Abstract
Light-chain amyloidosis (AL) is a plasma cell dyscrasia closely related to multiple myeloma. In multiple myeloma, the cancer-testis antigens (CTAs) CT7 (MAGE-C1), CT10 (MAGE-C2) and MAGE-A CTAs are expressed in up to 80% of cases. In this study, we investigated the expression and immunogenicity of several CTAs in patients with AL amyloidosis in a total of 38 bone marrow specimens by employing standard immunohistochemistry techniques on paraffin-embedded archival tissues. Plasma samples from 35 patients (27 with matched bone marrow samples) were also analyzed by ELISA for sero reactivity to a group of full-length CTA proteins. CT7 was present in 25/38 (66%) while CT10 was demonstrated in 3/38 and GAGE in 1/38 AL amyloid cases. The expression pattern was mostly focal. There were no significant differences with regard to organ involvement, response to treatment, or prognosis in CTA positive compared to negative cases. None of the specimens showed spontaneous humoral immunity to CT7, but sero reactivity was observed in individual patients to other CTAs. This study identifies CT7 as the prevalent CTA in plasma cells of patients with AL amyloidosis. Further analyses determining the biology of CTAs in AL amyloidosis and their value as potential targets for immunotherapy are warranted.
Keywords: AL amyloidosis, cancer-testis antigens, stem cell transplantation
Introduction
Immunoglobulin light-chain (AL) amyloidosis is a plasma cell dyscrasia closely related to multiple myeloma and is the most common form of systemic amyloidosis diagnosed in the developed world.1 In this disorder, there is aggregation of amyloid fibrils derived from a monoclonal immunoglobulin light chain, which deposit in tissues and organs leading to progressive organ dysfunction and often death. Left untreated, systemic AL amyloidosis has a median overall survival of 1–2 years and only ∼6 months for patients presenting with symptomatic cardiac involvement.2, 3
Current therapy for AL amyloidosis is aimed at eliminating the bone marrow-based pathologic plasma cells responsible for producing the toxic, amyloidogenic light chains. Strategies have largely been adopted from those used to treat multiple myeloma. At present, standard treatment includes the alkylating agent melphalan, either administered orally in combination with dexamethasone, or given at high doses followed by autologous stem cell transplantation. However, AL patients are prone to complications during stem cell transplantation, and this therapy is restricted to a carefully selected subset.4 The novel agents, thalidomide, lenalidomide and bortezomib, are also active in AL amyloidosis, but their role has not been clearly defined.5, 6, 7, 8 Allogeneic stem cell transplantation has been used to treat AL amyloidosis, but is limited by toxicity and potentially high treatment-related mortality.9 Although these approaches have markedly improved survival, they are highly toxic in patients with impaired organ function because of amyloid deposition, and many patients are diagnosed at too advanced a stage of disease to tolerate aggressive therapy. Consequently, improvement of the current treatment options for AL amyloidosis is warranted.
In recent years, immunotherapeutic approaches including cancer vaccines have been under investigation for various types of solid and hematologic malignancies. In amyloidosis, immunotherapy could offer a possible strategy by targeting the pathologic plasma cell; however, a specific antigen target has not yet been identified. Cancer-testis antigens (CTAs) are a family of tumor-associated antigens, which are expressed in a broad range of human tumors but not in normal adult tissues, except in testicular germ cells and occasionally placenta.10, 11 The prototypical CTA, MAGE-A1, was identified in a melanoma patient by its ability to elicit an autologous cytotoxic T-cell response.12 In most normal adult cells, CTAs are not expressed. However, male germ cells, which do express CTAs, lack major histocompatibility complex class I and consequently are protected from a T-cell-based (auto) immune response.11, 13, 14
Although originally thought to be limited to solid tumors, CTA expression has been increasingly identified in hematologic malignancies. MAGE antigens represent the largest gene family of CTAs. Multiple myeloma, a B cell neoplasm characterized by the clonal proliferation of malignant plasma cells and closely related to AL amyloidosis, is associated with high CTA expression. Several studies have demonstrated a high incidence of CT7/MAGE-C1, CT10/MAGE-C2 and MAGE-A3 expression in multiple myeloma, as well as in a variety of other malignant gammopathies.15, 16, 17, 18
Although the biologic role of CTAs in multiple myeloma is poorly understood, previous studies indicate that MAGE-A3 and CT7/MAGE-C1 expression impacts prognosis.19, 20 Moreover, in myeloma cells where MAGE-A3 and CT7/MAGE-C1 expression was knocked out using RNA interference, a gene silencing technique, lower numbers of viable cells were observed.21 Silencing of MAGE-A3 and CT7/MAGE-C1 resulted in spontaneous apoptosis in up to 80% of malignant cells. In addition, myeloma cells with impaired expression of CT7 and MAGE-A3 demonstrated increased response to treatment with conventional therapies including melphalan and bortezomib compared with cell lines with intact CTA expression.22
Multiple myeloma and amyloidosis are related diseases; however, little is known about the presence of CTAs in amyloidosis. Consequently, in this study, we evaluated bone marrow and sera for the presence of CTAs on a protein level by immunohistochemistry (IHC) in bone marrow biopsies from patients with AL amyloidosis treated at Memorial Sloan-Kettering Cancer Center and at the Amyloid Treatment and Research Program at Boston University. In addition, we tested for the presence of anti-CTA antibodies in the sera of several patients with AL amyloidosis by enzyme-linked immunosorbent assay.
