRANSPARENT TESTA16 locus encodes the ARABIDOPSIS BSISTER MADS domain protein and is required for proper development and pigmentation of the seed coat. Plant Cell 14: 2463–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Yanofsky MF. (2001) Function and evolution of the plant MADS-box gene family. Nat Rev Genet 2: 186–195 [DOI] [PubMed] [Google Scholar]
- O'Hara P, Slabas AR, Fawcett T. (2007) Antisense expression of 3-oxoacyl-ACP reductase affects whole plant productivity and causes collateral changes in activity of fatty acid synthase components. Plant Cell Physiol 48: 736–744 [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J. (1995) Lipid biosynthesis. Plant Cell 7: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458: 357–361 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Yu H-J, Ngo QA, Rajani S, Mayalagu S, Johnson CS, Capron A, Xie L-F, Ye D, Sundaresan V. (2005) Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132: 603–614 [DOI] [PubMed] [Google Scholar]
- Prasad K, Zhang X, Tobon E, Ambrose BA. (2010) The Arabidopsis B-sister MADS-box protein, GORDITA, represses fruit growth and contributes to integument development. Plant J 62: 203–214 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2010) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [Google Scholar]
- Shimizu KK, Okada K. (2000) Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development 127: 4511–4518 [DOI] [PubMed] [Google Scholar]
- Stellari GM, Jaramillo MA, Kramer EM. (2004) Evolution of the APETALA3 and PISTILLATA lineages of MADS-box-containing genes in the basal angiosperms. Mol Biol Evol 21: 506–519 [DOI] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ. (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98: 11806–11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G. (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol 4: 75–85 [DOI] [PubMed] [Google Scholar]
- Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Münster T, Winter K-U, Saedler H. (2000) A short history of MADS-box genes in plants. Plant Mol Biol 42: 115–149 [PubMed] [Google Scholar]
- Theissen G, Kim JT, Saedler H. (1996) Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J Mol Evol 43: 484–516 [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ. (2001) AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. (2009) Auxin: a trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M. (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17: 2676–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselake RJ, Taylor DC, Rahman MH, Shah S, Laroche A, McVetty PBE, Harwood JL. (2009) Increasing the flow of carbon into seed oil. Biotechnol Adv 27: 866–878 [DOI] [PubMed] [Google Scholar]
- Wu GZ, Xue HW. (2010) Arabidopsis β-ketoacyl-[acyl carrier protein] synthase I is crucial for fatty acid synthesis and plays a role in chloroplast division and embryo development. Plant Cell 22: 3726–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Zhang Y, Kang L, Roossinck MJ, Mysore KS. (2006) Computational estimation and experimental verification of off-target silencing during posttranscriptional gene silencing in plants. Plant Physiol 142: 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadegari R, Drews GN. (2004) Female gametophyte development. Plant Cell (Suppl) 16: S133–S141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J. (2001) Auxin modulates the degradation rate of Aux/IAA proteins. Proc Natl Acad Sci USA 98: 11795–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]