Abstract
A patient with dilated cardiomyopathy and no history of thromboembolic events received a surgically implanted axial-flow left ventricular assist device. After implantation, transesophageal echocardiography revealed a giant thrombus on the lateral and anterior aspects of the left ventricle. The inflow cannula inserted through the apex of the left ventricle was not obstructed, and the device generated satisfactory blood flow. Laboratory screening for thrombophilia showed protein S deficiency, heterozygous factor V Leiden mutation, and heterozygous MTHFR C667T mutation. During the entire duration of circulatory support, no significant suction events were detected, and the patient was listed for heart transplantation. Ventricular assist device implantation can unmask previously undiagnosed thrombophilia; therefore, it should be necessary to identify thrombophilic patients before cardiac support implantation.
Key words: Anticoagulants/therapeutic use, blood coagulation disorders/complications, factor V/genetics, factor V Leiden, left ventricular assist device, perioperative care, postoperative complications, thrombophilia/genetics, thrombus/etiology/prevention & control
Thrombophilia can be defined as a predisposition to form clots inappropriately. Especially in cardiac surgical patients, thrombophilia can cause severe complications. Moreover, the presence of artificial materials in the circulatory system renders prothrombotic disease even more dangerous. Currently, there is no published information about patients with factor V Leiden mutation who have undergone the implantation of mechanical circulatory support devices.
Case Report
We present a case of a 53-year-old man with dilated cardiomyopathy who was admitted to our hospital in severe heart failure: his left ventricular ejection fraction was <0.20 despite maximal pharmacologic support. The urgent implantation of a left ventricular assist device (LVAD)—in this case, a HeartMate® II Left Ventricular Assist System (Thoratec Corporation; Pleasanton, Calif)—was indicated. The patient had no history of thromboembolic events, and perioperative transesophageal echocardiography (TEE) did not show any thrombus in the cardiac chambers. Because of unsatisfactory coagulation values and bleeding after the procedure, no anticoagulation therapy was given and sternal closure was delayed until postoperative day 2.
On day 2, TEE revealed a giant thrombus (longitudinal diameter, 55 mm; maximal transverse diameter in the central part, 33 mm) on the lateral and anterior aspects of the left ventricle (Figs. 1 and 2). Nevertheless, the LVAD inflow cannula inserted through the apex of the left ventricle was not obstructed, and the assist device generated satisfactory blood flow (Figs. 3 and 4). Calculated flow ranged between 6.0 and 7.0 L/min. To minimize the risk of embolism of the thrombus, the speed of the LVAD was set in such a manner that the aortic valve was not allowed to open. Subsequent laboratory screening for a thrombophilic state showed protein S deficiency and heterozygosity for factor V Leiden and MTHFR C667T mutations. Combined anticoagulation therapy was initiated via the continuous administration of heparin and warfarin. The heparin was stopped 18 days after definitive chest closure, when the thrombus size had not changed. The international normalized ratio (INR) was then maintained in the range of 2.5 to 3.0 throughout mechanical support.
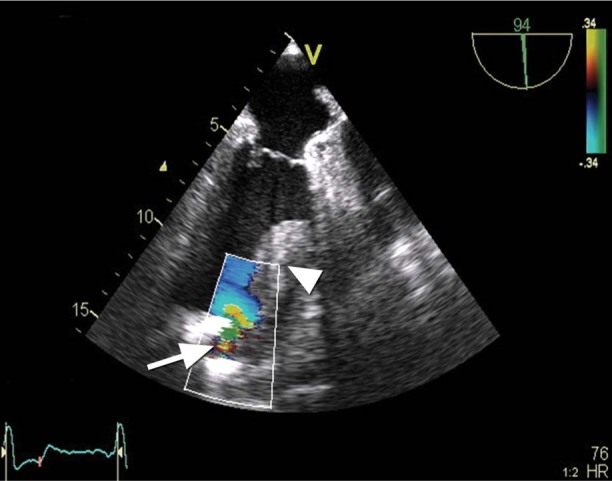
Fig. 1 Transesophageal echocardiogram (apical long-axis view with color-flow Doppler) shows some flow acceleration near the left ventricular assist device (LVAD) inflow cannula (arrow). Note also the adjacent thrombus (arrowhead).
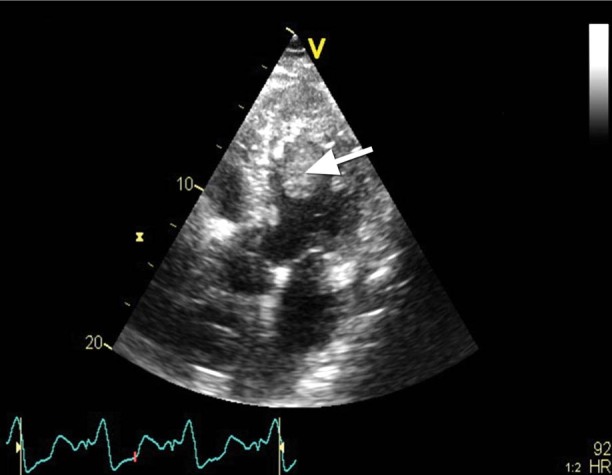
Fig. 2 Transesophageal echocardiogram (apical 5-chamber view) shows the left ventricular thrombus (arrow).
