Abstract
Different institutions have different strategies for managing both native and prosthetic aortic valves in recipients of left ventricular assist devices (LVADs). Anticoagulation protocols and pump-flow algorithms remain nonstandardized. We describe our institutional experience with thrombotic complications and our evolving approach to this important clinical problem. We report the cases of 4 HeartMate II LVAD recipients in whom, despite an anticoagulative regimen, thrombus formed on the noncoronary cusp of the aortic valve. The management of the closed aortic valve in LVAD-supported patients remains problematic.
Key words: Aortic valve/pathology; heart-assist devices/adverse effects; thrombosis; ventricular dysfunction, left
The first implantation of the current clinically approved HeartMate® II Left Ventricular Assist System (Thoratec Corporation; Pleasanton, Calif) was performed at our center in November 2003.1 Currently, the HeartMate II is the world's most widely used implantable left ventricular assist device (LVAD), both for bridging to transplantation and for destination therapy, and our center has had one of the largest single-center experiences with this pump to date. As of August 2012, we had performed 318 implantations of this device. The HeartMate II LVAD frequently works in parallel with the natural heart; as a result, in this operational mode, the aortic valve usually remains in a closed position, and the resulting stagnation of flow in the aortic root may favor thrombus formation. Although the HeartMate II yields better overall results and fewer complications than do pulsatile LVADs,2–4 thrombus on the noncoronary cusp of the aortic valve (which has never occurred in our experience with pulsatile pumps) has occurred in 4 of our patients who have had the HeartMate II implanted. We describe our experience with this unusual complication and its management.
Case Reports
Patient 1
A 61-year-old woman with a history of mucosa-associated lymphoid-tissue lymphoma of the stomach was admitted to our hospital in February 2005 with nonischemic cardiomyopathy (New York Heart Association functional class IV). She was approved for heart transplantation in March 2005. That same month, because of severe heart failure, she received a HeartMate II LVAD and was discharged from the hospital. Approximately one month later, an echocardiogram showed a 0.5 × 0.5-cm thrombus at the base of the noncoronary cusp of her aortic valve. We initiated a heparin infusion, and the LVAD's speed was increased from 8,600 rpm (at which the aortic valve opened) to 9,600 rpm (at which the aortic valve remained closed). The patient remained clinically stable.
However, in May 2005, an echocardiogram showed that the thrombus had grown to a size of 1.2 × 2 cm and filled the noncoronary cusp. The LVAD's speed was maintained at a rate of 9,000 rpm, and the aortic valve was not opening. Later that month, the patient was discharged from the hospital. The next day, she was readmitted with severe diarrhea, confusion, and dehydration. Her international normalized ratio (INR) was 1.0, and her prothrombin time was 11 sec. An echocardiogram showed a decompressed ventricle with arrhythmias and aortic root thrombus. Shortly after admission, the patient had cardiac arrest. Subsequently, ventricular fibrillation was induced and external cardiac massage was performed by an ill-informed shock team, resulting in dislodgment of the thrombus, bilateral cerebral infarctions, and brain death. This was believed to have been induced by the inlet catheter's touching the ventricular septum in the decompressed heart.
Patient 2
In April 2007, a 62-year-old man underwent HeartMate II implantation for end-stage heart failure. The patient had a history of basal-cell carcinoma of the face, polyarteritis nodosa that was in remission, and ischemic cardiomyopathy. He had undergone coronary artery bypass grafting in 1994. In 2002, he underwent a below-the-knee amputation because of peripheral arterial disease. In 2006, he had a percutaneous coronary intervention involving the ostial circumflex artery.
Shortly after admission, the patient's clinical condition further deteriorated, necessitating implantation of the HeartMate II device. Five days after implantation, an echocardiogram showed a 1 × 0.9-cm thrombus in the noncoronary cusp of the aortic valve. The LVAD's speed was 9,000 rpm, and the aortic valve was not opening. Anticoagulation was initiated with a continuous heparin infusion, which was adjusted according to the patient's partial thromboplastin time. He developed chronic renal failure postoperatively and needed continuous venovenous hemodialysis before and after LVAD implantation. In June 2007, his renal function recovered, and he was discharged from the hospital on anticoagulative therapy. In September 2007, echocardiography revealed that the thrombus had enlarged to a size of 1.6 × 1.3 cm. The aortic valve remained closed at pump speeds of 9,800 and 10,800 rpm. In November 2007, the patient again underwent echocardiography in the outpatient clinic, and the thrombus seemed to be stable and immobile. His prothrombin time was 18.9 sec, and his INR was 2.0. He died suddenly at home 3 days later, presumably because of his long-standing coronary disease and ventricular irritability. No autopsy was performed.
