Abstract
Purpose
The current working model of type II testicular germ cell tumor (TGCT) pathogenesis states that carcinoma in situ (CIS): arises during embryogenesis; is a necessary precursor; and always progresses to cancer. An implicit condition of this model is that only in utero exposures affect development of TGCT in later life. In an age-period-cohort analysis, this working model contends an absence of calendar period deviations. We tested this contention using data from the SEER registries of the United States.
Methods
We assessed age-period-cohort models of TGCTs, seminomas, and nonseminomas for the period 1973–2008. Analyses were restricted to whites diagnosed at ages 15 to 74 years. We tested whether calendar period deviations were significant in TGCT incidence trends adjusted for age deviations and cohort effects.
Results
This analysis included 32,250 TGCTs (18,475 seminomas, 13,775 nonseminomas). Seminoma incidence trends have increased with an average annual percentage change in log-linear rates (net drift) of 1.25%, relative to just 0.14% for nonseminoma. In more recent time periods, TGCT incidence trends have plateaued and then undergone a slight decrease. Calendar period deviations were highly statistically significant in models of TGCT (p=1.24−9) and seminoma (p=3.99−14), after adjustment for age deviations and cohort effects; results for nonseminoma (p=0.02) indicated that the effects of calendar period were much more muted.
Conclusion
Calendar period deviations play a significant role in incidence trends of TGCT which indicates that postnatal exposures are etiologically relevant.
Keywords: testicular cancer, age-period-cohort, carcinoma in-situ, calendar period deviations
Introduction
Testicular germ cell tumors constitute 98% of testicular cancers—the remaining 2% of tumors arise from stromal cells [1]. Type II testicular germ cell tumors (hereafter referred to as TGCT) are the seminomatous and nonseminomatous tumors which arise during adolescence and adulthood, and which are the focus of this study. Current evidence suggests that maldevelopment of the primordial germ cells gives rise to testicular carcinoma in situ (CIS), which is the recognized dormant precursor of TGCT [2,3]. The current working model of TGCT pathogenesis states that CIS: arises during embryogenesis [3]; is a necessary precursor of TGCT [4]; and, always progresses to TGCT [5–8]. An implicit condition of this model is that only in utero exposures can affect development of TGCT in later life.
Previous age-period-cohort (APC) models have indicated that TGCT incidence trends predominantly follow a birth cohort effect [9–18] and this is commonly interpreted as evidence for the current working model of TGCT pathogenesis. However, calendar period deviations (the non-linear and identifiable parameter of calendar period effects) in TGCT incidence rates have also been observed [11,17,18,12,9,15,19]; although such deviations may indicate that the current working model is incomplete, they are often not among the primary conclusions drawn. This may be due to the qualitative nature of APC modeling which renders the exercise somewhat subjective. In addition, or alternatively, the extent of calendar period deviations may have been missed due to lack of stratification by race and histology.
To overcome these limitations and provide a statistical framework to supplement qualitative interpretations of APC analyses, we utilize novel methods to test for the importance of elements of the model with a specific focus on the influence of calendar period deviations on TGCT incidence rates [20].
Methods
Cancer Incidence Data
To maximize the statistical power available for APC models of TGCTs, we concatenated populations and TGCT cases from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program of 9 registries (1973–1991), 13 registries (1992–1999) and 17 registries (2000–2008), a similar method utilized in studies of other malignancies [20,11,21–23]. As a sensitivity analysis to ensure calendar period deviations were not the result of concatenation of SEER databases, we also conducted models using data from SEER-9 for the full period (1973–2008). For APC models, SEER*Stat was used to prepare counts of TGCTs and underlying populations by age and year of diagnosis for whites for the period 1973 to 2008. The International Classification of Diseases for the Oncology third edition (ICD-O-3) [24] was used to classify histologic subtypes of TGCTs (topography code: C62) into seminoma (9060–9062, 9064) and nonseminoma (9065–9102). Spermatocytic seminomas (type III TGCTs) [25] were not included as they are considered to have an etiology distinct from type II TGCTs [26].