MATERIALS AND METHODS
Histologic samples
Bone marrow and sera were examined from patients with AL amyloidosis treated at Memorial Sloan-Kettering Cancer Center from 1 April 2007, until 1 September 2010. In addition, a subset of bone marrow specimens from patients with AL amyloidosis seen at the Amyloid Treatment and Research Program at Boston University Medical Center were evaluated. The diagnosis of AL amyloidosis was established by tissue biopsy and concordant confirmation of a plasma cell dyscrasia. Procurement of specimens and analyses were performed under valid protocols approved by the institutional review board of both institutions. The patient characteristics with regard to age, gender, light-chain type, cardiac stage and organ involvement are described in Table 1.
Table 1. Patient characteristics for selected bone marrow specimens.
| Status | Statistics |
|---|---|
| AL amyloidosis patients | N=38 |
| Median age | 55 y |
| Male/female | 21/17 |
| Light-chain isotype | |
| Kappa | 5 |
| Lambda | 33 |
| Cardiac stage | |
| I | 14 |
| II | 13 |
| III | 11 |
| Organ involvement | |
| Cardiac | 17 |
| Renal | 26 |
| PNS | 7 |
| GI tract | 3 |
| Liver | 2 |
| Lung | 1 |
Abbreviations: AL, amyloid light chain; PNS, peripheral nervous system.
Bone marrow core biopsies were decalcified in ethylene diamine tetra acetic acid for 1 h, then fixed in 10% formalin and embedded in paraffin. For IHC, 5 μm histologic sections were cut using a microtome and placed on slides for IHC (Superfrost plus, Menzel, Braunschweig, Germany). For conventional histological analysis, sections were stained with hematoxylin/eosin.
Immunohistochemical analysis
The presence of plasma cells was verified and quantified employing an anti-CD138 (clone MI15, DAKO, Carpenteria, CA, USA) immunostain. The following monoclonal antibodies (to the following CTAs) were used for the detection of CTAs: mAb MA454 (MAGE-A1), mAb 57B (MAGE-A4), mAb E978 (NY-ESO-1), mAb CT7-33(CT7/MAGE-C1), CT10 no. 5 (CT10/MAGE-C2), no. 26 (anti-GAGE; clone no. 26, Transduction Labs, Lexington, KY, USA) 16.23, 24, 25, 26, 27 Immunopositivity for CTA markers was graded on the basis of the amount of CD138-positive plasma cells. IHC was performed as described previously.23, 28 After deparaffinization and rehydration in xylene and a series of graded alcohols, antigen retrieval was performed by heating the slides in the appropriate buffer solution. DAKO high-pH solution and citrate buffer (10 mmol, pH 6.0) were used as the AGR solution for mAbs E978 and for mAb CT7-33, respectively. Ethylene diamine tetra acetic acid buffer (1 mmol, pH 8.0) was used for the remaining reagents.
