Abstract
Background
The aim of this study was to determine the incidence of complications associated with primary closure in surgical procedures performed for diabetic foot osteomyelitis compared to those healed by secondary intention. In addition, further evaluation of the surgical digital debridement for osteomyelitis with primary closure as an alternative to patients with digital amputation was also examined in our study.
Methods
Comparative study that included 46 patients with diabetic foot ulcerations. Surgical debridement of the infected bone was performed on all patients. Depending on the surgical technique used, primary surgical closure was performed on 34 patients (73.9%, Group 1) while the rest of the 12 patients were allowed to heal by secondary intention (26.1%, Group 2). During surgical intervention, bone samples were collected for both microbiological and histopathological analyses. Post-surgical complications were recorded in both groups during the recovery period.
Results
The average healing time was 9.9±SD 8.4 weeks in Group 1 and 19.1±SD 16.9 weeks in Group 2 (p=0.008). The percentage of complications was 61.8% in Group 1 and 58.3% in Group 2 (p=0.834). In all patients with digital ulcerations that were necessary for an amputation, a primary surgical closure was performed with successful outcomes.
Discussion
Primary surgical closure was not associated with a greater number of complications. Patients who received primary surgical closure had faster healing rates and experienced a lower percentage of exudation (p=0.05), edema (p<0.001) and reinfection, factors that determine the delay in wound healing and affect the prognosis of the surgical outcome. Further research with a greater number of patients is required to better define the cases for which primary surgical closure may be indicated at different levels of the diabetic foot.
Keywords: diabetic foot, diabetic foot infections, osteomyelitis, surgery
Diabetic foot infections are one of the main prognostic factors in patients suffering from diabetic foot ulcers, becoming the main cause for amputation, even more than peripheral arterial disease, and require an early diagnosis and aggressive management (1, 2). Surgical debridement to eradicate non-viable tissue has proven to be useful in improving the outcome of these patients as a necessary therapeutic choice (3). Diabetic foot infections can be classified into soft tissue infections that are usually more severe in the clinical setting and bone infections which are encountered more frequently (2). Bone infections represent approximately 50–60% of the total number of infected diabetic foot ulcers that can eventually lead to amputation in 10–30% of cases (4).
Treatment of osteomyelitis in the diabetic foot has been a subject of debate and controversy by researchers and clinicians who treat diabetic patients with this complication. There are authors who advocate drug treatment for osteomyelitis (5–8), but it is also generally accepted that surgery is necessary to treat this condition (3, 9, 10) in combination with antibiotic therapy. However, despite the relevance of this type of infection and its high prevalence, there are currently no evidence-based therapeutic protocols, given the lack of prospective and randomized clinical studies on the treatment of diabetic foot infections with clinically suspected osteomyelitis (6).
The American Diabetes Association (ADA) recommends removal of the entire bone or most of the infected parts of the bone if this can be performed and always in combination with antibiotic therapy (11). The objectives of the surgical treatment of osteomyelitis include an adequate drainage and extensive debridement of all non-viable tissues while attempting to preserve the greatest part of the foot in order to maintain functionality and avoid re-ulceration if possible (12).
Several classifications have been attempted on the surgical procedures performed for the diabetic foot and according to the urgent or emergent basis of the procedure, but there are still no standardized protocols to perform such techniques (6). However, an established surgical principle is that most of the infected wounds must be left open after their initial surgical debridement to facilitate drainage or evacuation of the exudate or purulent discharge. In addition, many studies describe how certain surgical techniques can be utilized to treat diabetic foot ulcers, with or without bone infection, in order to preserve foot function to the greatest extent possible (13–17).
The purpose of this manuscript was to determine the incidence of complications associated with primary closure in the surgical treatment of diabetic foot osteomyelitis compared to those healed by secondary intention. In addition, further evaluation on primary closure as a risk factor for the prognosis of the surgical outcome was done and surgical digital treatment for osteomyelitis with primary closure as an alternative to patients with digital amputation was also examined in our study.
