Abstract
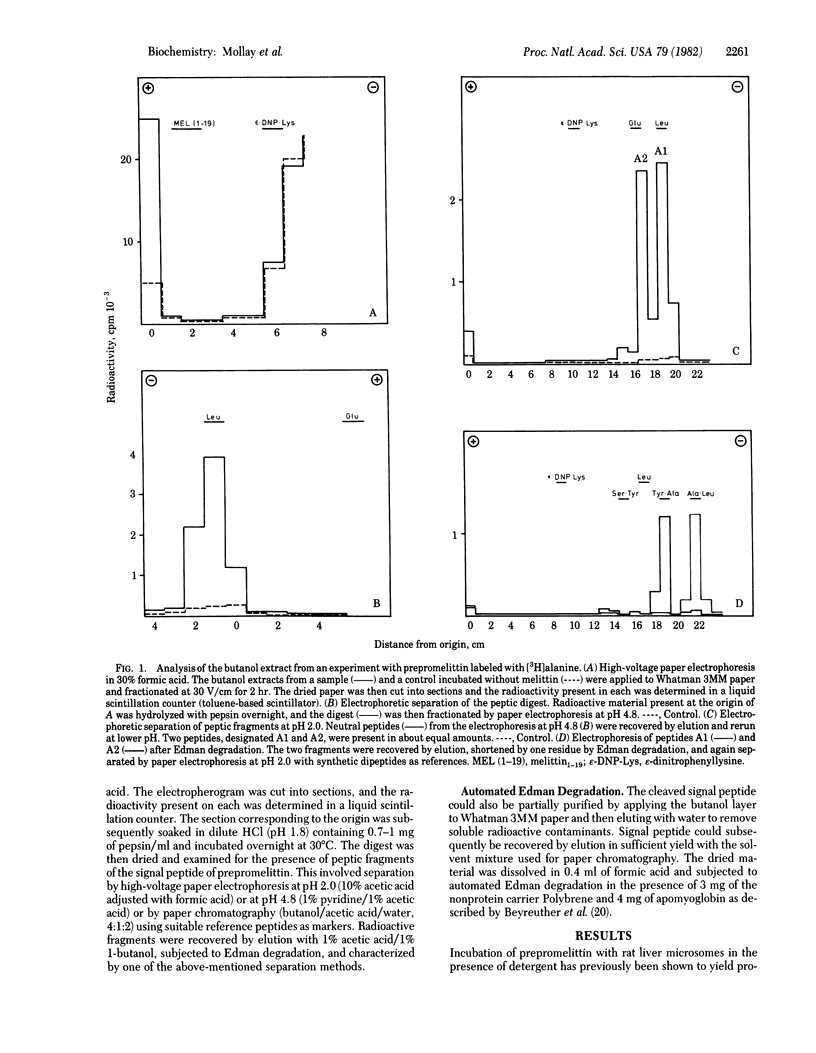
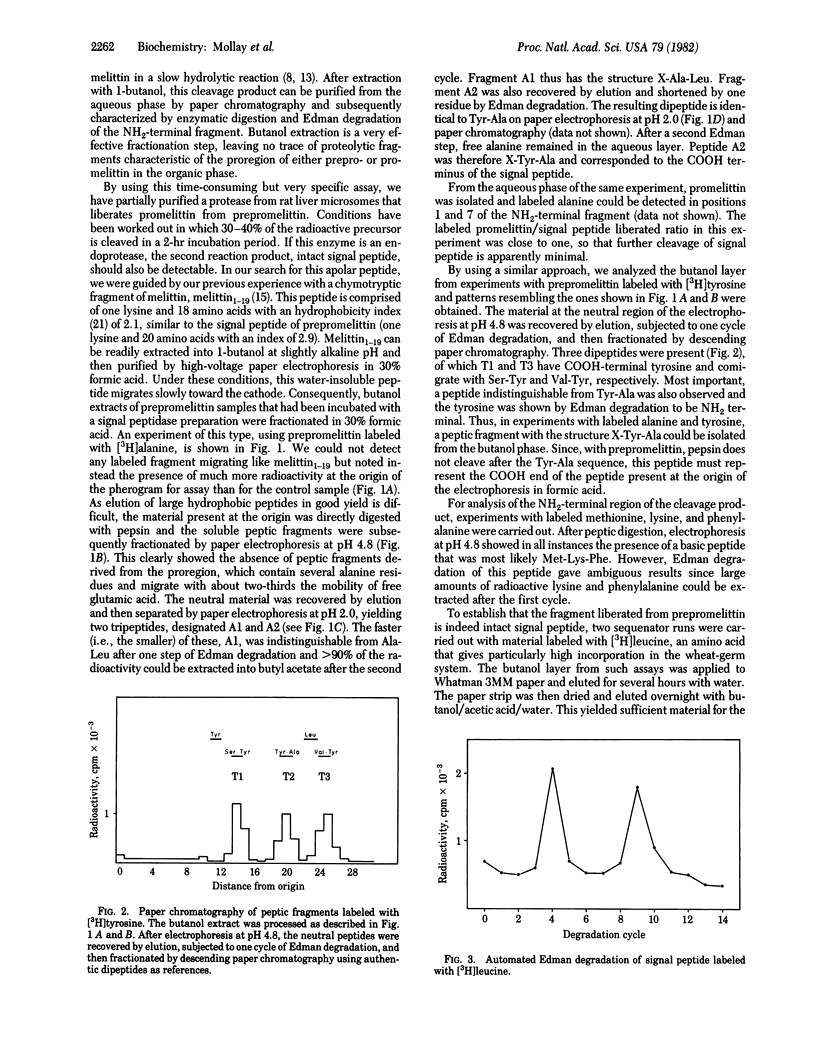
It has previously been shown that rat liver microsomes contain a proteolytic enzyme that cleaves honeybee prepromelittin to yield promelittin. This enzyme has now been further purified by centrifugation on a sucrose-deoxycholate gradient and then reconstituted into phospholipid vesicles. Incubation of prepromelittin with vesicles in the presence of melittin yields, in addition to promelittin, a hydrophobic peptide. The latter could be isolated by extraction with l-butanol and paper electrophoresis in 30% formic acid and was shown to be intact signal peptide by analysis of peptic fragments and automated Edman degradation. The microsomal enzyme is thus an endoprotease that hydrolyzes prepromelittin exclusively at the pre-pro junction. The precision of this cleavage of an insect preprotein by a rat liver enzyme indicates that we are dealing with the ubiquitous eukaryotic signal peptidase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Dee P. C., Potts J. T., Jr Cellular processing of pre-proparathyroid hormone involves rapid hydrolysis of the leader sequence. J Biol Chem. 1979 Nov 10;254(21):10596–10599. [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational processing of full-length presecretory proteins with canine pancreatic signal peptidase. Ann N Y Acad Sci. 1980;343:391–404. doi: 10.1111/j.1749-6632.1980.tb47268.x. [DOI] [PubMed] [Google Scholar]
- Kaderbhai M. A., Freedman R. B. Resolution of microsomal membranes into fractions differing in polypeptide composition. Biochim Biophys Acta. 1980 Dec 12;603(2):366–370. doi: 10.1016/0005-2736(80)90381-8. [DOI] [PubMed] [Google Scholar]
- Kaschnitz R., Kreil G. Processing of prepromelittin by subcellular fractions from rat liver. Biochem Biophys Res Commun. 1978 Aug 14;83(3):901–907. doi: 10.1016/0006-291x(78)91480-8. [DOI] [PubMed] [Google Scholar]