Patient 3
In January 2008, a 65-year-old man was admitted with ischemic cardiomyopathy and a history of anterolateral myocardial infarction. Because his left ventricular ejection fraction was only 0.15, an intra-aortic balloon pump and, subsequently, a HeartMate II LVAD were implanted. Left ventricular remodeling was also performed, and a left ventricular clot was removed. In February 2008, the patient's blood cultures were positive for vancomycin-resistant enterococci; he had a long hospitalization and was treated for LVAD infection. In September 2008, he was hospitalized for episodes of gastrointestinal bleeding from duodenal arteriovenous malformations.
In December 2008, the patient was admitted to the hospital with dyspnea, at which time his INR was 2.1. A chest radiograph showed mild pulmonary edema that was in part secondary to congestive heart failure. Echocardiography revealed an aortic valve thrombus and aortic insufficiency (Fig. 1A). He was taken to the operating room, where the aortic valve thrombus was removed and the aortic valve was oversewn with a double layer of bovine pericardium. Subsequently, the HeartMate II functioned normally, and the patient's heart failure resolved. He was discharged from the hospital in April 2009 and was listed for heart transplantation. In June 2009, the patient underwent orthotopic heart transplantation, during which the aortic valve appeared thrombus-free and remained oversewn with bovine pericardium in place (Fig. 1B). He recovered uneventfully and remained well.
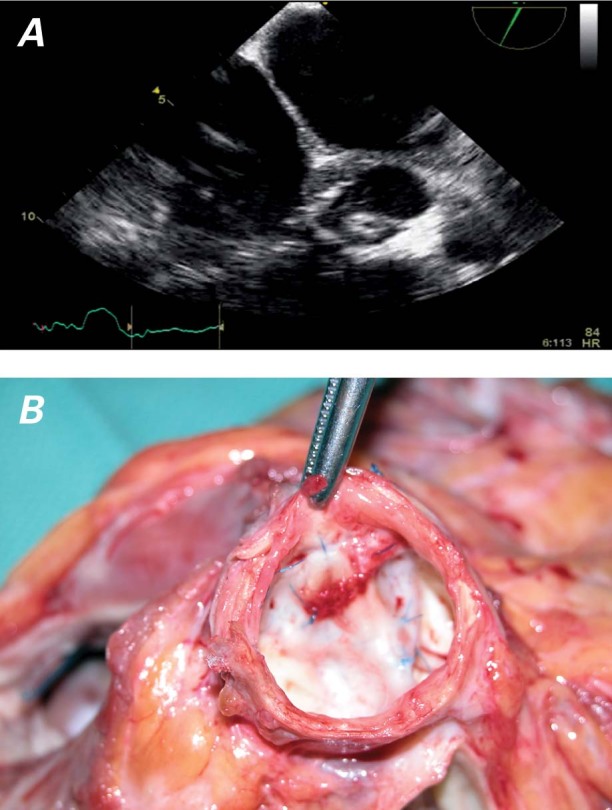
Fig. 1 Patient 3. Thrombus on the aortic valve, shown A) on an echocardiogram and B) at transplantation, at which time the aortic valve of the explanted heart was still oversewn with bovine pericardium. No thrombus formation was seen.
Patient 4
In June 2009, a 50-year-old man was admitted to our hospital with a history of familial cardiomyopathy and coronary artery disease, for which 2 stents had been implanted. He had previously been hospitalized elsewhere for acute decompensated heart failure and a left ventricular ejection fraction of 0.10 to 0.15. At that time, he developed acute tubular necrosis and underwent intermittent hemodialysis. Cardiogenic shock necessitated the implantation of an Impella® 2.5 catheter-based cardiac assist device (Abiomed, Inc.; Danvers, Mass).
Shortly after the patient's admission to our hospital, the Impella device was removed and a HeartMate II LVAD was implanted. Follow-up echocardiography showed a 1.1 × 0.3-cm thrombus that occupied a large part of the noncoronary cusp of the aortic valve (Fig. 2A). The patient had a partial thromboplastin time of 48.6 sec, a fibrinogen level of 640 mg/dL, a prothrombin time of 11.4 sec, and an INR of 1.1. He received a continuous heparin infusion and was listed for heart transplantation. His LVAD speed remained at 9,000 rpm, and his aortic valve was not opening. In July 2009, an orthotopic heart transplantation was performed; the thrombus on the noncoronary aortic valve cusp of the explanted heart was 2.5 × 1.3 cm in size (Fig. 2B). The patient was discharged from the hospital and remained well.