Methods
This is a comparative study that was performed at the Diabetic Foot Unit of the Complutense University of Madrid, Spain, between January and July of 2008. Patients received an informed written consent, and the study was approved by the Ethical Committee of San Carlos Clinic Hospital. Forty-six patients suffering from type 1 or 2 diabetes mellitus according to the diagnostic criteria described by the ADA were recruited (18), with a diabetic foot ulcer below the level of malleoli and with clinical signs of osteomyelitis established on the basis of the following criteria: presence of two or more inflammatory signs such as erythema, induration, edema and/or presence of exudate (19), ‘sausage toe’ (20) and ulcers that did not show progress with adequate local wound care and off-loading treatment for a period of at least 6 weeks (21). All patients did receive plain radiographs of at least three views for further assessment of the soft tissue and/or bone infection.
Of the 46 patients, 30 (65.21%) were males and 16 (34.79%) were females, with an average age of 62.65±18 years. Regarding the type of diabetes, 2 (4.3%) were diagnosed with type 1 diabetes mellitus and 44 (95.7%) were diagnosed with type 2 diabetes mellitus, with an average illness onset time of progression of 14.31±6.84 years. Fourteen patients (30.4%) had a history of diabetic foot ulceration, and 11 (23.9%) had a history of foot amputation.
Patients were subjected to neurological and vascular screening in accordance with the protocols defined in the international guidelines (22). Neurological examination was performed with the Semmens-Weinstein monofilament log 5.10 (Novalab Ibérica S.A.L, Alcalá de Henares, Madrid, Spain) and the Horwell Biothesiometer (Novalab Ibérica S.A.L) (23). All patients included in the study were subjected to at least one of the two tests.
The vascular screening consisted of physical examination of the posterior tibial and dorsalis pedis pulses, the ankle brachial index (ABI) using a Doppler probe and measurements of the transcutaneous pressure of oxygen (TcPO2). Pulses were present in 34 patients (73.91%). The average ABI was 0.84±0.33, and the TcPO2 was 39.14±12.66 mmHg. Patients with moderate vascular involvement but with data compatible with wound healing were included in cases of no distal pulses, when TcPO2 is >30 mmHg and/or the ABI was between 0.7 and 0.9. Patients with life-threatening infections and/or ischemia criteria under the description of the TASC II (24) were referred for proper hospitalization and treatment and thus excluded from the study.
According to the University of Texas classification (25), 51% of our diabetic foot ulcers were type IIIa and 49% were type IIIb. The most frequent locations of the diabetic foot ulcers in our study were the central metatarsals (n=13; 28.3%), followed by the lesser toes (n=11; 23.9%), the first metatarsal (n=9; 19.6%), the hallux (n=5; 10.9%) and fifth metatarsal (n=5; 10.9%). There was only one presentation (2.2%) at the midfoot level in the navicular and two (4.3%) in the calcaneus.
The surgical debridement of the infected bone was performed on all patients, under local anaesthesia, through the following procedures: bone curettage (n=9; 19.6%), digital amputation (n=1; 2.2%), metatarsal osteotomy (n=23; 50.2%), arthroplasty of the interphalangeal joint (n=12; 26.1%) (Fig. 1) and arthrodesis of the interphalangeal joint (n=1; 2.2%). During surgical intervention, bone cultures and biopsy samples were collected for further analysis by the microbiology and/or pathology laboratories. In all patients, the results of bone culture or biopsy were positive for osteomyelitis.
