Abstract
Epidermal growth factor (EGF) increases the phosphorylation of its receptor and other membrane proteins, and these proteins can be rapidly dephosphorylated by membrane-bound protein phosphatase [Carpenter, G., King L., Jr., & Cohen, S. (1979) J. Biol. Chem. 254, 4884]. We report that [35S]-adenosine 5'-[gamma-thio]triphosphate is as effective as [gamma-32P]ATP as substrate for the EGF receptor-associated protein kinase in A431 membranes. Both the kinetics and the extent of the EGF-dependent thiophosphorylation at 0 degrees C are similar to those obtained with [gamma-32P]ATP, provided that ATP hydrolysis by the membrane preparation is inhibited by addition of adenosine 5'-[beta, gamma-imino]-triphosphate. The thiophosphorylation reaction requires Mn2+ but differs from the phosphorylation reaction in the inability of Mg2+ to serve as cofactor. Both EGF-dependent phosphorylated and thiophosphorylated membrane proteins yield the same two major bands of Mr 145,000-160,000 in autoradiograms of NaDodSO4/polyacrylamide gel electrophorograms. The rate of dephosphorylation of membrane proteins that have been thiophosphorylated in the presence of EGF is dramatically slower (factors of 1/20 to 1/40) than that of the phosphorylated proteins at both 0 degrees C and 32 degrees C. This increased metabolic stability of the thiophosphorylated proteins will be useful for investigation of the role of phosphorylation in the biological effects of EGF.
Full text
