Abstract
An intra-aortic balloon pump is one of the most valuable tools in the cardiac surgeon's armament to assist in the management of the failing heart. Despite its widespread use, there are associated risks and complications, one of which is balloon rupture with associated entrapment. Numerous approaches for dealing with this complication have been described; here we review the previous experience with intra-aortic balloon pump entrapment and discuss potential management, with particular reference to a recent case of our own.
Key words: Assisted circulation/adverse effects, counterpulsation/mortality, entrapment, intra-aortic balloon pumping/adverse effects/methods/mortality/rupture/standards/statistics & numerical data, risk assessment
Cardiac surgery offers myriad interventions for possible use in an aging population that has a high prevalence of heart disease. This abundance of options has led to more complex cardiac surgery and to higher public expectations of successful outcomes.1 Against this background, any mechanism that facilitates survival is welcome.
The intra-aortic balloon pump (IABP), first used by Kantrowitz in 1967 in a patient with cardiogenic shock, provides mechanical cardiac support via insertion of an inflatable balloon into the descending aorta; it is the most commonly used supportive tool for temporary cardiac assistance.1,2 The IABP works by reducing afterload and actively increasing coronary perfusion.2 The indications are varied but include ongoing ischemia refractory to medical therapy, a need for prophylaxis in high-risk patients before cardiac surgery, and postoperative ischemia and low cardiac output despite inotropic support.3
Intra-aortic balloon pump use, although priceless in improving postoperative survival in high-risk cardiac surgical patients and those with ventricular dysfunction, is not without risks.1,2 Balloon rupture, aortic or iliac artery dissection, thromboembolism, distal ischemia, and thrombocytopenia due to the mechanical action of the balloon on platelets are all potential complications of IABP use.1,4 Despite these risks, there are over 70,000 insertions annually in the United States alone. Of all cardiac surgical patients, 5% to 10% undergo IABP placement.5
Intra-aortic balloon pump rupture with associated entrapment of the balloon within the arterial tree is very rare. Because numerous approaches to deal with this complication have been described, we review the previous experience and discuss the potential management of IABP entrapment, with specific reference to a case of our own.
Case Report
In September 2009, a 78-year-old man underwent coronary artery bypass grafting (CABG) after referral from a peripheral hospital, where he had presented with a non-ST-elevation myocardial infarction. Preoperative angiography revealed triple-vessel disease with severe stenosis of the left main stem but preserved left ventricular function. His medical history included previously stable angina, hypertension, chronic obstructive pulmonary disease, and a left carotid endarterectomy 3 years earlier. His medications included aspirin, clopidogrel, isosorbide mononitrate, Seretide® (Advair), simvastatin, lisinopril, nicorandil, and enoxaparin. Carotid Doppler sonograms showed <50% stenosis in the right carotid artery and no disease in the left carotid. Pulmonary function tests showed a forced expiratory volume in 1 sec (FEV1) of 78% and a forced vital capacity (FVC) of 87%. His EuroSCORE was 13.
He underwent standard CABG with a pedicled left internal mammary artery graft to the distal left anterior descending coronary artery (LAD) and long saphenous vein grafts to the mid-LAD, first diagonal branch, first obtuse marginal branch, acute marginal branch, and right coronary artery–posterior descending artery. Intraoperatively, the coronary arteries were noted to be very small and calcified, and the patient was very difficult to wean from cardiopulmonary bypass (CPB). This necessitated 6 grafts and substantial inotropic support. After the 2nd attempt to wean the patient from CPB failed—due to left ventricular distention with a falling blood pressure and heart rate, and a raised central venous pressure and pulmonary artery pressure—we inserted an IABP. The patient was unable to tolerate chest closure, but he was weaned from CPB on the 3rd attempt and then transferred (on significant inotropic support and with the IABP set at 1:1) to the cardiac intensive care unit with his chest splinted.
On postoperative day 1, he continued to require inotropic support with the IABP still set at 1:1. He had good urinary output with a falling serum lactate level, and transesophageal echocardiography (TEE) showed good biventricular function. Over the course of the day, the patient's norepinephrine level was slowly reduced. On postoperative day 2, his progress continued and he underwent formal chest closure. Later that day, blood in the inflation channel of the IABP indicated rupture of the balloon; however, the leakage alarm did not activate, and the IABP appeared to be functioning properly in automatic mode. There was no evidence of splanchnic or limb malperfusion. An attempt to remove the intra-aortic balloon proved unsuccessful, which indicated intra-aortic balloon entrapment.