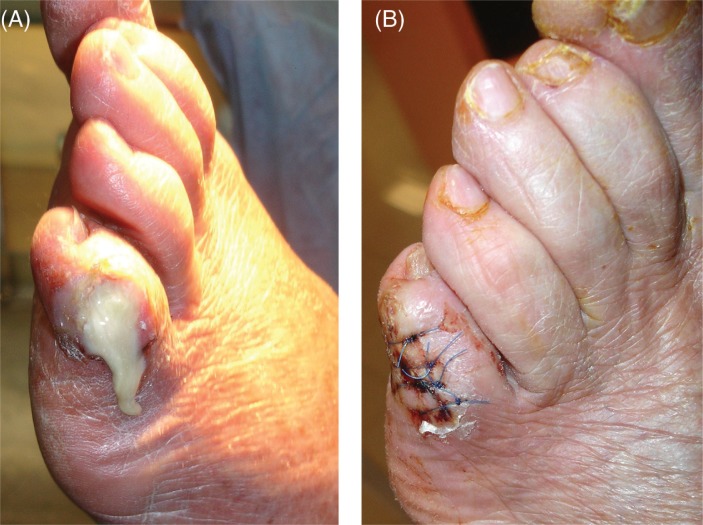
Fig. 1.
Example of an infected left fifth digit with significant amount of purulence and osteomyelitis (A) surgically treated with debridement, arthroplasty of the fifth interphalangeal joint and primary closure (B).
Depending on the surgical technique used, primary closure was performed in 34 (73.9%) patients by using a monofilament suture (Laboratorios Aragó SA®, Barcelona, Spain, Poliglecaprone) (Group 1). In the remaining 12 (26.1%) patients in Group 2, the initial surgical wound was left open, and local treatment was provided through frequent moist-to-dry dressings until complete healing was achieved in addition to the utilization of off-loading devices depending on the type and location of the surgical site. Both groups received instructions for post-operative off-loading of the surgical wounds with a post-operative shoe or other devices until complete healing was achieved.
All patients had received an initial empiric antibiotic treatment with oral amoxicillin/clavulanate for 4 weeks unless there was a known associated allergy with our antibiotic regimen. Once the results of microbiological cultures were available, the antibiotic regimen was modified according to the final intra-operative results. The type of pathogens identified from the surgical procedures is shown in Table 1.
Table 1.
Percentage and frequency of type of pathogens
| Group 1 | Group 2 | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Type of pathogens | Number | % | Number | % | p |
| Staphylococcus aureus | 18 | 52.9 | 6 | 50 | 0.8 |
| Pseudomonas aeruginosa | 7 | 20.6 | 1 | 8.3 | 0.3 |
| Staphylococcus epidermidis | 3 | 8.8 | 1 | 8.3 | 0.9 |
| Proteus mirabilis | 2 | 5.9 | 3 | 25 | 0.06 |
| Enterococcus species | 1 | 2.9 | 1 | 8.3 | 0.4 |
| Staphylococcus aureus+Proteus mirabilis | 2 | 5.9 | – | – | – |
| Staphylococcus aureus+Enterococcus species | 1 | 2.9 | – | – | – |
The following post-surgical complications were recorded for both groups during the post-operative period: hematoma, dehiscence, maceration, exudation, edema, reinfection, pain and necrosis. The statistical analysis was performed using the program SPSS for Windows, version 15.0 (SPSS, Chicago, IL). Descriptive analysis of the sample was made by distributing frequencies for the qualitative variables and determining the average and standard deviation for the quantitative variables. To compare the averages of the quantitative variables, a student's t-test was used for independent samples, and the association of qualitative variables was determined with a Chi-square test to compare proportions. Differences were assumed to be significant for values of p<0.05.
Results
The average healing time was 9.9±SD 8.4 weeks in Group 1 and 19.1±SD 16.9 weeks in Group 2 (p=0.008). The total percentage of complications was 61.8% in Group 1 and 58.3% in Group 2 (p=0.834). The different complications recorded in the study groups are shown in Table 2.
Table 2.
