Abstract
We present the case of a 55-year-old woman with giant cell myocarditis who experienced a rapid deterioration in her condition. As her heart failure progressed, she developed more ventricular ectopic beats, which culminated in a polymorphic ventricular tachycardia that did not improve despite immunosuppressive and antiarrhythmic therapy. Emergent biventricular assist device placement, however, did eliminate her arrhythmia.
Key words: Giant cells/pathology; heart-assist devices; myocarditis/complications/diagnosis/physiopathology; tachycardia, ventricular/etiology/physiopathology
Giant cell myocarditis (GCM) is a rare form of cardiomyopathy with a high mortality rate. In the largest GCM series published to date (63 patients),1 the rate of death or cardiac transplantation was 89%, with a median survival of 5.5 months from the onset of symptoms to the time of death or transplantation. Patients with GCM manifest a diffuse, infiltrative disease process and often have conduction system abnormalities such as heart block, bundle branch block, and ventricular tachycardia (VT). We present the case of a patient, diagnosed with GCM, whose ventricular arrhythmias were refractory to medical management but resolved after mechanical circulatory support.
Case Report
In 2011, a previously healthy 55-year-old Caucasian woman came to her primary care provider with progressive shortness of breath and abdominal fullness. A chest radiograph revealed pulmonary edema, and she was admitted to her local hospital. Her troponin I level was elevated, at 3.62 μg/L. An electrocardiogram revealed sinus rhythm with an intraventricular conduction delay resembling a left bundle branch block (Fig. 1). Cardiac catheterization showed normal coronary arteries. An echocardiogram revealed severe left ventricular systolic dysfunction (ejection fraction, 0.25), moderately decreased right ventricular function, and moderate-to-severe mitral regurgitation. She was treated with furosemide and nesiritide drips. Carvedilol and ramipril were also initiated. Her systolic blood pressure readings were between 70 and 90 mmHg, which made it difficult to accomplish diuresis and afterload reduction.
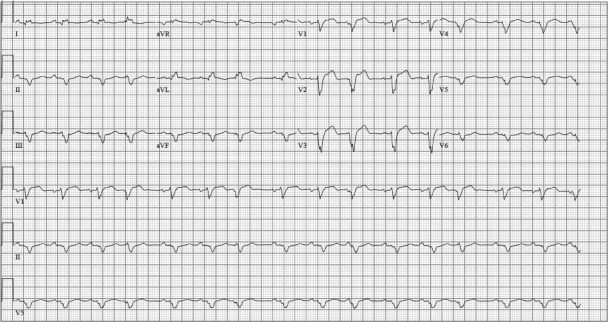
Fig. 1 The initial electrocardiogram reveals sinus rhythm, left-axis deviation, and a wide QRS that manifests an intraventricular conduction delay with the pattern of a left bundle branch block.
She was transferred to a tertiary care facility for further management. In the subsequent days, her troponin levels remained elevated and she developed runs of asymptomatic nonsustained VT. Her β-blocker and angiotensin-converting enzyme inhibitor were stopped due to hypotension, a Swan-Ganz catheter was placed, and milrinone and amiodarone drips were initiated. On a dosage of 0.1 μg/kg/min of milrinone, she had a Fick cardiac index of 1.4 L/min/m2, a pulmonary artery diastolic pressure of 30 mmHg, and a mixed venous oxygen saturation (SvO2) of 46%.
Evaluation of her cardiomyopathy included thyroid function tests, serum and urine protein electrophoresis, an iron panel, and a general viral serology panel, all of which yielded results within normal limits. Her antinuclear antibody test was positive at 1:80, which prompted additional consideration of an autoimmune origin. Cardiac magnetic resonance imaging was ordered to evaluate for an infiltrative process, but she was not able to lie flat for the time required to complete the examination. The patient was sent for cardiac biopsy, which subsequently provided the diagnosis of giant cell myocarditis (Fig. 2).
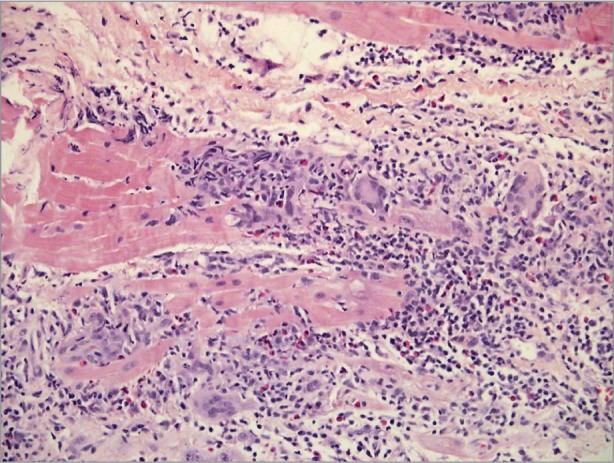
Fig. 2 Photomicrograph at myocardial biopsy reveals multinucleated giant cells and myocytic necrosis consistent with giant cell myocarditis.