The patient underwent urgent computed tomographic (CT) aortography (Fig. 1) to define the position of the balloon, the relevant anatomy, and the patency of the mesenteric arterial tree, and to determine if there was any aortic dissection. The CT scan showed the balloon just below the aortic bifurcation, extending through the right common iliac artery to the right external iliac artery, and to the right common femoral artery. Significant calcification was noted throughout the arterial tree.
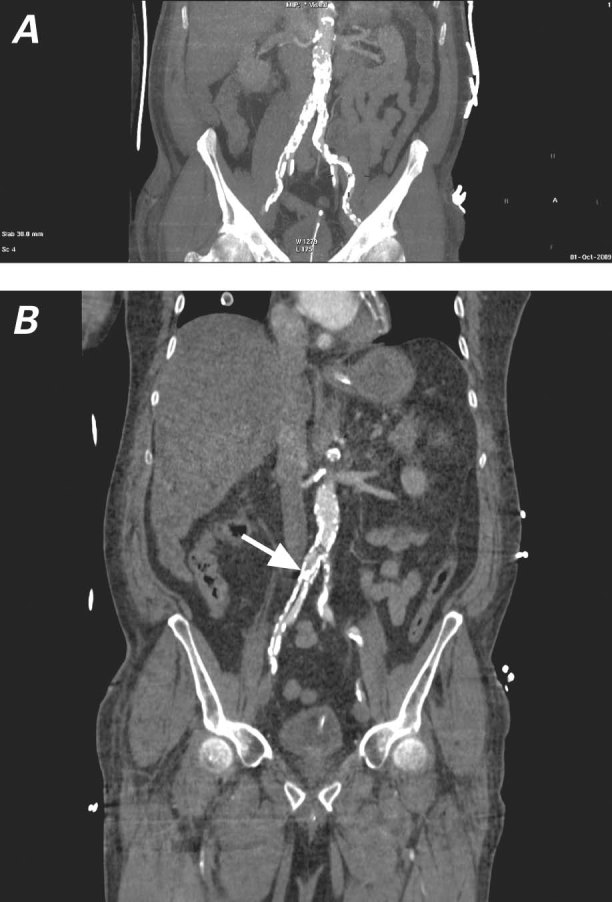
Fig. 1 Computed tomograms of the abdomen and pelvis show A) severely calcified aortic and iliac arteries and B) entrapment of the intra-aortic balloon pump (IABP) at the proximal third of the right common iliac artery (as indicated by the arrow at the IABP tip).
Because the usual means of IABP removal had failed, the vascular surgical team attempted percutaneous removal under imaging guidance. This revealed significantly calcified vessels and was ultimately unsuccessful. The patient then underwent open surgical removal from the right common femoral artery, which yielded good limb perfusion (Fig. 2). His subsequent postoperative recovery proceeded uneventfully, and on postoperative day 9 he was transferred to the referring hospital for convalescence. At outpatient review 6 weeks after discharge, he was symptom-free and was returned to the care of his primary care physician.
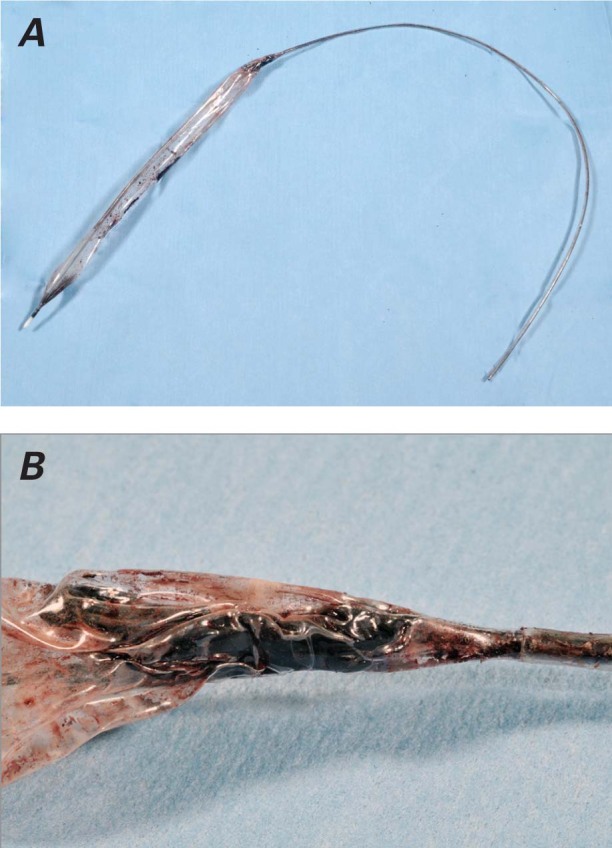
Fig. 2 A) Postoperative photograph of the ruptured intra-aortic balloon pump with B) a close-up of the balloon, showing hard clot at the distal end.