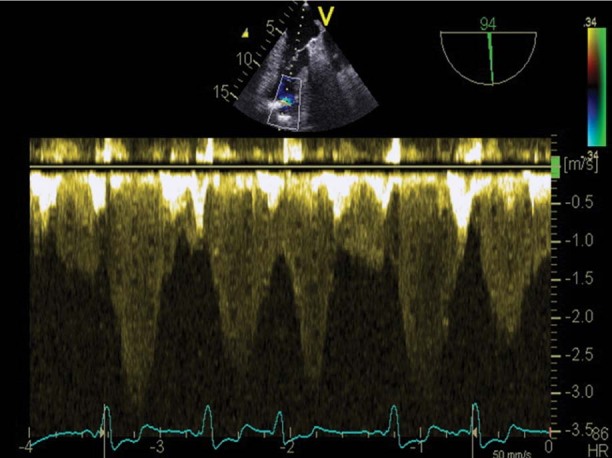
Fig. 3 Transesophageal echocardiogram (pulsed Doppler) shows the normal phasic flow pattern of the left ventricular assist device's inflow cannula, but at increased velocity (3 m/s), probably because of adjacent flow in the absence of cannula thrombosis.
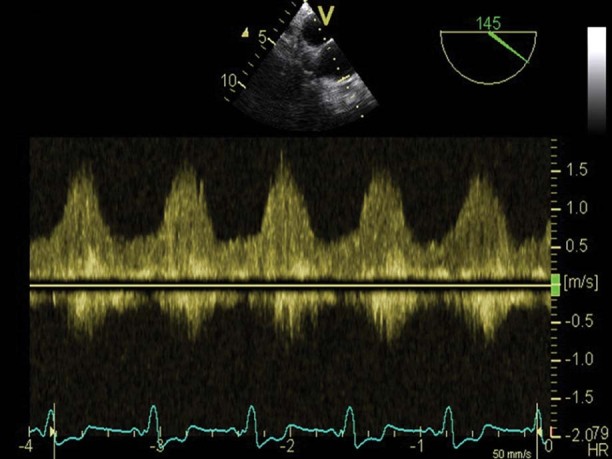
Fig. 4 Transesophageal echocardiogram (pulsed Doppler) focuses on the anastomosis of the left ventricular assist device's outlet cannula to the ascending aorta. Note the typical continuous but highly phasic flow pattern of lower peak systolic velocity (1.5 m/s) in the conduit, which implies the absence of a fixed internal obstruction.
During postoperative follow-up, the patient underwent transthoracic echocardiography once a week. This revealed the constant size of the thrombus. Throughout the entire duration of circulatory support, no significant LVAD suction events were detected and pump power consumption remained in the normal range (7–9.5 W, at a pump speed of 10,400 rpm). The patient was listed as a candidate for heart transplantation. We did not observe any signs of hemolysis, and the bilirubin levels throughout postoperative follow-up were within normal range; therefore, we did not measure the lactate dehydrogenase and plasma free-hemoglobin levels.
On postoperative day 170, drainage from the lower part of the sternotomy incision was noted. The patient had no signs of active systemic infection; however, cultures were positive for Enterococcus faecalis. We decided to explore the sternotomy wound and pump pocket in the operating room. After extensive débridement of the infected device pocket, the vacuum-assisted wound-closure system—V.A.C.® Therapy system (KCI Concepts, Inc.; San Antonio, Texas)—was applied around the pump and systemic antibiotics were given. Unfortunately, we did not achieve satisfactory healing of the device pocket infection. Despite long-term V.A.C. and antibiotic therapy, the patient died of sepsis on day 282 after HeartMate II implantation. Throughout the postoperative period, no clinical signs of embolism were observed. Autopsy revealed a large, well-organized thrombus adhering to the left myocardium (Fig. 5).
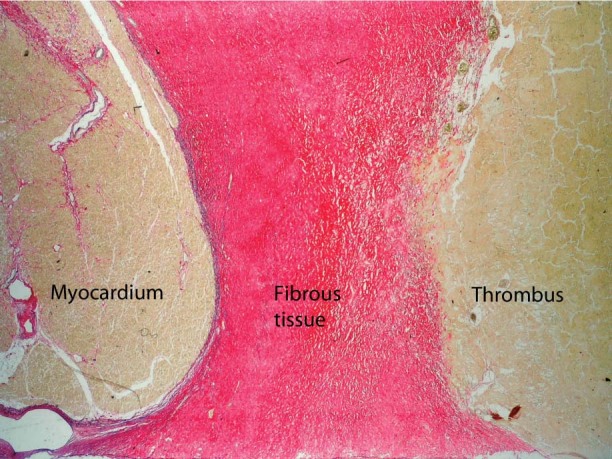
Fig. 5 Photomicrograph of transverse section of left myocardium shows the myocardium, fibrous tissue of the endocardium, and parietal thrombus (Sirius red with elastica, orig. ×40).
