Abstract
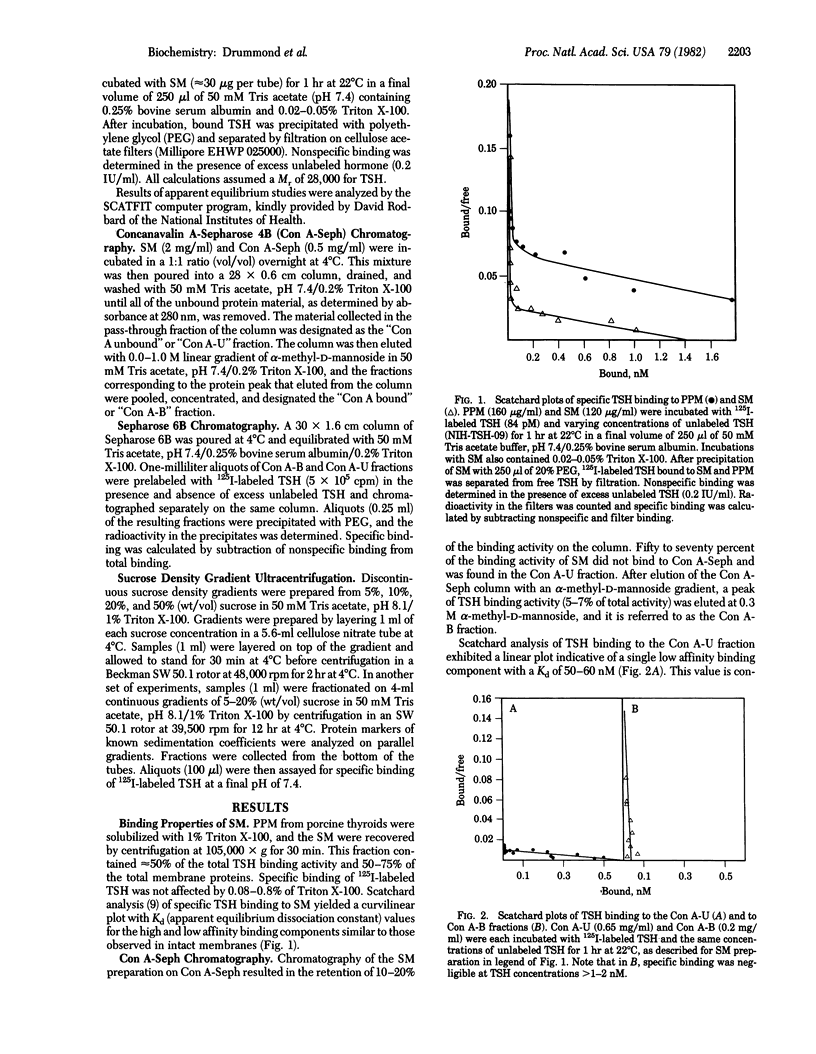
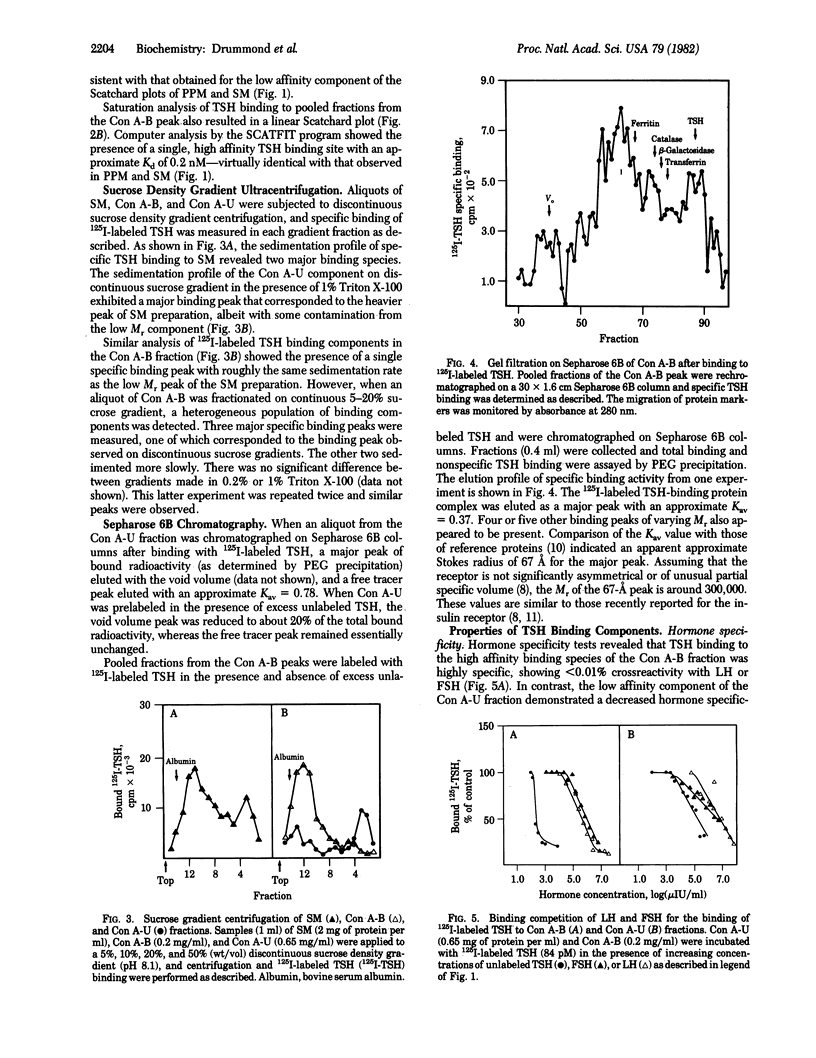
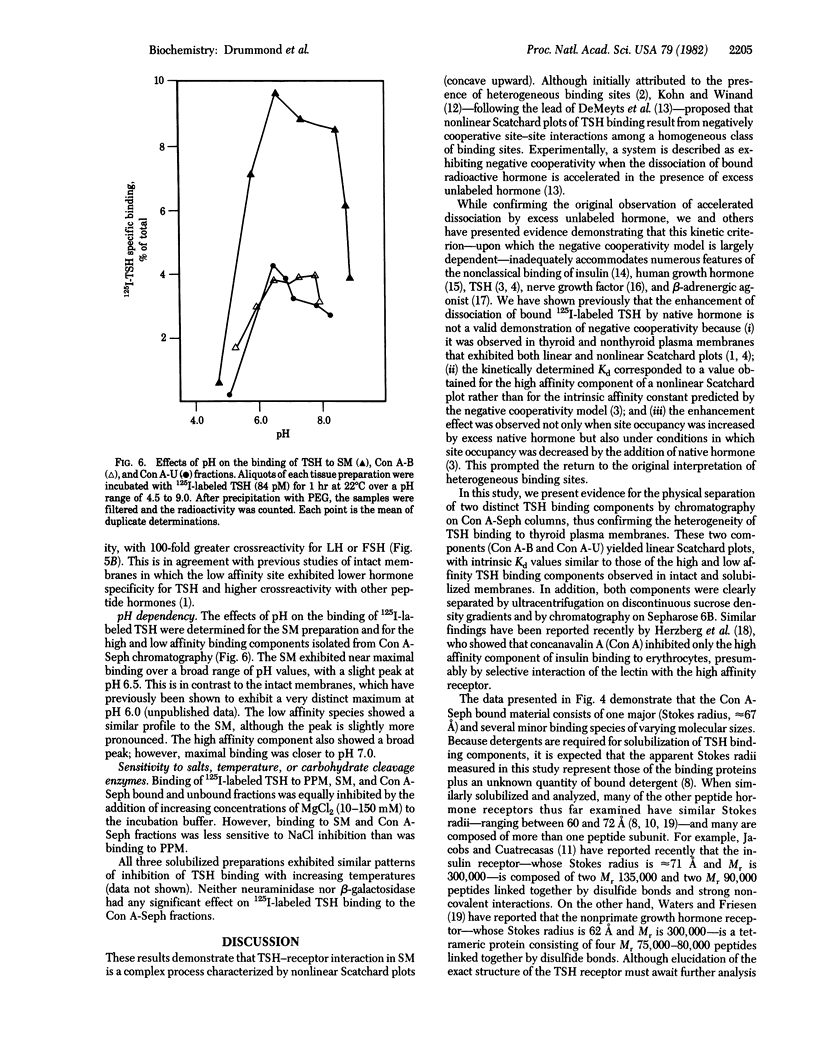
Two distinct thyrotropin (TSH) binding species have been separated from solubilized porcine thyroid membranes. Membranes was solubilized with 1% Triton X-100, and the supernatant was recovered by centrifugation at 105,000 X g. Scatchard analysis of thyrotropin binding to solubilized membranes (SM) yielded a nonlinear plot with Kd values for the high and low affinity components similar to those of intact membranes. Chromatography of the SM preparation on concanavalin A-Sepharose 4B resulted in the retention of 10-20% of the binding activity. Upon elution of the column, a peak of binding material (5-7% of total activity) was eluted at 0.3 M alpha-methyl-D-mannoside. This concanavalin A (Con A) bound fraction exhibited a linear Scatchard plot with a Kd value similar to that of the high affinity component of the SM. The protein fraction that did not bind to Con A (Con A unbound) also exhibited a linear Scatchard plot, but with affinity similar to that of the low affinity component of SM. Discontinuous sucrose density gradient ultracentrifugation revealed the presence of two major binding peaks in the solubilized membrane preparation. The slowly sedimenting peak corresponded to that seen in the Con A bound fraction, whereas the rapidly sedimenting peak corresponded to that of the Con A unbound fraction. Sepharose 6B chromatography indicated that in the case of the Con A unbound fraction, a single peak of specific binding activity was eluted in the void volume, and in the case of the Con A bound fraction, one major peak with an approximate Stokes radius of 67 A and several other minor peaks were eluted. These results demonstrate the physical separation of two distinct TSH binding species from thyroid membranes and provide further support for the model of multiple classes of binding sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir S. M., Goldfine I. D., Ingbar S. H. Properties of the interaction between bovine thyrotropin and bovine thyroid plasma membranes. J Biol Chem. 1976 Aug 10;251(15):4693–4699. [PubMed] [Google Scholar]