The Age-Period-Cohort Model
The APC model adjusts cancer incidence rates for age, calendar period, and cohort effects. The model can analyze populations separately or in pairwise comparisons. The “nonidentifiability” issue lies in the collinear APC relationship making it impossible to determine the independent influences (sum of linear and nonlinear components) of age, calendar period, and birth cohort. However, a restricted APC model can estimate certain parameters if age, calendar period, and cohort trends are orthogonally derived into their linear and nonlinear parts, following Holford [27]. Such APC estimable parameters are net drift (period trend + cohort trend) [28], longitudinal age trend (age trend + period trend) [29], cross-sectional age trend (age trend − period trend) [29], and age, period and cohort deviations [30].
APC models enabled fitted (smoothed or de-noised) rates to be estimated and graphed using maximum likelihood estimators into deconstructed age-period-cohort models with estimable parameters [31]; this method has consistently been shown to accurately represent the underlying data while providing gains in confidence about the estimates.
Statistical Analysis
We conducted APC models of TGCTs, seminomas, and nonseminomas for the period 1973–2008. Analyses were restricted to whites, in order to provide viable numbers of cancer cases from a single racial group, and to ages 15 to 74 years, in order to exclude etiologically distinct infantile TGCT cases and ensure viable numbers for analysis. These APC models used 15 4-year age at diagnosis groups (ages 15–74 years) and 9 4-year time periods (1973–2008) resulting in 23 partially overlapping 8-year birth cohorts from 1902 to 1990 (referred to by midyear of birth).
We evaluated APC models for TGCT, seminoma and nonseminoma. We also conducted pairwise statistical comparisons between the two histologic subgroups. As well as providing qualitative and quantitative interpretation of these models, we aimed to provide a focus on elucidating the effects, if any, of calendar period on incidence rates of these malignancies. This included assessment of the net drift, which is the sum of linear effects attributable to birth cohort and calendar period for all age groups combined [28]. The net drift is an estimate of the average annual percentage change in the logarithm of the incidence rates over time and is conceptually similar to the estimated annual percentage change of the age-standardized incidence rate. We graphed fitted cross-sectional age curves and fitted temporal trends [31]. We also graphed the calendar period rate ratio curve which provides an estimate of the rate ratio for each calendar period relative to an arbitrary reference period (here, the mid-calendar period [1989–1992]), adjusted for effects of age and cohort. Lastly, we formally tested whether the linear or nonlinear components of the calendar period rate ratio curves were statistically significant after adjustment for effects of age and cohort. It is important to realize that the linear component, modeled as net drift, represents the sum of linear effects attributable to calendar period and birth cohort. The nonlinear component is the calendar period deviations which can be uniquely attributed to effects of calendar period given adjustment for age deviations and cohort effects (net drift and cohort deviations) [27]. All tests were two-sided and p-values less than 0.05 were considered to be statistically significant. MATLAB R2011b (The MathWorks Inc., Natick, MA) was used for analysis with APC tools previously described [20,31].