Discussion
Due to the nature of the IABP, the chief adverse sequelae involve vascular injury; studies suggest that vascular ischemic sequelae occur at a rate of 8% to 18%, while major limb ischemia occurs less than 1% of the time.4–12 Rupture of the intra-aortic balloon is rare but can cause gas embolism and entrapment of the balloon within the arterial tree.13,14 Balloon rupture was first reported by Rajani and colleagues, followed by Aru and associates; however, it is very rare, occurring at a rate of possibly less than 0.5%.14–16 The proposed mechanism involves mechanical abrasion of the balloon by atherosclerotic plaque or a heavily calcified aortic wall. After perforation, the negative pressure created during deflation traps blood within the balloon. The blood rapidly reacts with the helium, causing a hard clot to form. This clot, together with the tortuous atherosclerotic aortic environment, entraps the semi-deflated balloon.15
Entrapment can be avoided by early diagnosis and prompt removal of the ruptured balloon. Balloon rupture can sometimes be recognized by the presence of blood in the catheter between the balloon and the safety chamber; if counterpulsation cannot be sustained, the console will sound the alarm, which enables prompt diagnosis of rupture and appropriate removal of the IABP (Fig. 3). However, care should be taken not to rely solely on the alarm, because Nishida and colleagues17 reported that the alarm indicated gas leakage in only 29% of IABP ruptures among patients in their case series. The main indicator of rupture remains blood within the catheter shaft, and this highlights the need for regular review of the shaft during use; 6% of cases of IABP rupture are detected only at the time of removal.17
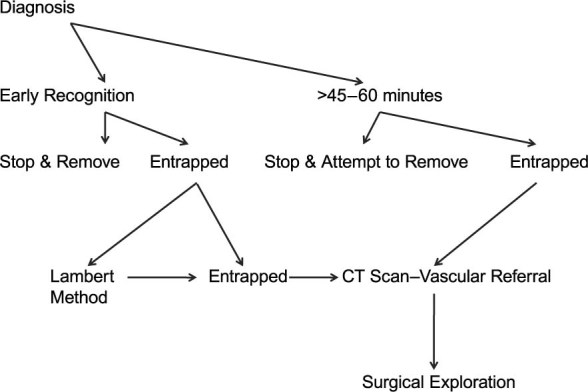
Fig. 3 Algorithm for the management of an entrapped intra-aortic balloon pump.