Immunosuppressive therapy was initiated with methylprednisolone and cyclosporine. Several hours later, she experienced multiple episodes of VT, with rates ranging from 100 to 170 beats/min. An amiodarone infusion was re-initiated (after having been discontinued when her previous run of ventricular ectopic beats had subsided). By the next morning, the patient was in polymorphic VT (Fig. 3), with a rate of approximately 140 beats/min. The tachycardia alternated between right and left axis and had varying forms of right bundle branch morphology. This rhythm was hemodynamically tolerated: she was alert and communicating throughout the episodes, which lasted several minutes before abating and then recurring. Even though her milrinone was increased to 0.4 μg/kg/min, her SvO2 was 40%.
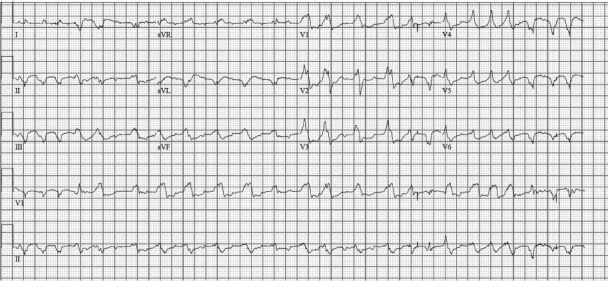
Fig. 3 Despite immunosuppressive and antiarrhythmic therapy, this electrocardiogram shows nonsustained polymorphic ventricular tachycardia, with a rate of approximately 140 beats/min.
The patient was emergently transported to the cardiac catheterization laboratory for placement of an intra-aortic balloon pump, which decreased, but did not eliminate, her ventricular ectopic arrhythmia. She was subsequently sent for emergent placement of a CentriMag® Blood Pump (Thoratec Corporation; Pleasanton, Calif) as a right and left ventricular assist device. After placement of the CentriMag, she had no further episodes of polymorphic VT. She achieved hemodynamic stability and was placed on the cardiac transplantation list. Unfortunately, after 19 days of successful ventricular assist device support, she suffered a hemorrhagic stroke and care was subsequently withdrawn.
Discussion
Arrhythmias are common in heart failure, but VT in giant cell myocarditis is highly unusual, because it is relatively slow (155 beats/min on average, in a series of 5 patients) and does not necessarily result in hemodynamic compromise.2 Although left ventricular function is moderately to severely depressed, the left ventricle might be only mildly enlarged, which reflects the rapid course and limited time for remodeling in this aggressive disease process.3 In a series of 9 patients with GCM,2 5 presented with sustained monomorphic VT and the remaining 4 experienced monomorphic VT during their hospitalization.
Our patient's case is unusual in that she experienced polymorphic VT in addition to the monomorphic VT that is more commonly seen in GCM patients. Although the mechanism for polymorphic VT has not been specifically explained, the underlying myocardial fibrosis might result in multiple foci of triggered automaticity and early after-depolarization.4 In monomorphic tachycardia, it has been suggested that the increased fibrosis and separated myocardial strands observed in histologic samples of inflammatory heart disease might provide the substrate for the unidirectional block and reentry that sustains the arrhythmia.5
Our patient is also unusual in that her polymorphic VT was refractory to immunosuppressive and antiarrhythmic therapy but did resolve once biventricular mechanical circulatory support was initiated. We considered the addition of procainamide to the amiodarone, but dual antiarrhythmic therapy was rejected due to our concern about worsening the conduction abnormalities with multiple levels of blockade. Although ventricular assist device support often provides increased hemodynamic stability, it does not routinely eliminate the occurrence of ventricular arrhythmias. In a series of 195 patients with left ventricular assist devices,6 36 had ventricular arrhythmias after implantation. In our patient, the mechanical off-loading of her right and left ventricles might have decreased wall stress, resulting in a decrease in triggered activity that originated from an underlying lymphocytic inflammatory infiltrate.
Footnotes
Address for reprints: Hannah Raasch, MD, CB #7075, 6th Fl., Burnett-Womack Bldg., 99 Manning Dr., Chapel Hill, NC 27599
E-mail: hraasch@unch.unc.edu
References
- 1.Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis–natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 1997;336(26):1860–6. [DOI] [PubMed]
- 2.Graner M, Lommi J, Kupari M, Raisanen-Sokolowski A, Toivonen L. Multiple forms of sustained monomorphic ventricular tachycardia as common presentation in giant-cell myocarditis. Heart 2007;93(1):119–21. [DOI] [PMC free article] [PubMed]
- 3.Lakdawala NK, Givertz MM. Dilated cardiomyopathy with conduction disease and arrhythmia. Circulation 2010;122(5):527–34. [DOI] [PMC free article] [PubMed]
- 4.Antzelevitch C, Sicouri S. Clinical relevance of cardiac arrhythmias generated by afterdepolarizations. Role of M cells in the generation of U waves, triggered activity and torsade de pointes. J Am Coll Cardiol 1994;23(1):259–77. [DOI] [PubMed]
- 5.Hsia HH, Marchlinski FE. Characterization of the electroanatomic substrate for monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy. Pacing Clin Electrophysiol 2002;25(7):1114–27. [DOI] [PubMed]
- 6.Genovese EA, Dew MA, Teuteberg JJ, Simon MA, Kay J, Siegenthaler MP, et al. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann Thorac Surg 2009;88(4):1162–70. [DOI] [PMC free article] [PubMed]