Results
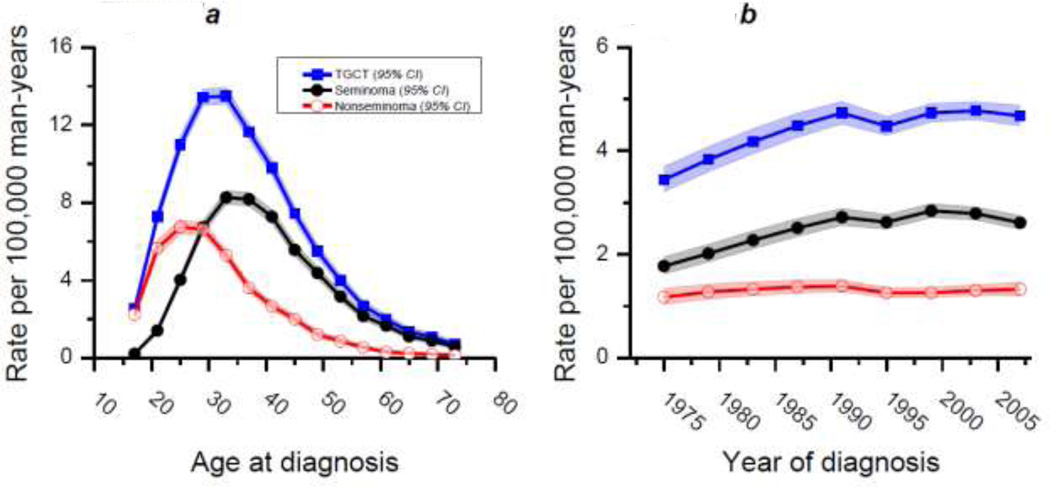
From 1973 to 2008, there were 32,250 diagnoses of TGCT in men aged 15 to 74 years in our SEER concatenated dataset. Of these, 18,475 were seminomas and 13,775 nonseminomas. These numbers were assessed using APC models, as described, and qualitative interpretations of normal probability plots of the deviance residuals indicated that the model fitted the data well (Supplementary Figures 1–3). APC models enabled fitted (smoothed or de-noised) rates to be estimated and graphed [31]. Figure 1A shows fitted cross-sectional age curves which serves to reiterate the well-understood fact that nonseminoma is an earlier onset disease with a median age of approximately 25 years, relative to the later onset seminoma which peaks at approximately 35 years of age.
Figure 1. Fitted cross-sectional age curves (A) and fitted temporal trends of testicular germ-cell tumor, seminoma, and nonseminoma.
Both plots are per 100,000 man-years and include data from a SEER concatenated dataset (SEER 9, 13, 17) which includes the period 1973–2008 and ages 15–74 years.
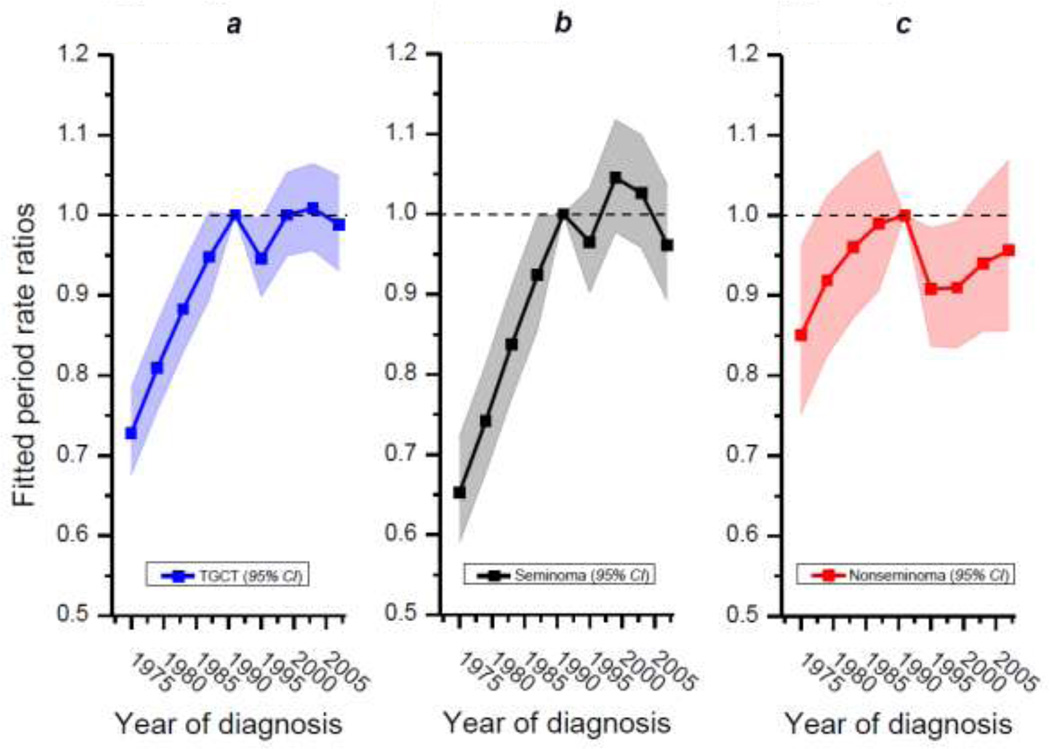
Figure 1B shows fitted temporal trends of TGCT, seminoma and nonseminoma. As can be seen, seminoma is the most incident testicular cancer histology for the age range included in this analysis. Furthermore over the 36-year period analyzed, it is clear that the incidence of seminoma has increased at a much greater rate compared with nonseminoma. Indeed, the net drift was 1.25% for seminoma and just 0.14% for nonseminoma, a difference which was statistically significant (Wald statistic=12.99, 1df, chi-square p value=0.0003). The net drift for TGCT was 0.89% for same time period. The calendar period relative risk curves (Figure 2) accentuate these patterns and emphasize the differences in net drift. From Figures 1B and 2 it can be seen that TGCT, and seminoma in particular, increased in incidence until approximately 1990 at which time it began to plateau followed, more recently, by a slight decrease. Incidence rates of nonseminoma, meanwhile, have varied much less and present a more stable pattern over the 36-year period.
Figure 2. Calendar period rate ratio curves of testicular germ-cell tumor (A), seminoma (B), and nonseminoma (C).
These plots include data from a SEER concatenated dataset (SEER 9, 13, 17) which includes the period 1973–2008 and ages 15–74 years. The 4-year period 1989–1992 was used as the referent as indicated by the confluence of each plot at the dotted line positioned at value 1 on the Y-axis.
