Abstract
Papillary fibroelastoma is a rare, benign cardiac tumor typically found on the heart valves. It is usually discovered incidentally on echocardiography. The clinical presentation of cardiac papillary fibroelastoma varies from no symptoms to severe embolic sequelae. We report the incidental finding of papillary fibroelastoma in 2 patients. In each, we chose to excise the tumor. The relevant medical literature provides little guidance regarding whether to excise a small papillary fibroelastoma in an asymptomatic patient. Multimodal imaging, which we discuss in the context of our patients' cases, aids the cardiologist and cardiovascular surgeon in more accurately evaluating papillary fibroelastoma preoperatively.
Key words: Chordae tendineae/pathology/ultrasonography; echocardiography; fibroma/epidemiology/pathology/surgery; heart neoplasms/diagnosis/epidemiology/pathology/surgery/ultrasonography; heart valves/pathology; incidental findings; tomography, x-ray computed
Papillary fibroelastoma is the third most common primary tumor, and it usually involves the cardiac valves. It can be asymptomatic, or it can cause major thromboembolic sequelae. Factors in deciding to proceed with surgery can be complex, because of difficulties in evaluating the tumor and predicting the patient's prognosis. Multimodal imaging enables a more accurate evaluation of papillary fibroelastoma. We report our incidental discovery of papillary fibroelastoma in 2 patients, discuss the role of multimodal imaging in our surgical decisions, and review ideas for managing these tumors in asymptomatic patients.
Case Reports
Patient 1
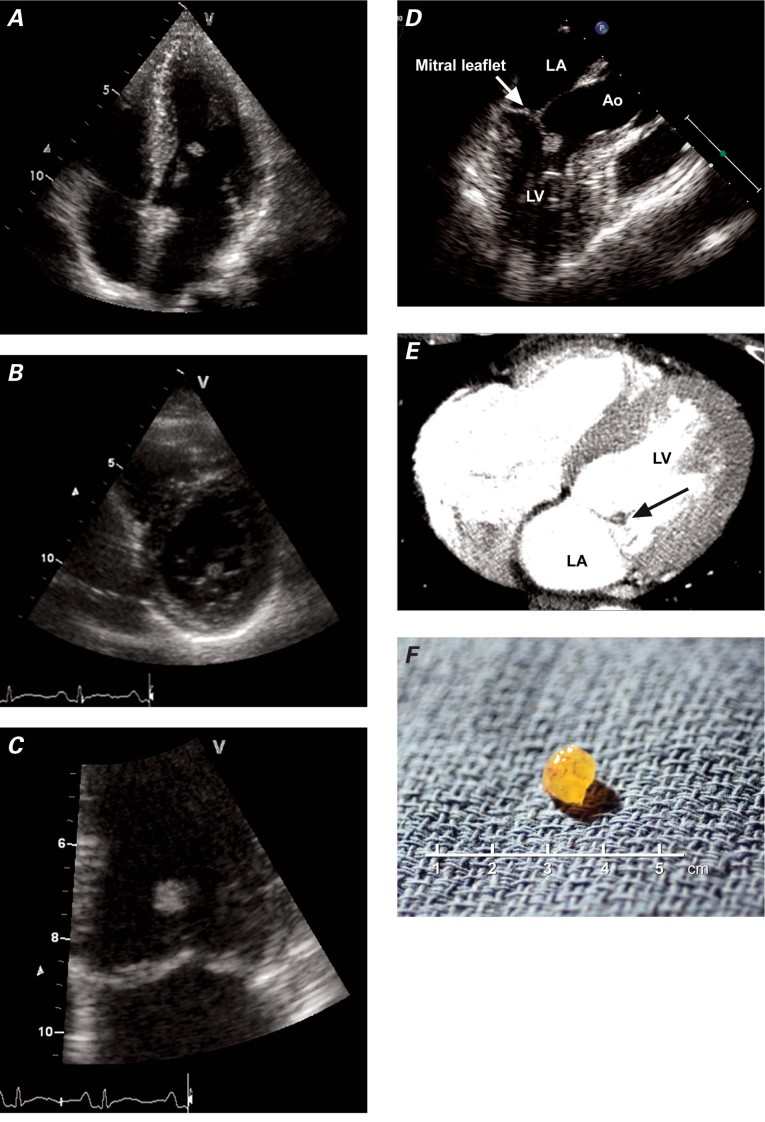
In August 2010, a 45-year-old asymptomatic woman with the human immunodeficiency virus was referred to our department to undergo routine echocardiographic screening for the early detection of pulmonary hypertension. The patient had been taking triple anti-retroviral medications for several years. Her medical history also included hypertension, chronic hepatitis C, and the excision of a cerebral aneurysm. At this presentation, her arterial blood pressure was 136/98 mmHg. Auscultation revealed no murmur or extra sound, an electrocardiogram showed sinus rhythm, and results of routine blood tests were normal. On transthoracic echocardiography (TTE), the patient's left ventricular function was normal, and no pulmonary hypertension was noted. However, a mass was detected near the edge of the anterior mitral leaflet (Fig. 1A–C). Transesophageal echocardiography (TEE) revealed a highly mobile 5.6 × 8-mm mass attached to the anterior mitral leaflet chordae tendineae, without significant mitral regurgitation or stenosis (Fig. 1D). Thoracic computed tomography (CT), performed to screen for coronary disease, excluded significant coronary stenosis but confirmed the presence of a round valvular mitral mass (Fig. 1E).