Briefly, primary antibodies were applied overnight at 4 °C. A biotinylated horse antimouse secondary antibody (1:200; Vector, Burlingame, CA, USA) followed by an avidin-biotin system (ABC-elite kit; Vector) was used to detect all primary antibodies except mAb E978, which was used in combination with the Powervision kit secondary reagent (Leica Microsystems, Buffalo Grove, IL, USA); 3,3-diaminobenzidine tetrahydrochloride (Liquid DAB, Biogenex, San Ramon, CA, USA) served as chromogen. Testis with intact spermatogenesis served as a control tissue. The extent of CTA expression was estimated on the basis of immunopositive plasma cells and graded as follows: focal, present but <5% +, 5–25% ++, >25–50% +++, >50–75% and ++++, >75%.
Serum analysis
Frozen serum samples, collected at the time of diagnosis of patients with AL amyloidosis evaluated at MSKCC, were tested for spontaneous antibody response to CTAs. Serum samples were analyzed by enzyme-linked immunosorbent assay for seroreactivity to a series of full-length cancer-testis protein antigens, including CT7/MAGE-C1, CT10/MAGE-C2, MAGE-A1, MAGE-A3, MAGE-A4, MAGE-A10, CT45, CT46/HORMAD, SSX1, SSX2, SSX4, PASD1, SAGE1, NXF2, CSAG2, ACTL7, CXorf48, GAGE-2 and NY-ESO-1. Serum was serially diluted from 1/100 to 1/100 000, and added to low-volume 96-well plates (Corning, NY, USA) coated with 1 μg/ml antigen and blocked with PBS containing 5% nonfat milk. After incubation, plates were washed (Bio-Tek, Winooski, VT, USA) with PBS containing 0.2% Tween and rinsed with PBS. Plasma IgG (total or subclasses) bound to antigens was detected with alkaline-phosphatase-conjugated specific monoclonal antibodies (Southern Biotech, Birmingham, AL, USA). Following addition of ATTOPHOS substrate (Fisher Scientific, Waltham, MA, USA), absorbance was measured using a fluorescence plate reader (Synergy 2, Bio-Tek, Winooski, VT, USA). A reciprocal titer was calculated for each serum sample as the maximal dilution still significantly reacting to a specific antigen. This value was then extrapolated by determining the intersection of a linear trend regression with a cutoff value. The cutoff is defined as 10X the average of OD values from the first four dilutions of a negative control pool made of five healthy donor sera. Sera with reciprocal titers >100 were considered significantly reactive, and specificity was determined by comparing seroreactivity among various antigens tested.
Results
Immunohistochemistry
A total of 38 bone marrow available specimens from patients with AL amyloidosis evaluated at Memorial Sloan-Kettering Cancer Center (n= 30) and Boston University Amyloidosis Treatment and Research Program (n=8) were examined by IHC for protein expression. The clinical data for all 38 patients are shown in Table 1.
Results of IHC are summarized in Table 2. The most prevalent CTA was CT7/MAGE-C1, which was present in 25/38 (66%) of the plasma cells of patients tested. The majority (21/ 25) of CT7-positive specimens displayed focal positivity, i.e., immunoreactivity in <5% of plasma cells. Expression pattern 1+ and 3+, corresponding to immunopositivity of 5–25% and 50–75%, respectively, was seen in one case each.
Table 2. Immunohistochemistry results for 38 bone marrow specimens including % plasma cells, light-chain restriction and degree expression following exposure to CTA-specific monoclonal antibodies (mAb/ CTA).