We also examined whether the linear or nonlinear components of the calendar period rate ratio curves were statistically significant after adjustment for effects of age and cohort (see Table). Linear and nonlinear components were highly statistically significant in models of TGCT and seminoma cancer incidence; for nonseminoma the results were much more muted—the linear effect p value was 0.5 while the non-linear effect p value was 0.02.
Table 1.
Assessment of the linear and nonlinear components of calendar period rate ratio curves for testicular germ-cell tumor, seminoma, and nonseminoma (SEER 9, 13 and 17, 1973–2008, Ages 15–74 years).
| Model | TGCT | Seminoma | Nonseminoma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Raw Deviance |
df | P value | Raw Deviance |
df | P value | Raw Deviance |
df | P value |
|
| Linear Effects1 | 48.73 | 1 | 2.94E-12 | 58.30 | 1 | 2.24E-14 | 0.45 | 1 | 0.50 |
| Non-linear Effects2 | 55.40 | 7 | 1.24E-09 | 77.73 | 7 | 3.99E-14 | 16.34 | 7 | 0.02 |
Modelled as net drift adjusted for age deviations and cohort deviations.
Modelled as period deviations adjusted for age deviations, net drift and cohort deviations.
Abbreviations: df, degress of freedom; TGCT, testicular germ-cell tumor.
The sensitivity analyses restricted to SEER-9 data included 19,933 TGCTs (11,382 seminomas, 8,551 nonseminomas) and provided similar results (Supplementary Figures 3 and 4, and Supplementary Table) and identical inferences.
Discussion
In this analysis of SEER data, we have found evidence to suggest that period deviations play a significant role in incidence trends of TGCT. This is an important observation given the current working model of TGCT pathogenesis which states that only in utero exposures affect development of TGCT—such a model predicts an absence of period effects in APC models of TGCT incidence rates. Therefore, our finding that period deviations are a significant component of TGCT incidence rates indicates that postnatal risk factors are etiologically relevant, a thesis supported by qualitative observations made in previous studies [11,17,18,12,9,15,19].
It is interesting to note that the evidence we present also suggests that period deviations are more important in seminoma compared with nonseminoma. As noted herein, and is well understood by the field, seminoma has a later age of diagnosis. If such is a proxy for age of carcinogenic transformation, then maybe this presents a greater time window for postnatal exposures to mediate risk of seminoma development relative to that for nonseminoma. This is commensurate with seminoma having a greater net drift, a more pronounced calendar period rate ratio curve, and highly statistically significant nonlinear effects of calendar period after adjustment for age deviations and cohort effects, relative to nonseminoma.