Current management approaches recommend against forceful extraction of the retained balloon, because the probable result is severe vascular injury. When there is early detection of entrapment, a conservative approach would be the Lambert method. Lambert first reported the use of intraluminal streptokinase with heparinized saline flush for clot dissolution; in his 3 patients, he injected streptokinase into the gas-driven lumen of the intra-aortic balloon catheter and thereby avoided surgery.18 This approach has since been successfully replicated at other institutions, which shows that injection of a thrombolytic agent (tissue plasminogen activator or streptokinase) into the gas lumen of the balloon catheter can dissolve the clot, enabling evacuation of the balloon chamber and normal removal of the intra-aortic balloon catheter.18–21
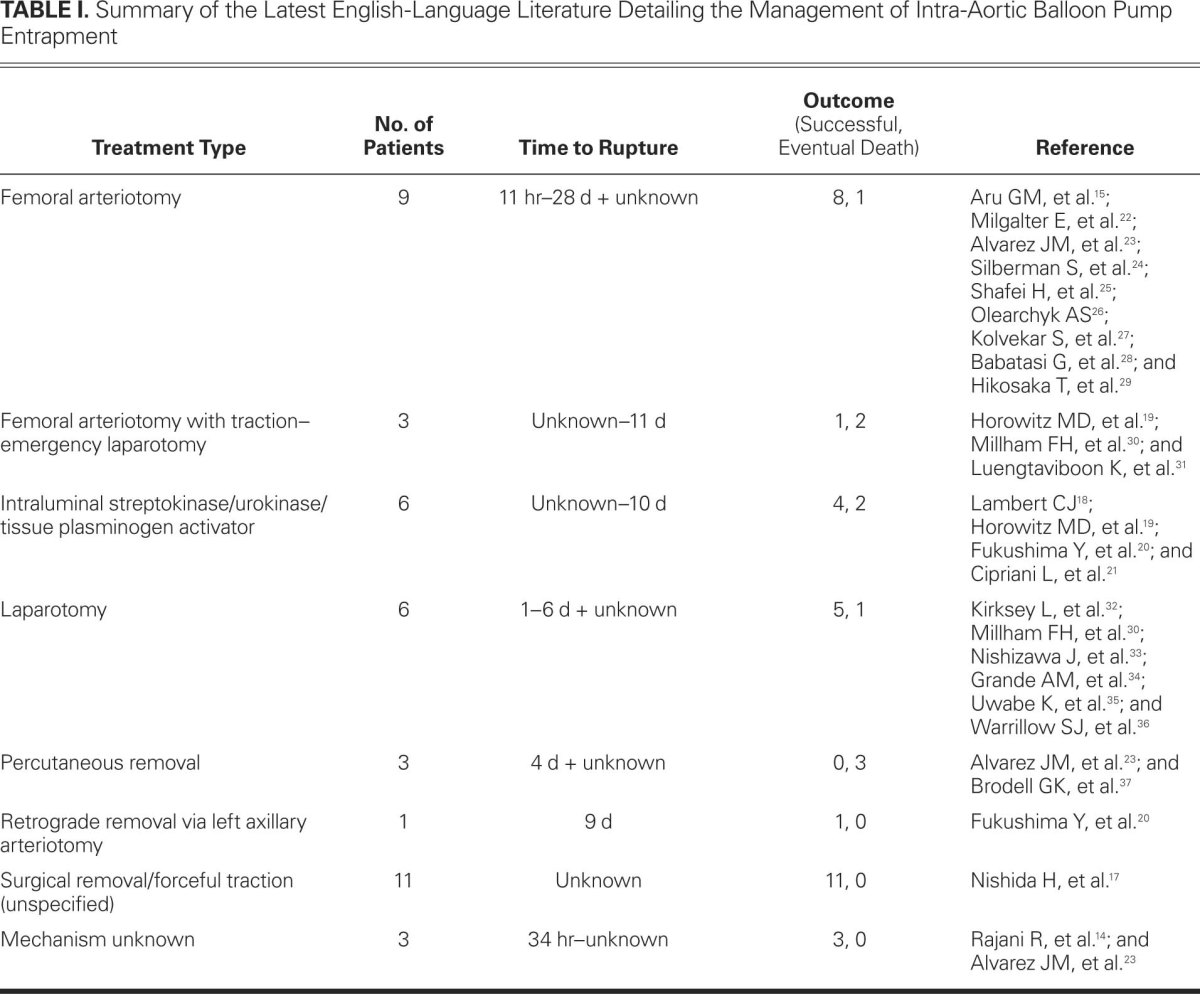
If the detection of the “entrapped clotted balloon” is recognized late (>60 min), the thrombus is unlikely to resolve with thrombolysis. We therefore recommend CT imaging and open vascular exploration with femoral, femoroiliac, or abdominal aortotomy (Table I.14,15,17–37). In particular, to avoid massive bleeding at extraction, an entrapped catheter should be removed tip-first (preferably) through a proximal arteriotomy.15
TABLE I. Summary of the Latest English-Language Literature Detailing the Management of Intra-Aortic Balloon Pump Entrapment
Because there is a significant potential for morbidity and death associated with IABP entrapment, it is useful to evaluate risk factors. Ferguson and colleagues38 reported that independent predictors of major complications associated with IABP use included female sex, peripheral artery disease, body surface area (<1.65 m2), and age >75 yr. Kirksey and co-authors32 also found that the risk of complications associated with IABP use was highest among women and patients with diabetes mellitus, and that infectious sequelae were greatly reduced if insertion of the IABP took place in the operating room. Erdogan and associates4 reported the additional modifiable risk factor of use of the introducer-sheath technique for IABP insertion and recommended sheathless insertion in high-risk patients, particularly those with peripheral artery disease and diabetes mellitus. It is difficult to know the specific risk factors for balloon entrapment due to the scarcity of cases; however, it is reasonable to think along the lines of Ferguson and colleagues.38