- Burke G. On the role of adenyl cyclase activation and endocytosis in thyroid slice metabolism. Endocrinology. 1970 Feb;86(2):353–359. doi: 10.1210/endo-86-2-353. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner D. B., Martin D. W., Sonenberg M. Accumulation of a slowly dissociable peptide hormone binding component by isolated target cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):672–676. doi: 10.1073/pnas.75.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimerl S., Neufeld G., Korner M., Schramm M. Functional implantation of a solubilized beta-adrenergic receptor in the membrane of a cell. Proc Natl Acad Sci U S A. 1980 Feb;77(2):760–764. doi: 10.1073/pnas.77.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg V., Boughter J. M., Carlisle S., Hill D. E. Evidence for two insulin receptor populations on human erythrocytes. Nature. 1980 Jul 17;286(5770):279–281. doi: 10.1038/286279a0. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptor: structure and function. Endocr Rev. 1981 Summer;2(3):251–263. doi: 10.1210/edrv-2-3-251. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. The mobile receptor hypothesis and "cooperativity" of hormone binding. Application to insulin. Biochim Biophys Acta. 1976 May 21;433(3):482–495. doi: 10.1016/0005-2736(76)90275-3. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D. Characterization of the thyrotropin receptor and its involvement in Graves' disease. Horiz Biochem Biophys. 1977;3:123–163. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Olsen R. W., Changeux J. P. Studies on the cholinergic receptor protein from Electrophorus electricus. Effect of detergents on some hydrodynamic properties of the receptor protein in solution. FEBS Lett. 1972 Jul 15;24(1):63–68. doi: 10.1016/0014-5793(72)80827-5. [DOI] [PubMed] [Google Scholar]
- Oseid S., Beck-Nielsen H., Pedersen O., Sovik O. Decreased binding of insulin to its receptor in patients with congenital generalized lipodystrophy. N Engl J Med. 1977 Feb 3;296(5):245–248. doi: 10.1056/NEJM197702032960503. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Hormone-receptor interactions are noncooperative: application to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4340–4344. doi: 10.1073/pnas.77.7.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Insulin binding to the human lymphocyte receptor. Evaluation of the negative cooperativity model. J Biol Chem. 1977 Aug 25;252(16):5828–5834. [PubMed] [Google Scholar]