| Patient | % plasma cells | Light Chain κ/λ | MA454/MAGE-A1 | 57B/ MAGE-A4 | E978/ NY-ESO-1 | CT7-33/ CT7 | CT10 no. 5/ CT10 | No.26 GAGE/ GAGE |
|---|---|---|---|---|---|---|---|---|
| 1a | 5 | λ | neg | neg | neg | +++ | neg | neg |
| 2 | 10 | λ | neg | neg | neg | neg | neg | neg |
| 3 | 10 | λ | neg | neg | neg | focal | neg | neg |
| 4 | 5 | λ | neg | neg | neg | focal | neg | neg |
| 5 | 10 | λ | neg | neg | neg | focal | neg | neg |
| 6 | 5–10 | λ | neg | neg | neg | focal | neg | neg |
| 7 | 5 | κ | neg | neg | neg | focal | neg | neg |
| 8 | 10–15 | κ | neg | neg | neg | focal | neg | neg |
| 9 | 9 | λ | neg | neg | neg | focal | neg | neg |
| 10 | 14 | λ | neg | neg | neg | neg | neg | neg |
| 11a | 9 | λ | neg | neg | neg | focal | neg | neg |
| 12 | 15 | λ | neg | neg | neg | focal | neg | neg |
| 13 | 21 | λ | neg | neg | neg | focal | neg | neg |
| 14 | 29 | λ | neg | neg | neg | + | foc | neg |
| 15 | 15 | λ | neg | neg | neg | focal | neg | neg |
| 16 | 9 | λ | neg | neg | neg | neg | neg | neg |
| 17 | 5 | λ | neg | neg | neg | focal | neg | neg |
| 18 | 2 | κ | neg | neg | neg | neg | neg | neg |
| 19 | 22 | λ | neg | neg | neg | neg | neg | neg |
| 20 | 10 | λ | neg | neg | neg | neg | neg | neg |
| 21 | 4 | λ | neg | neg | neg | focal | neg | neg |
| 22 | 24 | λ | neg | neg | neg | neg | neg | neg |
| 23 | 7 | κ | neg | neg | neg | neg | neg | neg |
| 24 | 7 | λ | neg | neg | neg | focal | neg | neg |
| 25 | 7 | λ | neg | neg | neg | focal | foc | neg |
| 26 | 26 | λ | neg | neg | neg | +++ | foc | foc |
| 27 | 4 | λ | neg | neg | neg | focal | neg | neg |
| 28 | 16 | λ | neg | neg | neg | neg | neg | neg |
| 29 | 4 | λ | neg | neg | neg | neg | neg | neg |
| 30 | 22 | λ | neg | neg | neg | neg | neg | neg |
| 31 | 10 | λ | neg | neg | neg | focal | neg | neg |
| 32 | 10 | κ | neg | neg | neg | + | neg | neg |
| 33 | 13 | λ | neg | neg | neg | focal | neg | neg |
| 34 | 7 | λ | neg | neg | neg | neg | neg | neg |
| 35 | 9 | λ | neg | neg | neg | focal | neg | neg |
| 36 | 14 | λ | neg | neg | neg | focal | neg | neg |
| 37 | 19 | λ | neg | neg | neg | focal | neg | neg |
| 38 | 7 | λ | neg | neg | neg | neg | neg | neg |
Abbreviations: neg, negative; focal, <5% positive; +, 5–25% expression; ++, 26–49% expression; +++, 50–75% expression.
See Figure 1.
The median number of CD138-positive plasma cells in all patients studied was 10%. Likewise, the CD138 expression was 10% for those with or without any CT7 expression. The median CD138 expression was 9% for those with focal positivity, 20% for those with+positivity**, and 16% for those with +++ CT7 expression.
Among the other CTAs, one case was positive, with mAb no. 26 to GAGE displaying focal reactivity. This case was also positive for CT7 (3+) and had 26% plasma cells in the bone marrow aspirate. Three cases showed focal positivity for mAb CT10no. 5 to CT10/MAGE-C2; all three specimens were also positive for mAb CT7-33. One case showed focal CT7 expression (patient no. 25); another showed+CT7 expression (patient no. 14), and a third had +++ CT7 expression (patient no. 26). The percentage of plasma cells (CD138 expression) in the three bone marrows that expressed CT10/MAGE-C2 ranged from 7 to 29%.