Percentage and frequency of post-surgical complications
| Post-surgical complications | Group 1a, % (n) | Group 2b, % (n) | p |
|---|---|---|---|
| Hematoma | 5.9 (2) | – | – |
| Dehiscence | 23.5 (8) | – | – |
| Maceration | 11.8 (4) | 25 (3) | 0.272 |
| Exudation | 20.6 (7) | 50 (6) | 0.05 |
| Edema | – | 16.7 (2) | – |
| Reinfection | 32.4 (10) | 41.7 (15) | 0.560 |
| Pain | 3.1 (1) | 16.7 (2) | 0.112 |
| Necrosis | 9.4 (3) | 8.3 (1) | 0.915 |
n=34
n=12
Discussion
Most patients with diabetic foot syndrome would most likely develop ulcerations at the digital level, given that these ulcers occur mainly due to friction with shoe gear. When these diabetic ulcers affect the bone, the primary surgical indication is usually amputation of the entire affected digit. Digital amputation entails a biomechanical alteration with the resulting risk of infection relapse and re-ulceration at the surgical site, given that the digital absence can cause increased pressure on that ray and contiguous ones (26).
Several studies have proven that performing advanced surgical techniques on patients with diabetic foot osteomyelitis allow for managing the septic process, while preserving foot function and preventing the loss of the toe, which will supposedly reduce subsequent complications (13–17). In our study, it was proven that primary closure was not associated with a greater number of complications. Patients who received primary closure healed faster than those with closure by secondary intention, 9.9±SD 8.4 vs. 19.1±SD 16.9 weeks (p=0.008). Additionally, healing by primary closure had a lower percentage of exudation (p=0.05), edema (p<0.001) and, more importantly, re-infection. These are all factors that determine the time required for the wounds to heal and affect the prognosis of the surgical outcome (27). Our study limitations included its comparative consecutive analysis without randomization and the fact that the original ulcer severity and location may have influenced the prognosis of re-ulceration and post-operative outcome.
Conclusion
Patients who suffer from osteomyelitis located at the digital level can benefit from advanced surgical techniques, and primary closure after surgical debridement at this level may not lead to a greater number of complications. These wounds may heal faster, and the overall foot structure and function is also preserved with these surgical techniques. However, studies with a greater number of patients are required to better define the cases for which primary surgical closure may be indicated at different levels of the diabetic foot.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.Parra EJ, Below JE, Krithika S, Valladares A, Barta JL, Cox NJ, et al. Genome-wide association study of type 2 diabetes in a sample from Mexico City and a meta-analysis of a Mexican-American sample from Starr County, Texas. Diabetologia. 2011;54:2038–46. doi: 10.1007/s00125-011-2172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragon-Sanchez J, Quintana-Marrero Y, Lazaro-Martinez JL, Hernandez-Herrero MJ, Garcia-Morales E, Beneit-Montesinos JV, et al. Necrotizing soft-tissue infections in the feet of patients with diabetes: outcome of surgical treatment and factors associated with limb loss and mortality. Int J Low Extrem Wounds. 2009;8:141–6. doi: 10.1177/1534734609344106. [DOI] [PubMed] [Google Scholar]
- 3.Aragon-Sanchez J, Lazaro-Martinez JL, Hernandez-Herrero C, Campillo-Vilorio N, Quintana-Marrero Y, Garcia-Morales E, et al. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabet Med. 2012;29:813–8. doi: 10.1111/j.1464-5491.2011.03528.x. [DOI] [PubMed] [Google Scholar]
- 4.Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev. 2008;24:S145–61. doi: 10.1002/dmrr.836. [DOI] [PubMed] [Google Scholar]
- 5.Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39:S115–22. doi: 10.1086/383272. [DOI] [PubMed] [Google Scholar]
- 6.Game FL, Jeffcoate WJ. Primarily non-surgical management of osteomyelitis of the foot in diabetes. Diabetologia. 2008;51:962–7. doi: 10.1007/s00125-008-0976-1. [DOI] [PubMed] [Google Scholar]