PDF
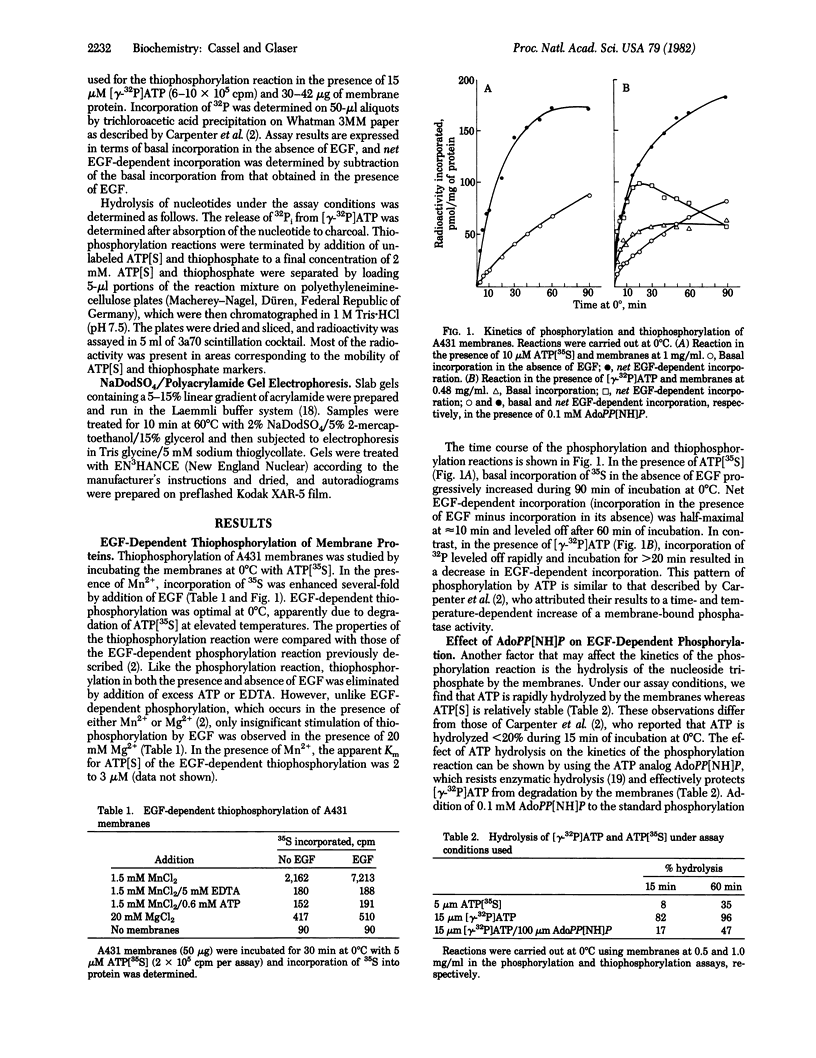
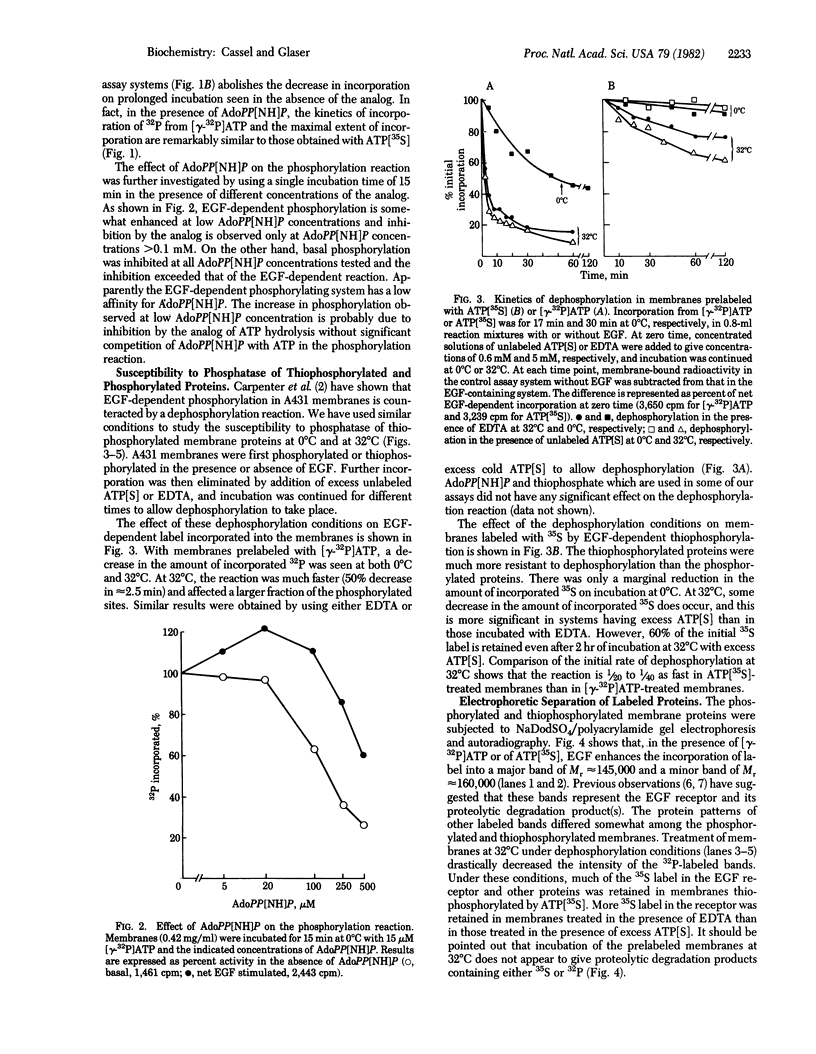
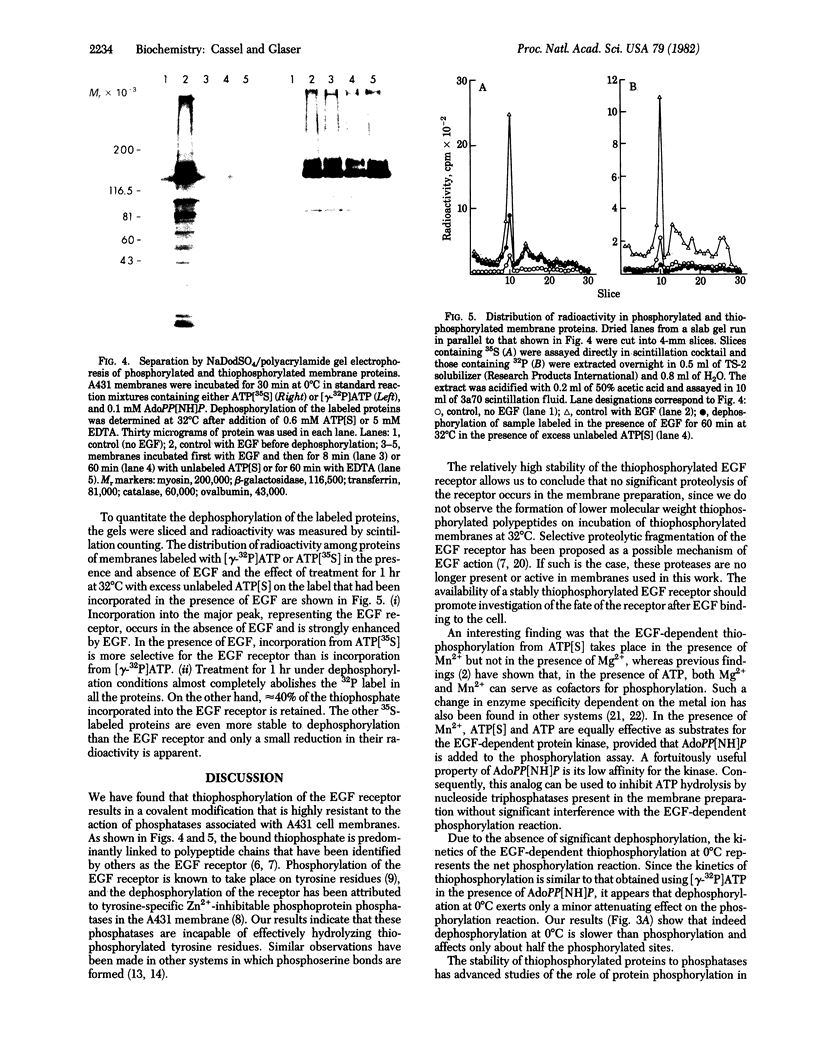

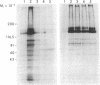
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brautigan D. L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981 Jul 10;256(13):6519–6522. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Pol J. A. Rapid and specific enhancement of phosphorylation of two normal rat kidney cell membrane polypeptides of Mr = 170,000 and 150,000 by epidermal growth factor in vitro. J Biol Chem. 1981 Sep 25;256(18):9742–9749. [PubMed] [Google Scholar]
- Fox C. F., Das M. Internalization and processing of the EGF receptor in the induction of DNA synthesis in cultured fibroblasts: the endocytic activation hypothesis. J Supramol Struct. 1979;10(2):199–214. doi: 10.1002/jss.400100210. [DOI] [PubMed] [Google Scholar]
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. E., Jr, Carpenter G., Cohen S. Characterization by electrophoresis of epidermal growth factor stimulated phosphorylation using A-431 membranes. Biochemistry. 1980 Apr 1;19(7):1524–1528. doi: 10.1021/bi00548a040. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Fox C. F. Controlled proteolysis of EGF receptors: evidence for transmembrane distribution of the EGF binding and phosphate acceptor sites. J Supramol Struct. 1980;14(4):461–471. doi: 10.1002/jss.400140405. [DOI] [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]