In the evaluation of balloon rupture, several risk factors have been identified. The balloon itself is susceptible to damage if a guidewire is not used or if there is forceful insertion against resistance; insertion of the guidewire at an angle greater than 45° predisposes the balloon to kinking at the posterior arterial wall; and inadequate wrapping and insufficient aspiration of air from the balloon can damage the membrane, while excessive inflation can also predispose to rupture. Nevertheless, the main risk remains an atheromatous arterial tree.23
From this perspective, several recommendations can be made in an effort to reduce the risk of IABP rupture. Routine arteriography of the aorta and iliac vessels can be carried out at the time of cardiac angiography in high-risk patients to accurately identify vessels distorted by atherosclerotic disease and thereby enable preoperative planning of IABP insertion approaches.39 Rastan and co-authors40 provided the most comprehensive advice regarding risk management. They advocated use of the smallest possible balloon size, use of a sheathless IABP insertion technique, and removal of the IABP at the earliest opportunity. They also showed the unreliability of plain chest radiography in the determination of IABP position and recommended a low threshold for CT imaging (dependent upon clinical judgment) in application to patients with an IABP and a rising serum lactate level, regardless of IABP positioning on the chest radiograph. They further advised considering the use of CT imaging or TEE for the definitive evaluation of IABP placement in patients who exhibit clinical signs suggestive of IABP malposition, and especially in patients with significant underlying atherosclerotic disease who are at risk of visceral compromise independent of IABP use.40 Finally, Silberman and associates24 advocated using the lowest augmentation setting to achieve the desired cardiac support, thus reducing the risk of contact between the balloon and the arterial plaques at higher settings.
Conclusion
In the case that we present here, there was evidence of blood inside the IABP tubing, but it is probable that this was initially overlooked because the IABP was functioning normally in automatic mode and there was no triggering of the leakage alarm. We believe that the clot formation occurred after the balloon was punctured and that the puncture was caused by prolonged contact between the balloon and the atheromatous aortic wall. Had an attempt been made at forceful extraction of the clot and balloon, aortic laceration and uncontrollable exsanguination most likely would have resulted.
In conclusion, we suggest immediate removal of the intra-aortic balloon when there is any evidence of blood, even a few drops, in the tubing; delay can produce clot and, possibly, balloon entrapment.
Footnotes
Address for reprints: Haralambos Parissis, PhD, Department of Cardiac Surgery, The Royal Victoria Hospital, Grosvenor Rd., Belfast BT12 6BA, Northern Ireland.
