Abstract
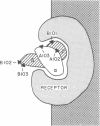
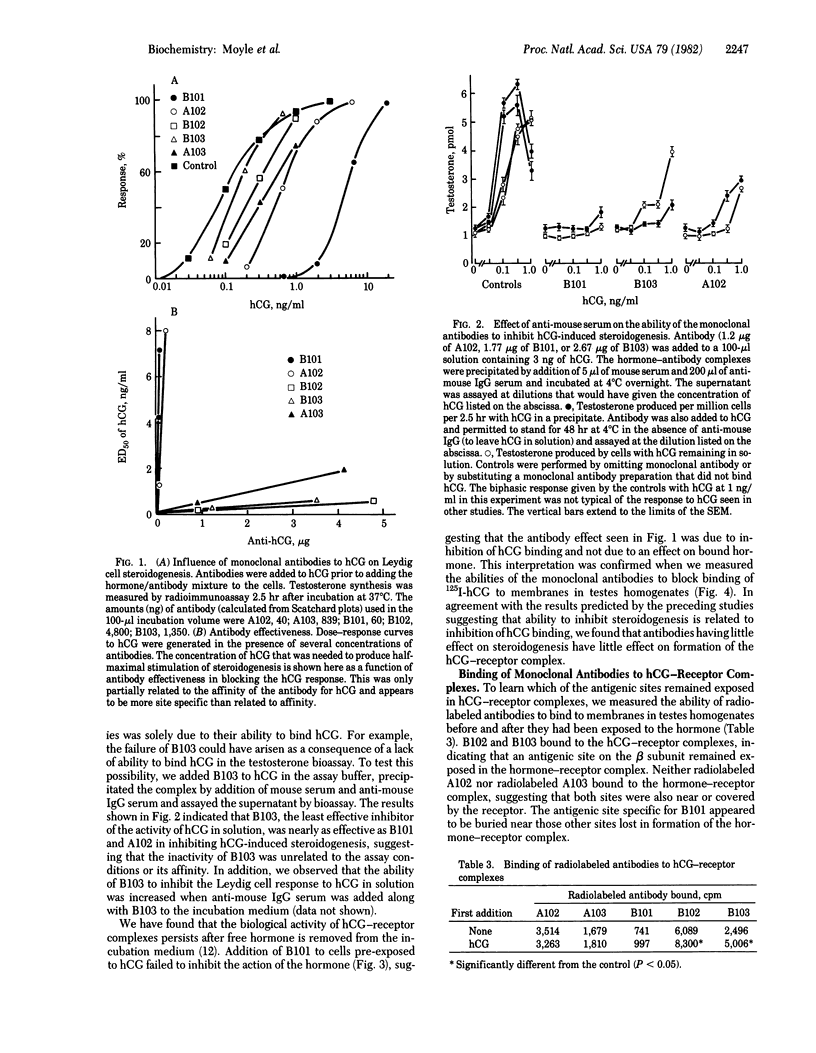
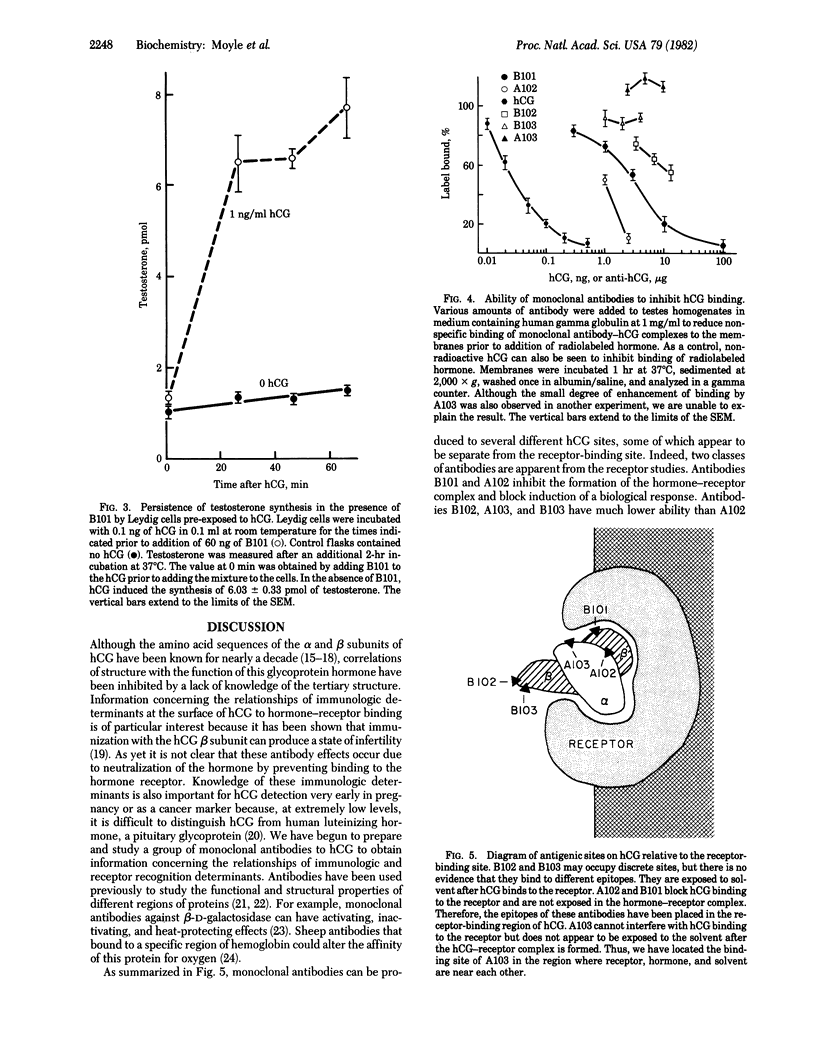
Monoclonal antibodies were prepared against the alpha and beta subunits of human chorionic gonadotropin (hCG). Although all were selected on the basis of their ability to bind the intact hormone, each also bound one of the two subunits but not both. Using a solid phase double antibody system to measure the relative binding to sites on the surface of hCG, we observed that four of the five antibodies bound to different sites on the molecule. This information was correlated with the ability of each antibody to inhibit the biological activity of hCG. Of the five antibodies tested for their ability to inhibit hCG-induced stimulation of rat testes steroidogenesis in vitro, two proved to be potent inhibitors, whereas the other three had almost no effect. This inhibition of steroidogenesis was highly correlated with the ability of the antibodies to inhibit hCG binding to testes homogenates. Thus, we have begun to derive a scheme that describes the relative binding positions of individual monoclonal antibodies and receptor on hCG. The purified monoclonal antibodies were iodinated and employed to evaluate which antigenic sites on hCG remained free in hCG-receptor complexes. The data indicated that portions of the beta subunit in hCG-receptor complexes were buried (i.e., failed to bind radiolabeled antibody), whereas other portions remained exposed (i.e., they bound radiolabeled antibody). Those antibodies that interacted with portions of hCG that became inaccessible in the receptor complex also blocked the biological actions of hCG, whereas those that interacted with exposed sites had little or no effect on activity. Although we did not find antibodies to the alpha subunit that would bind to the hormone-receptor complex, we found that one of the two antibodies specific to alpha subunit epitopes blocked the actions of the hormone. Both antigenic determinants on the alpha subunit appeared to be lost after the hCG-receptor complex had formed. These studies suggest that each hCG subunit participates in the hormone-receptor complex and that portions of the beta subunit project from the surface of the receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloj S. M., Edelhoch H., Ingham K. C., Morgan F. J., Canfield R. E., Ross G. T. The rates of dissociation and reassociation of the subunits of human chorionic gonadotropin. Arch Biochem Biophys. 1973 Nov;159(1):497–504. doi: 10.1016/0003-9861(73)90480-3. [DOI] [PubMed] [Google Scholar]