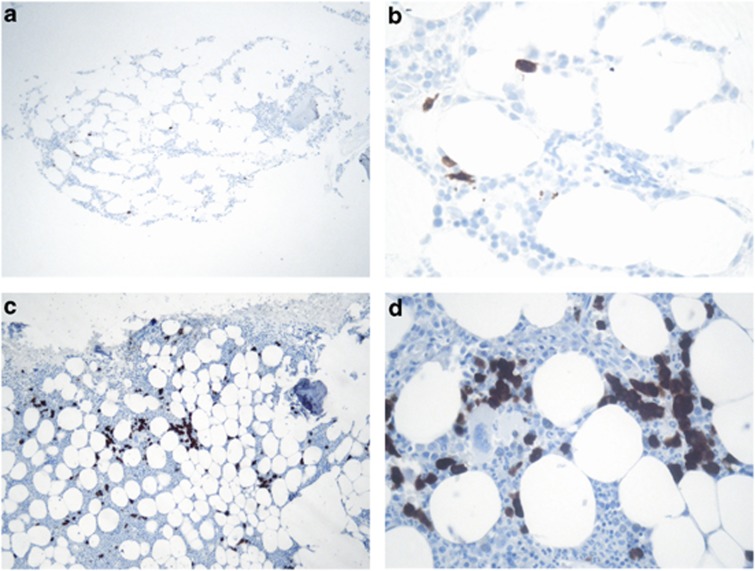
None of the bone marrow specimens stained positive for either of the remaining reagents: mAb MA454 or mAb 57B. Typical CTA expression patterns are illustrated in Figures 1a–d.
Figure 1.
CT7 expression pattern in bone marrow biopsies of two AL amyloid patients with low (5X; A, C) and high (20X; B, D) magnification. (a, b): Case 11; focal expression of CT7-positive plasma cells. (c, d): Case 1; homogeneous expression with 50–75% CT7-positive plasma cells (+++).
Serum analysis
A total of 35 frozen serum samples from patients evaluated at Memorial Sloan-Kettering were tested for a spontaneous antibody response to CTAs, including 27/30 patients who provided bone marrow samples. In addition, nine serum samples were available from patients with AL amyloidosis, but bone marrow specimens were not. There were no serum samples available for analysis from patients at Boston University.
Of 35 serum specimens tested, none demonstrated immunity to CT7/MAGE-C1 or CT10/MAGE-C2. One patient showed a serum antibody response to MAGE-A3 (titer 1/400), another to NY-ESO-1 (1/2000) and another to GAGE-2 (1/2000). Spontaneous immune responses were not seen for any of the other CTAs tested. Bone marrow specimens were not available for either the patient with MAGE-A3 response or the NY-ESO-1 serum antibody. The bone marrow specimen from the GAGE-2 seropositive patient was found negative for GAGE expression by IHC.
CT7 staining, survival and organ involvement
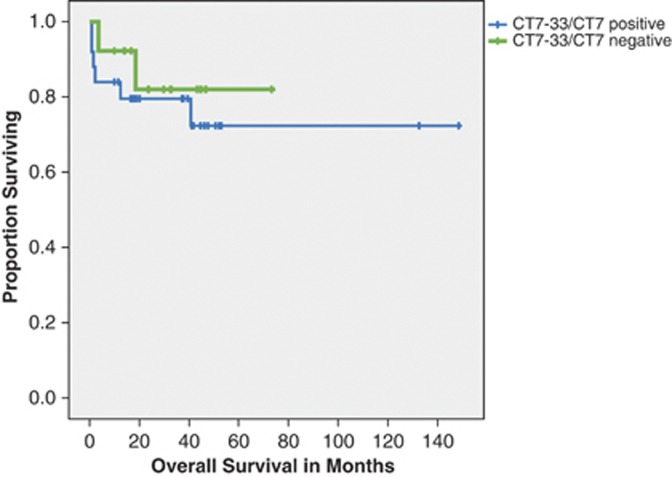
There was no significant difference in overall survival among the patients who stained positive for CT7 compared with those who did not. The median survival was not reached for either group. In addition to lack of survival impact, CT7 positivity also had no association with organ involvement or cardiac stage (Figure 2).
Figure 2.
Overall survival of patients on the basis of CT7 expression.