Fig. 1 Patient 1. Two-dimensional transthoracic echocardiograms show an oval mass attached to the anterior leaflet of the mitral valve in A) 4-chamber apical view, B) short-axis view at the level of the mitral leaflet, and C) apical view. D) Preoperative transesophageal echocardiogram shows the mass attached to the anterior mitral chordae tendineae (arrow). E) Contrast computed tomogram shows a filling defect (tumor) at the anterior leaflet of the mitral valve (arrow). F) Photograph shows the resected tumor.
Ao = aorta; LA = left atrium; LV = left ventricle
We thought that the patient had a substantial clinical likelihood of embolism because of the size of the fibroelastoma, so surgery was scheduled. After a limited sternotomy and a transaortic approach, the tumor (Fig. 1F) was seen to be attached to one of the secondary chordae of the anterior mitral leaflet. The mass was resected with preservation of the chordae. Histologic analysis revealed a typical fibroelastoma with branching papillary fronds, a central avascular and sparsely cellular collagenous core surrounded by myxoid tissue, and a lining of hyperplastic endothelial cells. Three months after surgery, the patient was doing well and had not experienced any embolic event.
Patient 2
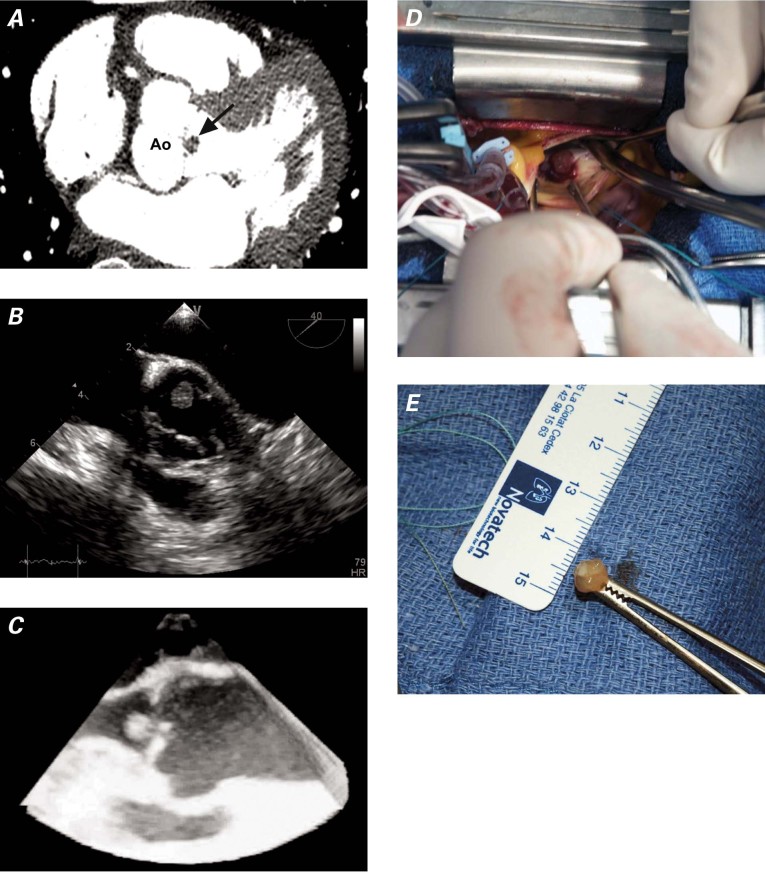
In February 2011, a 63-year-old woman was admitted for evaluation of anterior chest pain. Her medical history was notable only for smoking. The pain was mild, compressive, and not associated with effort. On admission, the patient was in no physical distress. Results of physical examination were within normal ranges. An electrocardiogram showed sinus rhythm of 70 beats/min with no ST-T-wave abnormalities, and results of chest radiography were within normal limits. Routine blood tests, including troponin, yielded normal results, and a stress test was inconclusive. A contrast-enhanced CT scan of the chest showed a mobile, 7-mm lesion attached to the noncoronary aortic valve leaflet, without coronary artery lesions (Fig. 2A). Subsequently, TTE and TEE both revealed a mobile mass, 10 × 8 mm in size, attached to the aortic valve. The tumor was not pedunculated (Figs. 2B and 2C), and other cardiac structures were normal. Contrast echocardiography was used to better delineate the endocardial borders. Because of the location and gross appearance of the tumor, the presumptive diagnosis was papillary fibroelastoma. The likelihood of embolism was high, so surgical resection was performed (Fig. 2D). The tumor was excised (Fig. 2E), and the histologic findings were similar to those for Patient 1 and were consistent with papillary fibroelastoma. After discharge from the hospital, the patient was asymptomatic and doing well.