E-mail: hparissis@yahoo.co.uk
References
- 1.Lavana JD, Fraser JF, Smith SE, Drake L, Tesar P, Mullany DV. Influence of timing of intraaortic balloon placement in cardiac surgical patients. J Thorac Cardiovasc Surg 2010;140 (1):80–5. [DOI] [PubMed]
- 2.Parissis H. Haemodynamic effects of the use of the intraaortic balloon pump. Hellenic J Cardiol 2007;48(6):346–51. [PubMed]
- 3.Parissis H, Leotsinidis M, Akbar MT, Apostolakis E, Dougenis D. The need for intra aortic balloon pump support following open heart surgery: risk analysis and outcome. J Cardiothorac Surg 2010;5:20. [DOI] [PMC free article] [PubMed]
- 4.Erdogan HB, Goksedef D, Erentug V, Polat A, Bozbuga N, Mansuroglu D, et al. In which patients should sheathless IABP be used? An analysis of vascular complications in 1211 cases. J Card Surg 2006;21(4):342–6. [DOI] [PubMed]
- 5.Cohen M, Urban P, Christenson JT, Joseph DL, Freedman RJ Jr, Miller MF, et al. Intra-aortic balloon counterpulsation in US and non-US centres: results of the Benchmark Registry. Eur Heart J 2003;24(19):1763–70. [DOI] [PubMed]
- 6.Pennington DG, Swartz M, Codd JE, Merjavy JP, Kaiser GC. Intraaortic balloon pumping in cardiac surgical patients: a nine-year experience. Ann Thorac Surg 1983;36(2):125–31. [DOI] [PubMed]
- 7.Naunheim KS, Swartz MT, Pennington DG, Fiore AC, McBride LR, Peigh PS, et al. Intraaortic balloon pumping in patients requiring cardiac operations. Risk analysis and long-term follow-up. J Thorac Cardiovasc Surg 1992;104(6):1654–61. [PubMed]
- 8.Arafa OE, Pedersen TH, Svennevig JL, Fosse E, Geiran OR. Intraaortic balloon pump in open heart operations: 10-year follow-up with risk analysis. Ann Thorac Surg 1998;65(3):741–7. [DOI] [PubMed]
- 9.Allen RC, Schneider J, Longenecker L, Kosinski AS, Smith RB 3rd, Lumsden AB. Acute lower extremity ischemia after cardiac surgery. Am J Surg 1993;166(2):124–9. [DOI] [PubMed]
- 10.Gol MK, Bayazit M, Emir M, Tasdemir O, Bayazit K. Vascular complications related to percutaneous insertion of intraaortic balloon pumps. Ann Thorac Surg 1994;58(5):1476–80. [DOI] [PubMed]
- 11.Tatar H, Cicek S, Demirkilic U, Ozal E, Suer H, Aslan M, Ozturk OY. Vascular complications of intraaortic balloon pumping: unsheathed versus sheathed insertion. Ann Thorac Surg 1993;55(6):1518–21. [DOI] [PubMed]
- 12.Meharwal ZS, Trehan N. Vascular complications of intra-aortic balloon insertion in patients undergoing coronary revascularization: analysis of 911 cases. Eur J Cardiothorac Surg 2002;21(4):741–7. [DOI] [PubMed]
- 13.Gottlieb SO, Brinker JA, Borkon AM, Kallman CH, Potter A, Gott VL, Baughman KL. Identification of patients at high risk for complications of intraaortic balloon counterpulsation: a multivariate risk factor analysis. Am J Cardiol 1984;53(8):1135–9. [DOI] [PubMed]
- 14.Rajani R, Keon WJ, Bedard P. Rupture of an intra-aortic balloon. A case report. J Thorac Cardiovasc Surg 1980;79(2):301–2. [PubMed]
- 15.Aru GM, King JT Jr, Hovaguimian H, Floten HS, Ahmad A, Starr A. The entrapped balloon: report of a possibly serious complication. J Thorac Cardiovasc Surg 1986;91(1):146–9. [PubMed]
- 16.Sutter FP, Joyce DH, Bailey BM, Laub GW, Fernandez J, Pollock SB, et al. Events associated with rupture of intra-aortic balloon counterpulsation devices. ASAIO Trans 1991;37(1):38–40. [DOI] [PubMed]
- 17.Nishida H, Koyanagi H, Abe T, Arai H, Hirayama H, Hirayama T, et al. Comparative study of five types of IABP balloons in terms of incidence of balloon rupture and other complications: a multi-institutional study. Artif Organs 1994; 18(10):746–51. [DOI] [PubMed]
- 18.Lambert CJ. Intraaortic balloon entrapment. Ann Thorac Surg 1987;44(4):446. [DOI] [PubMed]