Discussion
In the last decade, CTAs have been recognized as promising targets for immunotherapy. The interest in CTAs is based primarily on their almost exclusive tumor-associated pattern of expression. Their presence in normal adult tissues is restricted to male germ cells, although they show variable expression in several types of cancer. In solid cancers, such as carcinomas of the head and neck, non-small cell lung cancers, and melanoma, CTA expression is prevalent, although other types of malignancies such as renal cell and colorectal carcinomas show very low CTA expression.11 Most hematologic malignancies appear to have a low level of CTA expression, yet there are exceptions. Several studies indicated that myelomatous plasma cells highly express several CTAs at both the molecular and protein level.15, 16, 17, 18 Most interestingly, expression of CTAs in clonal plasma cells parallels the activity of disease.15, 16 Less CTA expression was found in monoclonal gammopathy of undetermined significance, whereas increased expression was found in early-stage symptomatic myeloma, and even more so in advanced disease at 13%, 75% and 82%, respectively.16
In addition to the actual presence of CTA in multiple myeloma, spontaneous humoral responses against CTAs have been documented. In a previous study of 46 patients with multiple myeloma, antibodies to CTAs NY-ESO-1, CT7, CT10 and SSX4 were found. In another analysis of 67 newly diagnosed multiple myeloma patients, antibody response to NY-ESO1 was associated with inferior overall survival independent of other prognostic factors.29 Consequently, CTAs are currently under investigation as potential vaccine targets in myeloma.30
The pathologic plasma cell in AL amyloidosis is thought to be similar to the plasma cell seen in multiple myeloma, with common molecular defects.31 We found that CTAs are, in fact, present in AL amyloidosis. The most prevalent CTA is CT7/MAGE-C1, which we identified in 25/38 (66%) cases. The expression pattern was predominantly focal, i.e., <5% of tumor cells were immunopositive. A high distribution of CT7 expression, i.e., in >50% of the plasma cells, was seen in only two cases. In those cases, there was no difference in percent plasma cell burden with any CT7 expression compared with those with none. However, those patients with >5% CT7 expression had a higher percent plasma cell burden compared with those with just focal expression by IHC. The high prevalence of CT7/MAGE-C1 expression in our AL series shares similarities with previous studies in myeloma, where incidences of >80% positivity were observed.15, 17 However, although the expression in myeloma expression usually encompasses a majority of plasma cells, immunopositivity is generally restricted to only few plasma cells in amyloidosis.
There was a markedly low incidence of CTA expression other than CT7 in our series. CT10 and GAGE were expressed in only 3/38 (7%) and 1/38 (3%) cases, respectively. For both antigens, only single cells were immunopositive. There was no expression for any of the other CTAs tested. Conversely, in myeloma, a much higher incidence of positivity was reported for several CTAs that were analyzed in our study, such as CT10/MAGE-C2 (>50%), NY-ESO-1 (>20%) and MAGE-A1 (>20%).15, 17 Taken together at a protein level, AL amyloidosis and myeloma share the prevalence of CT7 expression, albeit in a much smaller fraction of cells, whereas other CTAs are mostly absent. Low CTA protein expression may reflect the biological nature of AL amyloidosis resembling pathologic plasma cells with a low proliferative index. Nevertheless, this finding calls into question whether the pathologic plasma cells in patients with AL amyloidosis are truly molecularly identical to plasma cells seen in multiple myeloma.
Treatments for amyloidosis mirror those for multiple myeloma, and, similarly, response rates are high but relapse is frequent. Light-chain amyloidosis differs from myeloma in that patients present with a plasma cell clone that has a low proliferative index and therefore a lower tumor burden.32 The disease is equally devastating, however, because of unique organ dysfunction caused by the amyloidogenic monoclonal proteins released from a small number of abnormal cells. Immunotherapy may hold even more promise than in multiple myeloma, with a small number of malignant cells to target in amyloidosis. While it is reasonable to consider CTAs as a therapeutic option for targeted therapy in amyloidosis, an appropriate next step would be to evaluate the presence of CT7 in order to determine if CT7 expression levels change following treatment.
There was no detectable spontaneous humoral immune response to CT7 or CT10, the most prevalent CTAs in our study. This is not surprising, as CT7 and CT10 have not been particularly immunogenic in prior studies investigating a variety of other tumors. In these previous studies, the most immunogenic antigen was NY-ESO-1; however, NY-ESO-1 expression was not detected in the present series.33, 34 Lack of seroreactivity to CT7 may further reason to induce immune responses against this antigen with immunotherapeutic approaches.
Evidence of seroreactivity to MAGE-A3, NY-ESO-1 or GAGE-2 in individual patients indicates that AL may be considered an immunogenic disease. Unfortunately, no tumor specimen was available for patients with serological responses to MAGE-A3 and NY-ESO-1, and we therefore could not correlate immunogenicity with antigen expression. Of note, the bone marrow specimen from the GAGE-2 seropositive patient was found to be negative for GAGE by IHC. This may be explained by sample error because of known heterogeneity of GAGE expression.