Fig. 2 Patient 2. A) Multislice computed tomogram shows a papillary fibroelastoma of the aortic valve (arrow). B) Transesophageal echocardiogram (short-axis view) reveals an aortic valve papillary fibroelastoma. C) Three-dimensional transesophageal echocardiogram (longitudinal view) of the aortic valve shows the fibroelastoma attached to the noncoronary aortic valve leaflet. D) Operative photograph of the fibroelastoma. E) Gross specimen of the resected mass. Ao = aorta
Discussion
Primary tumors of the heart are rare, ranging from 0.002% to 0.02% in prevalence, according to large autopsy series.1,2 Papillary fibroelastoma is the third most common primary cardiac tumor (after myxoma and lipoma), accounting for 7% to 9% of benign primary tumors.1–3 Fibroelastoma usually involves the aortic or mitral valve, and less frequently the pulmonary or tricuspid valve. It can also develop from the papillary muscles, chordae tendineae, ventricular septum, or endocardial surface.3 Men and persons older than 40 years of age are chief among those who are diagnosed with papillary fibroelastoma; however, this tumor has also been described in neonates with congenital cardiac abnormalities. The histogenesis of papillary fibroelastoma is still unclear, with several theories about its origin. Some consider these tumors to be neoplasms or hamartomas, whereas others believe them to be endocardial responses to infection or hemodynamic trauma.2
Most patients with this tumor are asymptomatic. Clinical presentation varies, depending upon which side of the heart is involved. Right-side papillary fibroelastomas are asymptomatic and rarely cause pulmonary embolism. Conversely, left-side fibroelastomas can cause life-threatening sequelae such as transient ischemic attacks or stroke, myocardial infarction, mesenteric ischemia, renal infarction, or limb ischemia. In younger individuals, sudden death has occurred from coronary obstruction due to emboli from the aortic valve. Embolism is due to the brittle nature of the tumor and to the tendency for easy platelet aggregation.2
Diagnosis is usually made with use of echocardiography. In patients with a cardiac mass, the echocardiographic differential diagnosis includes myxoma, vegetations, and mural thrombus. Features that distinguish papillary fibroelastoma from other intracardiac masses are suggestive, rather than definitive: a small lesion less than 1 cm in diameter, but perhaps as large as 3 to 4 cm; and a highly mobile mass, with a pedicle attached to the valve or endocardium. In the detection of papillary fibroelastoma, the sensitivity and specificity of TTE are 88.9% and 87.8%, respectively, when the tumor is larger than 2 mm. However, when the tumor is smaller, the overall sensitivity of TTE is only 61.9%, compared with 76.6% for TEE.2–4 Newer imaging techniques, such as contrast echocardiography, can help to better delineate cardiac masses. Three-dimensional echocardiography has a role in routine echocardiographic evaluation5 and appears to be useful in characterizing papillary fibroelastomas, yielding a comprehensive approach to the lesion and the facilitation of operative planning. On CT, small papillary fibroelastomas are more difficult to see, because they move quickly with the valves. However, since the introduction of multislice scanners, CT has become equivalent to echocardiography in the depiction of small moving structures. On CT,6 papillary fibroelastoma may appear as a small homogeneous mass attached to a cardiac valve, sometimes with a small pedicle. The increasingly common clinical use of cardiac CT could more frequently reveal papillary fibroelastoma in the future. However, tachycardia and arrhythmias can severely degrade image quality, and CT does not enable true real-time imaging. Multimodal imaging affords the cardiologist and cardiovascular surgeon the capability of more accurately evaluating papillary fibroelastoma preoperatively.