Discussion
Surgery and immobilization are themselves triggers for thromboembolic events in otherwise healthy and asymptomatic patients with hereditary thrombophilia. Especially in cardiac surgical patients, thrombophilia can cause severe complications. The potential major thromboembolic events in these patients include pulmonary embolism, graft occlusion, deep venous embolism, and coagulation of the extracorporeal circuit.1,2
Factor V Leiden mutation is the most common cause of thrombophilia. In Caucasians, the frequency of this mutation is relatively high—from 2% to 15% in the general population and up to 50% in selected patients with venous thromboembolism.3 The influence of the heterozygous factor V Leiden mutation in patients undergoing cardiac surgery is still under discussion.4,5 In a 2009 study, Massoudy and colleagues6 showed a high incidence of perioperative and postoperative thromboembolic events in patients with symptomatic heterozygous factor V Leiden mutation. Nevertheless, there is no information about patients with factor V Leiden mutation who have undergone the implantation of mechanical circulatory support devices; one can only assume that prothrombotic disease is very dangerous in these patients.
Because the frequency of thromboembolic events in HeartMate II patients is extremely low, the general recommendation for a target INR is 1.5 to 2.5; some centers add aspirin therapy.7 Although 40% of ischemic strokes in HeartMate II patients occurred in those with an INR <1.5, 33% of hemorrhagic strokes were in patients with an INR >3.0. The highest incidence of bleeding was observed in patients with an INR >2.5. Nevertheless, the management approach to patients with factor V Leiden mutation should be different during cardiac surgery. Massoudy and colleagues6 reviewed the records of patients with symptomatic factor V Leiden mutation who had undergone coronary artery bypass grafting and concluded that early postoperative reuptake of phenprocoumon, or even continued anticoagulation throughout surgery, is safe and might reduce the incidence of perioperative thromboembolic events. We are convinced that the optimal postoperative anticoagulation approach in these patients is to bridge with continuous heparin as soon as possible after implantation and maintain a target INR of 2.5 to 3.0 for the duration of mechanical circulatory support.
In conclusion, we believe this to be the first report describing the implantation of a continuous-flow LVAD and an associated perioperative thromboembolic complication in a patient with factor V Leiden mutation. We suggest that thrombophilic patients be identified before assist device implantation and that more aggressive anticoagulation become standard during the support of such patients. The optimal regimen, however, must be defined in a larger series of patients.
Footnotes
Address for reprints: Ondrej Szarszoi, MD, PhD, Department of Cardiovascular Surgery, Institute for Clinical and Experimental Medicine, Vídenska 1958/9, 140 21 Prague 4, Czech Republic
E-mail: onsz@medicon.cz
References
- 1.Massoudy P, Cetin SM, Thielmann M, Kienbaum P, Piotrowski JA, Marggraf G, et al. Antiphospholipid syndrome in cardiac surgery-an underestimated coagulation disorder? Eur J Cardiothorac Surg 2005;28(1):133–7. [DOI] [PubMed]
- 2.Varela ML, Adamczuk YP, Martinuzzo ME, Forastiero RR, Klein FR, Rossi AS, Carreras LO. Early occlusion of coronary by-pass associated with the presence of factor V Leiden and the prothrombin 20210A allele: case report. Blood Coagul Fibrinolysis 1999;10(7):443–6. [DOI] [PubMed]
- 3.Martinelli I. Risk factors in venous thromboembolism. Thromb Haemost 2001;86(1):395–403. [PubMed]
- 4.Donahue BS. The response to activated protein C after cardiopulmonary bypass: impact of factor V Leiden. Anesth Analg 2004;99(6):1598–603. [DOI] [PubMed]
- 5.Moor E, Silveira A, van't Hooft F, Tornvall P, Blomback M, Wiman B, et al. Coagulation factor V (Arg506–>Gln) mutation and early saphenous vein graft occlusion after coronary artery bypass grafting. Thromb Haemost 1998;80(2):220–4. [PubMed]
- 6.Massoudy P, Thielmann M, Muller-Beissenhirtz H, Gorlinger K, Dietrich W, Herget-Rosenthal S, Jakob H. Thrombophilia in cardiac surgery-patients with symptomatic factor V Leiden. J Card Surg 2009;24(4):379–82. [DOI] [PubMed]
- 7.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant 2009;28(9):881–7. [DOI] [PubMed]