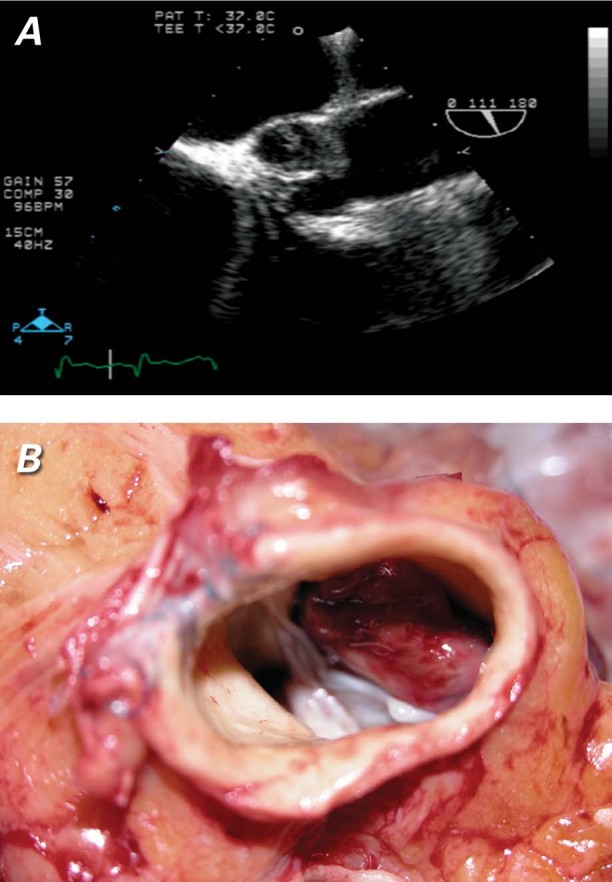
Fig. 2 Patient 4. Thrombus on the aortic valve, shown A) on an echocardiogram and B) at transplantation. The explanted heart had thrombus on the noncoronary cusp of the aortic valve.
Discussion
For patients awaiting heart transplantation, a continuous-flow LVAD such as the HeartMate II can provide effective hemodynamic support; compared with pulsatile LVADs, continuous-flow pumps substantially improve the probability of survival free of stroke and advanced heart failure. However, the new physiology introduced by constant flow throughout the cardiac cycle introduces unique complications. For example, the constant active pressure may result in persistent aortic valve closure. In addition to leaflet fusion and aortic insufficiency, stasis in the noncoronary sinus may predispose patients to thrombus formation.
The native aortic valve is not accustomed to the constant pressure to which it is subjected during LVAD support when the LVAD is parallel with the heart. Our patients were monitored monthly, and the level of LVAD support (the pump speed) was reduced stepwise by 1,000 rpm per visit. The end-diastolic dimension, the degree of mitral regurgitation, and the aortic valve opening times were analyzed after each such reduction. In this fashion, the pump speed was gradually reduced to a level that would enable control of the mitral regurgitation and aortic valve opening times. Adjusting the pump speed and enabling the valve to open intermittently might prevent leaflet fusion and thrombus formation. In a previous report of our experience with acquired commissural fusion of the aortic valves in LVAD recipients, we stated that this detrimental effect can be lessened by carefully controlling the systemic blood pressure and decreasing the pump rate to enable the pump to function in parallel with the native heart. This acts to prevent not only commissural fusion but also thrombus formation on the immobile aortic valve.5,6
Footnotes
Address for reprints: O.H. Frazier, MD, MC 3-147, Texas Heart Institute, P.O. Box 20345, Houston, TX 77225-0345
E-mail: lschwenke@texasheart.org
Dr. Demirozu is now at Istanbul Florence Nightingale Hospital, Istanbul, Turkey.
References
- 1.Frazier OH, Delgado RM 3rd, Kar B, Patel V, Gregoric ID, Myers TJ. First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report [published erratum appears in Tex Heart Inst J 2004;31(3): 333]. Tex Heart Inst J 2004;31(2):157–9. [PMC free article] [PubMed]
- 2.John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg 2008;86(4):1227–35. [DOI] [PubMed]
- 3.Rao V, Slater JP, Edwards NM, Naka Y, Oz MC. Surgical management of valvular disease in patients requiring left ventricular assist device support. Ann Thorac Surg 2001;71(5):1448–53. [DOI] [PubMed]
- 4.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361(23):2241–51. [DOI] [PubMed]
- 5.Connelly JH, Abrams J, Klima T, Vaughn WK, Frazier OH. Acquired commissural fusion of aortic valves in patients with left ventricular assist devices. J Heart Lung Transplant 2003; 22(12):1291–5. [DOI] [PubMed]
- 6.Letsou GV, Connelly JH, Delgado RM 3rd, Myers TJ, Gregoric ID, Smart FW, Frazier OH. Is native aortic valve commissural fusion in patients with long-term left ventricular assist devices associated with clinically important aortic insufficiency? J Heart Lung Transplant 2006;25(4):395–9. [DOI] [PubMed]