- 19.Horowitz MD, Otero M, de Marchena EJ, Neibart RM, Novak S, Bolooki H. Intraaortic balloon entrapment. Ann Thorac Surg 1993;56(2):368–70. [DOI] [PubMed]
- 20.Fukushima Y, Yoshioka M, Hirayama N, Kashiwagi T, Onitsuka T, Koga Y. Management of intraaortic balloon entrapment. Ann Thorac Surg 1995;60(4):1109–11. [DOI] [PubMed]
- 21.Cipriani L, Baldereschi G, Boncinelli L, Marchionni N. Vascular entrapment of a ruptured intra-aortic balloon: a case report of successful removal without surgery. Cathet Cardiovasc Diagn 1998;44(2):218–9. [DOI] [PubMed]
- 22.Milgalter E, Mosseri M, Uretzky G, Romanoff H. Intraaortic balloon entrapment: a complication of balloon perforation. Ann Thorac Surg 1986;42(6):697–8. [DOI] [PubMed]
- 23.Alvarez JM, Brady PW, McWilson R. Intra-aortic balloon rupture causing femoral entrapment. Aust N Z J Surg 1993; 63(1):72–4. [DOI] [PubMed]
- 24.Silberman S, Merin O, Fink D, Bitran D. Intraaortic balloon rupture: a possible means of prevention. Ann Thorac Surg 1994;58(3):915–6. [DOI] [PubMed]
- 25.Shafei H, Webb G, Lennox SC. Entrapping of the clotted intra-aortic balloon in the descending aorta. Eur J Cardiothorac Surg 1991;5(3):165–6. [DOI] [PubMed]
- 26.Olearchyk AS. Retained intraaortic balloon assist device. J Thorac Cardiovasc Surg 1992;103(6):1231–2. [PubMed]
- 27.Kolvekar S, Griffin S, Fisher A, O'Riordan J. Retained intraaortic balloon. Ann Thorac Surg 1993;55(6):1598–9. [DOI] [PubMed]
- 28.Babatasi G, Massetti M, Bhoyroo S, Le Page O, Khayat A. Aortic balloon entrapment complicating intra-aortic balloon counterpulsation. ASAIO J 1999;45(5):514–5. [DOI] [PubMed]
- 29.Hikosaka T, Ito K, Takuji T, Zen K, Adachi Y, Kato S, et al. Surgical removal of intra-aortic balloon catheter with fractured nitinol central lumen: a case report. Circ J 2002;66(4):419–22. [DOI] [PubMed]
- 30.Millham FH, Hudson HM, Woodson J, Menzoian JO. Intraaortic balloon pump entrapment. Ann Vasc Surg 1991;5 (4):381–4. [DOI] [PubMed]
- 31.Luengtaviboon K, Signhathanadgige S, Chartlaorng B, Surapongse K. Intraaortic balloon entrapment–a rare complication of intraaortic balloon pump. J Med Assoc Thai 2002; 85 Suppl 1:S153–5. [PubMed]
- 32.Kirksey L, Woody DJ, Plazk L. Ruptured intra-aortic balloon pump. A case report. J Cardiovasc Surg (Torino) 2002;43(4):461–4. [PubMed]
- 33.Nishizawa J, Konishi Y, Matsumoto M, Yuasa S. Intraaortic balloon entrapment–a case report and a review of the literature. Jpn Circ J 1991;55(6):563–6. [DOI] [PubMed]
- 34.Grande AM, Martinelli L, Graffigna A, Vigano M. Retained intraaortic balloon. Case report and review of the literature. Tex Heart Inst J 1995;22(4):332–4. [PMC free article] [PubMed]
- 35.Uwabe K, Kurihara H, Koyanagi H. Intra-aortic balloon entrapment in the descending aorta. J Cardiovasc Surg (Torino) 2007;48(2):255–6. [PubMed]
- 36.Warrillow SJ, Fealy NG, Houston SA. Intra-aortic balloon pump microperforation resulting in balloon entrapment. Anaesth Intensive Care 2008;36(6):916–7. [PubMed]
- 37.Brodell GK, Tuzcu EM, Weiss SJ, Simpfendorfer C. Intra-aortic balloon-pump rupture and entrapment. Cleve Clin J Med 1989;56(7):740–2. [DOI] [PubMed]
- 38.Ferguson JJ 3rd, Cohen M, Freedman RJ Jr, Stone GW, Miller MF, Joseph DL, Ohman EM. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol 2001;38(5):1456–62. [DOI] [PubMed]
- 39.Bahn CH, Vitikainen KJ, Anderson CL, Whitney RB. Vascular evaluation for balloon pumping. Ann Thorac Surg 1979;27(5):474–7. [DOI] [PubMed]
- 40.Rastan AJ, Tillmann E, Subramanian S, Lehmkuhl L, Funkat AK, Leontyev S, et al. Visceral arterial compromise during intra-aortic balloon counterpulsation therapy. Circulation 2010;122(11 Suppl):S92–9. [DOI] [PubMed]