Although protein expression could not be demonstrated in patients with humoral responses to CTAs, it is unlikely that an antibody would be induced without an antigen present. It should be noted that antibody responses to CTA are largely absent in healthy donors. In patients with solid tumors, CTA-specific serum antibody presence is usually correlated with tumor expression of the cognate antigen, and there is a trend for increased immunogenicity of CTA with advanced stages and grades of disease. Finding serum antibodies to CTAs in AL patients is therefore surprising, given the relatively low tumor burden in AL, but it forecasts further evidence of CTA expression in AL amyloidosis.
Taken together, our study demonstrates the prevalence of CT7/MAGE-C1 CTAs are the most commonly expressed in AL amyloidosis. In addition, we demonstrate that there is poor to no expression of other CTAs, such as MAGE-A, NY-ESO-1 or CT10, which are commonly seen in multiple myeloma. On the basis of limited knowledge of CTA biology, we speculate that this could be a reflection of the low proliferation of plasma cells in amyloidosis. Evidence of CTA expression and immunogenicity in AL, albeit not frequent, provides a rationale for further investigation in this disease of potential immunotherapy-based strategies. Because of the low tumor burden, systemic amyloidosis may be susceptible to immunotherapeutic treatment. On the basis of the present findings, CT7/MAGE-C1 appears to be the most reasonable target for future research.
Acknowledgments
This work was supported by a grant from Empire Clinical Research Investigator Program (ECRIP) and partially by the Cancer Research Institute.
The authors declare no conflict of interest.
References
- Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O'Fallon WM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–1822. [PubMed] [Google Scholar]
- Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- Gertz MA, Zeldenrust SR. Treatment of immunoglobulin light chain amyloidosis. Curr Hematol Malig Rep. 2009;4:91–98. doi: 10.1007/s11899-009-0013-6. [DOI] [PubMed] [Google Scholar]
- Seldin DC, Choufani EB, Dember LM, Wiesman JF, Berk JL, Falk RH, et al. Tolerability and efficacy of thalidomide for the treatment of patients with light chain-associated (AL) amyloidosis. Clin Lymphoma. 2003;3:241–246. doi: 10.3816/clm.2003.n.005. [DOI] [PubMed] [Google Scholar]
- Sanchorawala V, Wright DG, Rosenzweig M, Finn KT, Fennessey S, Zeldis JB, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109:492–496. doi: 10.1182/blood-2006-07-030544. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Lacy MQ, Rajkumar SV, Geyer SM, Witzig TE, Fonseca R, et al. Poor tolerance to high doses of thalidomide in patients with primary systemic amyloidosis. Amyloid. 2003;10:257–261. doi: 10.3109/13506120309041743. [DOI] [PubMed] [Google Scholar]
- Comenzo RL, Hegenbart U, Sanchorawala V. High rates of overall and complete haematologic response in a prospective phase 1/2 study of weekly and twice-weekly bortezomib in relapsed AL amyloidosis. Amyloid J Protein Folding Disord. 2010;17:83–84. [Google Scholar]
- Schonland SO, Lokhorst H, Buzyn A, Leblond V, Hegenbart U, Bandini G, et al. Allogeneic and syngeneic hematopoietic cell transplantation in patients with amyloid light-chain amyloidosis: a report from the European Group for Blood and Marrow Transplantation. Blood. 2006;107:2578–2584. doi: 10.1182/blood-2005-06-2462. [DOI] [PubMed] [Google Scholar]
- Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- Knuth A, Wolfel T, Klehmann E, Boon T, Meyer zum Büschenfelde KH. Cytolytic T-cell clones against an autologous human melanoma: specificity study and definition of three antigens by immunoselection. Proc Natl Acad Sci USA. 1989;86:2804–2808. doi: 10.1073/pnas.86.8.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszer D, Kurpisz M. Major histocompatibility complex expression on human, male germ cells: a review. Am J Reprod Immunol. 1998;40:172–176. doi: 10.1111/j.1600-0897.1998.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Westbrook VA, Schoppee PD, Diekman AB, Klotz KL, Allietta M, Hogan KT, et al. Genomic organization, incidence, and localization of the SPAN-x family of cancer-testis antigens in melanoma tumors and cell lines. Clin Cancer Res. 2004;10:101–112. doi: 10.1158/1078-0432.ccr-0647-3. [DOI] [PubMed] [Google Scholar]