If calendar period effects are important in TGCT incidence trends and this reflects exposures which can mediate TGCT risk across a broad range of ages, then what epidemiologic evidence is there to support such? Exposures such as endogenous hormones [1], adult height [32], early age at puberty [33,15], consumption of fats, milk, and dairy products [33,15] and marijuana use [34,35] have been associated with TGCT, but it could be argued that these “exposures” are determined prenatally and/or are mere confounders of a prenatal exposure. Associations with occupation would be less easily dismissed if these associations were more consistent [1]. The evidence that surgical intervention for cryptorchidism affects TGCT risk is provocative and supports the idea that postnatal factors can affect TGCT risk [36]. Overall, however, there is a paucity of identified risk factors for TGCT whose effects can be confined to the postnatal period. It is possible that some of the inconsistency in postnatal risk factors, such as occupation, could be due to combining seminoma and nonseminoma into a single analytic group, especially given that the results presented herein argue for a stronger effect of period deviations in seminoma compared with nonseminoma. However, to-date there is limited evidence to suggest that there exists a histologic dissimilarity in risk factors [1]; this may be partly attributable to the low statistical power of subgroup analyses which have been conducted of this rare malignancy.
Alternatively the prevalence, and thus effect, of postnatal risk factors for TGCT could vary by geography. Indeed, an APC analysis of Norwegian data (1958–1992, ages15–44) found that a statistical model with “acceptable best fit” could be achieved by including the variables age and birth cohort only [37]. Subsequent work following this paper found that a frailty model, whereby a small proportion of individuals are deemed as having extremely high risk of TGCT from birth and a larger group deemed as having extremely low risk, fit Norwegian and Danish TGCT incidence data fairly well [8,38]. While these studies re-emphasize the idea that key, likely necessary, carcinogenic events for future TGCT development take place during pre- or perinatal development—something which we do not dispute—they do not deem these carcinogenic events as definitive in the risk they confer for TGCT, and thus do not contravene the thesis laid-out here that postnatal exposures are etiologically relevant.
Strengths of this analysis include the use of high-quality cancer registry data; SEER has extensive quality control procedures that have been in place for many years [39,40]. In addition, these data are large-scale and population-based which increases the generalizability of results to the wider US population. We also used novel statistical pairwise comparisons of APC models [20] which strengthens the validity and objectivity of the differences detected by adding a quantitative level to something which has often been a qualitative exercise. Limitations of our analysis include the use of concatenated SEER databases which causes expansion of the US population under study at 1992 and 2000. However, analyses restricted to SEER 9 registries provided similar results both qualitatively and quantitatively. In addition, we restrict our analyses to whites as other races provide very low numbers of cases for analysis, given the relative rarity of this tumor in such groups and the potential for etiologic heterogeneity by race. Lastly, the well documented identifiability constraint precludes changes in incidence rates being uniquely assigned to influences (sum of linear and nonlinear components) of age, period or cohort, due to the co-linear nature of these metrics [30]. However, the statistical significance of nonlinear effects (deviations) of calendar period, once adjusted for age deviations and cohort influences, can be uniquely attributed to the influence of calendar period.
In conclusion, we have found evidence to suggest that period deviations play a significant role in incidence trends of TGCT which indicate that postnatal exposures are etiologically relevant. This is especially true for seminoma, thus future epidemiologic studies should strive to elucidate histologic heterogeneity in risk factor profiles.
Supplementary Material
Acknowledgments
FUNDING
Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
There are no financial disclosures from any of the authors.