- Powell-Jones C. H., Thomas C. G., Jr, Nayfeh S. N. Contribution of negative cooperativity to the thyrotropin-receptor interaction in normal human thyroid: kinetic evaluation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):705–709. doi: 10.1073/pnas.76.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Jones C. H., Thomas C. G., Jr, Nayfeh S. N. Thyrotropin receptors in normal human thyroid. Nonclassical binding kinetics not explained by the negative cooperativity model. J Biol Chem. 1980 May 10;255(9):4001–4010. [PubMed] [Google Scholar]
- Saltiel A. R., Powel-Jones C. H., Thomas C. G., Jr, Nayfeh S. N. Thyrotropin receptor-adenylate cyclase function in human thyroid neoplasms. Cancer Res. 1981 Jun;41(6):2360–2365. [PubMed] [Google Scholar]
- Saltiel A. R., Powell-Jones C. H., Thomas C. G., Jr, Nayfeh S. N. Apparent "negative cooperativity" kinetics in the absence of a nonlinear Scatchard plot of thyrotropin-receptor interaction in a human thyroid adenoma. Biochem Biophys Res Commun. 1980 Jul 16;95(1):395–403. doi: 10.1016/0006-291x(80)90751-2. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Chang K. J., Jacobs S., Cuatrecasas P. Modulation of binding and bioactivity of insulin by anti-insulin antibody: relation to possible role of receptor self-aggregation in hormone action. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2720–2724. doi: 10.1073/pnas.76.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Tate R. L., Holmes J. M., Kohn L. D., Winand R. J. Characteristics of a solubilized thyrotropin receptor from bovine thyroid plasma membranes. J Biol Chem. 1975 Aug 25;250(16):6527–6533. [PubMed] [Google Scholar]
- Waters M. J., Friesen H. G. Purification and partial characterization of a nonprimate growth hormone receptor. J Biol Chem. 1979 Jul 25;254(14):6815–6825. [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]