- Dhodapkar MV, Osman K, Teruya-Feldstein J, Filippa D, Hedvat CV, Iversen K, et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun. 2003;3:9. [PubMed] [Google Scholar]
- Jungbluth AA, Ely S, DiLiberto M, Niesvizky R, Williamson B, Frosina D, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Caballero OL, Gnjatic S, Andrade VC, Colleoni GW, Vettore AL, et al. Physical interaction of two cancer-testis antigens, MAGE-C1 (CT7) and NY-ESO-1 (CT6) Cancer Immun. 2006;6:12. [PubMed] [Google Scholar]
- Andrade VC, Vettore AL, Felix RS, Almeida MS, Carvalho F, Oliveira JS, et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- van Duin M, Broyl A, de Knegt Y, Goldschmidt H, Richardson PG, Hop WC, et al. Cancer testis antigens in newly diagnosed and relapse multiple myeloma: prognostic markers and potential targets for immunotherapy. Haematologica. 96:1662–1669. doi: 10.3324/haematol.2010.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D, Hildebrandt Y, Jadczak A, Cao Y, Luetkens T, Meyer S, et al. Cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 promote the survival of multiple myeloma cells. Haematologica. 2010;95:785–793. doi: 10.3324/haematol.2009.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D, Luetkens T, Hildebrandt Y, Arfsten J, Bartels K, Horn C, et al. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin Cancer Res. 2009;15:1343–1352. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- Jungbluth AA, Stockert E, Chen YT, Kolb D, Iversen K, Coplan K, et al. Monoclonal antibody MA454 reveals a heterogeneous expression pattern of MAGE-1 antigen in formalin-fixed paraffin embedded lung tumours. Br J Cancer. 2000;83:493–497. doi: 10.1054/bjoc.2000.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry C, Brasseur F, Spagnoli GC, Marbaix E, Boon T, Coulie P, et al. Monoclonal antibody 57B stains tumor tissues that express gene MAGE-A4. Int J Cancer. 2000;86:835–841. doi: 10.1002/(sici)1097-0215(20000615)86:6<835::aid-ijc12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jungbluth AA, Chen YT, Busam KJ, Coplan K, Kolb D, Iversen K, et al. CT7 (MAGE-C1) antigen expression in normal and neoplastic tissues. Int J Cancer. 2002;99:839–845. doi: 10.1002/ijc.10416. [DOI] [PubMed] [Google Scholar]
- Zhuang R, Zhu Y, Fang L, Liu XS, Tian Y, Chen LH, et al. Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun. 2006;6:7. [PubMed] [Google Scholar]
- Rimoldi D, Salvi S, Schultz-Thater E, Spagnoli GC, Cerottini JC. Anti-MAGE-3 antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int J Cancer. 2000;86:749–751. doi: 10.1002/(sici)1097-0215(20000601)86:5<749::aid-ijc24>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Lu P, Gnjatic S, Hoffman J, Ritter E, Zhou P, et al. MAGE-A3 or NY-ESO1 expression and spontaneous antibody responses to NY-ESO1 in newly diagnosed multiple myeloma patients are associated with worse overall survival. ASH Annual Meeting Abstracts. 2008;112:5110. [Google Scholar]
- Anderson LD, Cook DR, Yamamoto TN, Berger C, Maloney DG, Riddell SR, et al. Identification of MAGE-C1 (CT-7) epitopes for T-cell therapy of multiple myeloma. Cancer Immunol Immunother. 2011;60:985–997. doi: 10.1007/s00262-011-1009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochtler T, Hegenbart U, Cremer FW, Heiss C, Benner A, Hose D, et al. Evaluation of the cytogenetic aberration pattern in amyloid light chain amyloidosis as compared with monoclonal gammopathy of undetermined significance reveals common pathways of karyotypic instability. Blood. 2008;111:4700–4705. doi: 10.1182/blood-2007-11-122101. [DOI] [PubMed] [Google Scholar]
- Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108:2520–2530. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]