References
- 1.McGlynn KA, Cook MB. Etiologic factors in testicular germ-cell tumors. Future Oncol. 2009;5(9):1389–1402. doi: 10.2217/fon.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skakkebaek NE, Berthelsen JG, Muller J. Carcinoma-in-situ of the undescended testis. Urol Clin North Am. 1982;9(3):377–385. [PubMed] [Google Scholar]
- 3.Sonne SB, Almstrup K, Dalgaard M, Juncker AS, Edsgard D, Ruban L, Harrison NJ, Schwager C, Abdollahi A, Huber PE, Brunak S, Gjerdrum LM, Moore HD, Andrews PW, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res. 2009;69(12):5241–5250. doi: 10.1158/0008-5472.CAN-08-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oosterhuis JW, Kersemaekers AM, Jacobsen GK, Timmer A, Steyerberg EW, Molier M, Van Weeren PC, Stoop H, Looijenga LH. Morphology of testicular parenchyma adjacent to germ cell tumours. An interim report. APMIS. 2003;111(1):32–40. doi: 10.1034/j.1600-0463.2003.11101061.x. discussion 41-32. [DOI] [PubMed] [Google Scholar]
- 5.von der Maase H, Rorth M, Walbom-Jorgensen S, Sorensen BL, Christophersen IS, Hald T, Jacobsen GK, Berthelsen JG, Skakkebaek NE. Carcinoma in situ of contralateral testis in patients with testicular germ cell cancer: study of 27 cases in 500 patients. Br Med J (Clin Res Ed) 1986;293(6559):1398–1401. doi: 10.1136/bmj.293.6559.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skakkebaek NE. Carcinoma in situ of the testis: frequency and relationship to invasive germ cell tumours in infertile men. Histopathology. 1978;2(3):157–170. doi: 10.1111/j.1365-2559.1978.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 7.Giwercman A, Skakkebaek NE. Carcinoma in situ of the testis: biology, screening and management. Eur Urol. 1993;23(Suppl 2):19–21. doi: 10.1159/000474694. [DOI] [PubMed] [Google Scholar]
- 8.Aalen OO, Tretli S. Analyzing incidence of testis cancer by means of a frailty model. Cancer Causes Control. 1999;10(4):285–292. doi: 10.1023/a:1008916718152. [DOI] [PubMed] [Google Scholar]
- 9.Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Moller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer. 2006;118(12):3099–3111. doi: 10.1002/ijc.21747. [DOI] [PubMed] [Google Scholar]
- 10.Moller H. Decreased testicular cancer risk in men born in wartime. Journal of the National Cancer Institute. 1989;81(21):1668–1669. doi: 10.1093/jnci/81.21.1668-a. [DOI] [PubMed] [Google Scholar]
- 11.McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97(1):63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 12.Baade P, Carriere P, Fritschi L. Trends in testicular germ cell cancer incidence in Australia. Cancer Causes Control. 2008;19(10):1043–1049. doi: 10.1007/s10552-008-9168-z. [DOI] [PubMed] [Google Scholar]
- 13.Bray F, Richiardi L, Ekbom A, Forman D, Pukkala E, Cuninkova M, Moller H. Do testicular seminoma and nonseminoma share the same etiology? Evidence from an age-period-cohort analysis of incidence trends in eight European countries. Cancer Epidemiol Biomarkers Prev. 2006;15(4):652–658. doi: 10.1158/1055-9965.EPI-05-0565. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Wen SW, Mao Y, Mery L, Rouleau J. Birth cohort effects underlying the increasing testicular cancer incidence in Canada. Can J Public Health. 1999;90(3):176–180. doi: 10.1007/BF03404502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richiardi L, Bellocco R, Adami HO, Torrang A, Barlow L, Hakulinen T, Rahu M, Stengrevics A, Storm H, Tretli S, Kurtinaitis J, Tyczynski JE, Akre O. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2157–2166. [PubMed] [Google Scholar]
- 16.Liu S, Semenciw R, Waters C, Wen SW, Mery LS, Mao Y. Clues to the aetiological heterogeneity of testicular seminomas and non-seminomas: time trends and age-period-cohort effects. Int J Epidemiol. 2000;29(5):826–831. doi: 10.1093/ije/29.5.826. [DOI] [PubMed] [Google Scholar]