- 7.Embil JM, Rose G, Trepman E, Math MC, Duerksen F, Simonsen JN, et al. Oral antimicrobial therapy for diabetic foot osteomyelitis. Foot Ankle Int. 2006;27:771–9. doi: 10.1177/107110070602701003. [DOI] [PubMed] [Google Scholar]
- 8.Senneville E, Lombart A, Beltrand E, Valette M, Legout L, Cazaubiel M, et al. Outcome of diabetic foot osteomyelitis treated nonsurgically: a retrospective cohort study. Diabetes Care. 2008;31:637–42. doi: 10.2337/dc07-1744. [DOI] [PubMed] [Google Scholar]
- 9.Aragon-Sanchez FJ, Cabrera-Galvan JJ, Quintana-Marrero Y, Hernandez-Herrero MJ, Lazaro-Martinez JL, Garcia-Morales E, et al. Outcomes of surgical treatment of diabetic foot osteomyelitis: a series of 185 patients with histopathological confirmation of bone involvement. Diabetologia. 2008;51:1962–70. doi: 10.1007/s00125-008-1131-8. [DOI] [PubMed] [Google Scholar]
- 10.Ha Van G, Siney H, Danan JP, Sachon C, Grimaldi A. Treatment of osteomyelitis in the diabetic foot. Contribution of conservative surgery. Diabetes Care. 1996;19:1257–60. doi: 10.2337/diacare.19.11.1257. [DOI] [PubMed] [Google Scholar]
- 11.Consensus Development Conference on Diabetic Foot Wound Care: 7–8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care. 1999;22:1354–60. doi: 10.2337/diacare.22.8.1354. [DOI] [PubMed] [Google Scholar]
- 12.Aragon-Sanchez J. Seminar review: a review of the basis of surgical treatment of diabetic foot infections. Int J Low Extrem Wounds. 2011;10:33–65. doi: 10.1177/1534734611400259. [DOI] [PubMed] [Google Scholar]
- 13.Berner A, Sage R, Niemela J. Keller procedure for the treatment of resistant plantar ulceration of the hallux. J Foot Ankle Surg. 2005;44:133–6. doi: 10.1053/j.jfas.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Wieman TJ, Mercke YK, Cerrito PB, Taber SW. Resection of the metatarsal head for diabetic foot ulcers. Am J Surg. 1998;176:436–41. doi: 10.1016/s0002-9610(98)00235-9. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths GD, Wieman TJ. Metatarsal head resection for diabetic foot ulcers. Arch Surg. 1990;125:832–5. doi: 10.1001/archsurg.1990.01410190024003. [DOI] [PubMed] [Google Scholar]
- 16.Resch S. Corrective surgery in diabetic foot deformity. Diabetes Metab Res Rev. 2004;20:S34–6. doi: 10.1002/dmrr.436. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong DG, Rosales MA, Gashi A. Efficacy of fifth metatarsal head resection for treatment of chronic diabetic foot ulceration. J Am Podiatr Med Assoc. 2005;95:353–6. doi: 10.7547/0950353. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shank CF, Feibel JB. Osteomyelitis in the diabetic foot: diagnosis and management. Foot Ankle Clin. 2006;11:775–89. doi: 10.1016/j.fcl.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Rajbhandari SM, Sutton M, Davies C, Tesfaye S, Ward JD. ‘Sausage toe’: a reliable sign of underlying osteomyelitis. Diabet Med. 2000;17:74–7. doi: 10.1046/j.1464-5491.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 21.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg. 2006;117:S212–38. doi: 10.1097/01.prs.0000222737.09322.77. [DOI] [PubMed] [Google Scholar]
- 22.Apelqvist J, Bakker K, van Houtum WH, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) Prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2008;24:S181–7. doi: 10.1002/dmrr.848. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischi JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289–92. doi: 10.1001/archinte.158.3.289. [DOI] [PubMed] [Google Scholar]
- 24.Feldman EL, Stevens MJ. Clinical testing in diabetic peripheral neuropathy. Can J Neurol Sci. 1994;21:S3–7. doi: 10.1017/s0317167100040671. [DOI] [PubMed] [Google Scholar]
- 25.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21:855–9. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 27.Lavery LA, Peters EJ, Armstrong DG. What are the most effective interventions in preventing diabetic foot ulcers? Int Wound J. 2008;5:425–33. doi: 10.1111/j.1742-481X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]