The optimal management of incidental papillary fibroelastoma, especially when the tumor is small, left-sided, and has no stalk, is unknown; the lack of clear indications for surgery makes treatment decisions difficult. At present, it is generally agreed that excision should be performed in symptomatic patients. Decisions to proceed with surgery should take into account the presence and severity of symptoms and the risks of life-threatening sequelae, such as embolism. The exact role of the morphologic features of papillary fibroelastoma (such as mobility, size, and valvular involvement) remains undetermined in the prediction of embolic sequelae. Ngaage and colleagues7 found no difference in the incidence of thromboembolism between aortic and mitral papillary fibroelastoma. We think that lessons can be learned from other diseases. For example, in patients with infective endocarditis, vegetation length above 10 mm tends to be associated with new embolism.8 In accordance with this guideline, papillary fibroelastomas larger than 10 mm should be considered for excision, especially in comparatively younger patients who are undergoing another cardiovascular surgical procedure. In patients who have a papillary fibroelastoma smaller than 10 mm, peripheral embolism should be carefully ruled out by means of thorough clinical examination and whole-body imaging. If the results are negative, we recommend serial echocardiographic evaluation rather than elective surgery. This then raises the question of systemic oral anticoagulation. In an analysis of 725 reported cases,9 tumor size was not an independent predictor of fibroelastoma-related nonfatal embolization. However, due to tumor migration, size as measured echocardiographically might have been underestimated and inappropriately incorporated in statistical analyses. The only independent predictor of embolization was tumor mobility.9 Similarly, in patients with infective endocarditis, severe mobility of vegetation was a predictor of new embolism.8 In one series,4 22% of papillary fibroelastoma patients who were treated without surgery experienced neurologic events, and 4% had peripheral embolic events during the follow-up period. Conversely, no patient in that study's surgical cohort showed evidence of central or peripheral embolic events during the follow-up period. Despite the small size of the tumors, the likelihood of both central and peripheral embolic events was high. There was no tumor recurrence and no tumor-related late morbidity or death during a mean follow-up period of 31 months (range, 1–77 mo).4
In regard to our 2 patients, our decision-making process took into account their ages, their histories of brain disease, and the mobility of each mass, as well as our uncertainty regarding further tumor growth and the consequent embolic risk. Multimodal imaging helped to characterize these papillary fibroelastomas and clarify our decisions to proceed with surgery.
Footnotes
Address for reprints: Najime Bouhzam, MD, Service de Cardiologie, CHU de Rouen, 1 rue de Germont, 76031 Rouen, France
E-mail: bouhzamnajime@yahoo.fr
References
- 1.Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991;52(5):1127–31. [DOI] [PubMed]
- 2.Moustafa S, Sauve C, Page P, Serri K. Incidental finding of a papillary fibroelastoma of the mitral valve chordae. Eur J Echocardiogr 2008;9(5):745–6. [DOI] [PubMed]
- 3.al-Mohammad A, Pambakian H, Young C. Fibroelastoma: case report and review of the literature. Heart 1998;79(3):301–4. [DOI] [PMC free article] [PubMed]
- 4.Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol 1997;30(3):784–90. [DOI] [PubMed]
- 5.Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J 2011;38(3):261–2. [PMC free article] [PubMed]
- 6.Hoey ET, Mankad K, Puppala S, Gopalan D, Sivananthan MU. MRI and CT appearances of cardiac tumours in adults. Clin Radiol 2009;64(12):1214–30. [DOI] [PubMed]
- 7.Ngaage DL, Mullany CJ, Daly RC, Dearani JA, Edwards WD, Tazelaar HD, et al. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg 2005;80(5):1712–8. [DOI] [PubMed]
- 8.Thuny F, Di Salvo G, Belliard O, Avierinos JF, Pergola V, Rosenberg V, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study [published erratum appears in Circulation 2002;112(9):e125]. Circulation 2005;112(1):69–75. [DOI] [PubMed]
- 9.Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003;146(3):404–10. [DOI] [PubMed]