- Ascoli M., Puett D. Degradation of receptor-bound human choriogonadotropin by murine Leydig tumor cells. J Biol Chem. 1978 Jul 25;253(14):4892–4899. [PubMed] [Google Scholar]
- Bellisario R., Carlsen R. B., Bahl O. P. Human chorionic gonadotropin. Linear amino acid sequence of the alpha subunit. J Biol Chem. 1973 Oct 10;248(19):6796–6809. [PubMed] [Google Scholar]
- Canfield R. E., Morgan F. J., Kammerman S., Bell J. J., Agosto G. M. Studies of human chorionic gonadotropin. Recent Prog Horm Res. 1971;27:121–164. doi: 10.1016/b978-0-12-571127-2.50028-6. [DOI] [PubMed] [Google Scholar]
- Carlsen R. B., Bahl O. P., Swaminathan N. Human chorionic gonadotropin. Linear amino acid sequence of the beta subunit. J Biol Chem. 1973 Oct 10;248(19):6810–6827. [PubMed] [Google Scholar]
- Carlsen R. B., Bahl O. P. The reaction of tetranitromethane with human chorionic gonadotropin. Arch Biochem Biophys. 1976 Jul;175(1):209–220. doi: 10.1016/0003-9861(76)90501-4. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L., Tsuruhara T. Absence of intrinsic biological activity in LH and hCG subunits. J Clin Endocrinol Metab. 1973 Jan;36(1):73–80. doi: 10.1210/jcem-36-1-73. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Conti M., Harwood J. P., Dufau M. L., Catt K. J. Internalisation of gonadotrophin--receptor complex in ovarian luteal cells. Nature. 1978 Aug 10;274(5671):598–600. doi: 10.1038/274598a0. [DOI] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Conformation-specific antibodies to the alpha chain COOH terminus of hemoglobin A0. J Biol Chem. 1979 Sep 25;254(18):9185–9193. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Rotman B. Functional diversity of antibodies elicited by bacterial beta-D-galactosidase. Monoclonal activating, inactivating, protecting, and null antibodies to normal enzyme. J Biol Chem. 1980 Jun 10;255(11):5286–5290. [PubMed] [Google Scholar]
- Furie B., Schechter A. N., Sachs D. H., Anfinsen C. B. An immunological approach to the conformational equilibrium of staphylococcal nuclease. J Mol Biol. 1975 Mar 15;92(4):497–506. doi: 10.1016/0022-2836(75)90305-8. [DOI] [PubMed] [Google Scholar]