- 17.Zheng T, Holford TR, Ma Z, Ward BA, Flannery J, Boyle P. Continuing increase in incidence of germ-cell testis cancer in young adults: experience from Connecticut, USA, 1935–1992. Int J Cancer. 1996;65(6):723–729. doi: 10.1002/(SICI)1097-0215(19960315)65:6<723::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Moller H. Trends in incidence of testicular cancer and prostate cancer in Denmark. Hum Reprod. 2001;16(5):1007–1011. doi: 10.1093/humrep/16.5.1007. [DOI] [PubMed] [Google Scholar]
- 19.Sincic N, Kulis T, Znaor A, Bray F. Time trends in testicular cancer in Croatia 1983–2007: rapid increases in incidence, no declines in mortality. Cancer Epidemiol. 2012;36(1):11–15. doi: 10.1016/j.canep.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg PS, Anderson WF. Proportional hazards models and age-period-cohort analysis of cancer rates. Stat Med. 2010;29(11):1228–1238. doi: 10.1002/sim.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson WF, Camargo MC, Fraumeni JF, Jr., Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303(17):1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. Journal of Clinical Oncology. 2010;28(2):232–239. doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimley PM, Matsuno RK, Rosenberg PS, Henson DE, Schwartz AM, Anderson WF. Qualitative age interactions between low-grade and high-grade serous ovarian carcinomas. Cancer Epidemiology, Biomarkers and Prevention. 2009;18(8):2256–2261. doi: 10.1158/1055-9965.EPI-09-0240. [DOI] [PubMed] [Google Scholar]
- 24.Fritz AG. International classification of diseases for oncology : ICD-O. 3rd edn. Geneva: World Health Organization; 2000. [Google Scholar]
- 25.World Health Organization. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: 2004. IARC Press ; Oxford : Oxford University Press [distributor] [Google Scholar]
- 26.Waheeb R, Hofmann MC. Human spermatogonial stem cells: a possible origin for spermatocytic seminoma. Int J Androl. 2011;34(4 Pt 2):e296–e305. doi: 10.1111/j.1365-2605.2011.01199.x. discussion e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39(2):311–324. [PubMed] [Google Scholar]
- 28.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med. 1987;6(4):469–481. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 29.Robertson C, Gandini S, Boyle P. Age-period-cohort models: a comparative study of available methodologies. J Clin Epidemiol. 1999;52(6):569–583. doi: 10.1016/s0895-4356(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 30.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiology, Biomarkers and Prevention. 2011;20(7):1263–1268. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerro CC, McGlynn KA, Cook MB. A systematic review and meta-analysis of the relationship between body size and testicular cancer. Br J Cancer. 2010;103(9):1467–1474. doi: 10.1038/sj.bjc.6605934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGlynn KA, Cook MB. The Epidemiology of Testicular Cancer. In: Cooney KA, Foulkes WD, editors. Male Reproductive Cancers: Epidemiology, Pathology and Genetics. vol Cancer Genetics series. New York: Springer Publications; 2009. [Google Scholar]
- 34.Daling JR, Doody DR, Sun X, Trabert BL, Weiss NS, Chen C, Biggs ML, Starr JR, Dey SK, Schwartz SM. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115(6):1215–1223. doi: 10.1002/cncr.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer. 2011;117(4):848–853. doi: 10.1002/cncr.25499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med. 2007;356(18):1835–1841. doi: 10.1056/NEJMoa067588. [DOI] [PubMed] [Google Scholar]
- 37.Wanderas EH, Tretli S, Fossa SD. Trends in incidence of testicular cancer in Norway 1955–1992. Eur J Cancer. 1995;31A(12):2044–2048. doi: 10.1016/0959-8049(95)00321-5. [DOI] [PubMed] [Google Scholar]
- 38.Moger TA, Aalen OO, Halvorsen TO, Storm HH, Tretli S. Frailty modelling of testicular cancer incidence using Scandinavian data. Biostatistics. 2004;5(1):1–14. doi: 10.1093/biostatistics/5.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76(11):2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15(2):415–423. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.