- Ingham K. C., Tylenda C., Edelhoch H. Structural studies of human chorionic gonadotropin and its subunits using tyrosine fluorescence. Arch Biochem Biophys. 1976 Apr;173(2):680–690. doi: 10.1016/0003-9861(76)90306-4. [DOI] [PubMed] [Google Scholar]
- Ji I., Ji T. H. Macromolecular photoaffinity labeling of the lutropin receptor on granulosa cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7167–7170. doi: 10.1073/pnas.77.12.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet J. P., Ross G. T., Birken S., Canfield R. E. Absence of neutralizing effect of antisera to the unique structural region of human chorionic gonadotropin. J Clin Endocrinol Metab. 1974 Dec;39(6):1155–1158. doi: 10.1210/jcem-39-6-1155. [DOI] [PubMed] [Google Scholar]
- Morgan F. J., Birken S., Canfield R. E. Letter: Human chorionic gonadotropin: a proposal for the amino acid sequence. Mol Cell Biochem. 1973 Nov 15;2(1):97–99. doi: 10.1007/BF01738683. [DOI] [PubMed] [Google Scholar]
- Morgan F. J., Birken S., Canfield R. E. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975 Jul 10;250(13):5247–5258. [PubMed] [Google Scholar]
- Morgan F. J., Canfield R. E. Nature of the subunits of human chorionic gonadotropin. Endocrinology. 1971 Apr;88(4):1045–1053. doi: 10.1210/endo-88-4-1045. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Bahl O. P., März L. Role of carbohydrate of human chorionic gonadotropin in the mechanism of hormone action. J Biol Chem. 1975 Dec 10;250(23):9163–9169. [PubMed] [Google Scholar]
- Moyle W. R., Netburn M., Cosgrove A. E., Krieger J., Bahl O. P. hCG-Leydig cell functional binding kinetics: threshold and nonlinearity in response. Am J Physiol. 1980 Mar;238(3):E293–E302. doi: 10.1152/ajpendo.1980.238.3.E293. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J. L., Braunstein G. D., Ross G. T. A radioimmunoassay which specifically measures human chorionic gonadotropin in the presence of human luteinizing hormone. Am J Obstet Gynecol. 1972 Jul 15;113(6):751–758. doi: 10.1016/0002-9378(72)90553-4. [DOI] [PubMed] [Google Scholar]
- Wands J. R., Zurawski V. R., Jr High affinity monoclonal antibodies to hepatitis B surface antigen (HBsAg) produced by somatic cell hybrids. Gastroenterology. 1981 Feb;80(2):225–232. [PubMed] [Google Scholar]