- Kreil G., Mollay C., Kaschnitz R., Haiml L., Vilas U. Prepromelittin: specific cleavage of the pre- and the propeptide in vitro. Ann N Y Acad Sci. 1980;343:338–346. doi: 10.1111/j.1749-6632.1980.tb47262.x. [DOI] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Champion J., Haiml L., Kreil G. The sequestration, processing and retention of honey-bee promelittin made in amphibian oocytes. Eur J Biochem. 1981 Jan;113(2):273–281. doi: 10.1111/j.1432-1033.1981.tb05063.x. [DOI] [PubMed] [Google Scholar]
- Mollay C. Effect of melitin and melittin fragments on the thermotropic phase transition of dipalmitoyllecithin and on the amount of lipid-bound water. FEBS Lett. 1976 Apr 15;64(1):65–68. doi: 10.1016/0014-5793(76)80250-5. [DOI] [PubMed] [Google Scholar]
- Mollay C., Kreil G., Berger H. Action of phospholipases on the cytoplasmic membrane of Escherichia coli. Stimulation by melittin. Biochim Biophys Acta. 1976 Mar 5;426(2):317–324. doi: 10.1016/0005-2736(76)90340-0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Sandberg P. O., Marzella L., Glaumann H. A method for rapid isolation of rough and smooth microsomes and Golgi apparatus from rat liver in the same sucrose gradient. Exp Cell Res. 1980 Dec;130(2):393–400. doi: 10.1016/0014-4827(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Feldmann R. J. Membrane proteins: amino acid sequence and membrane penetration. J Mol Biol. 1974 Aug 25;87(4):853–858. doi: 10.1016/0022-2836(74)90090-4. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P., Watts C., Wickner W. Membrane assembly from purified components. I. Isolated M13 procoat does not require ribosomes or soluble proteins for processing by membranes. Cell. 1981 Aug;25(2):341–345. doi: 10.1016/0092-8674(81)90052-0. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Zimmerman M., Mumford R. A., Alberts A. W. Processing of pre-proalbumin and pre-placental lactogen. Ann N Y Acad Sci. 1980;343:168–179. doi: 10.1111/j.1749-6632.1980.tb47250.x. [DOI] [PubMed] [Google Scholar]
- Suchanek G., Kreil G., Hermodson M. A. Amino acid sequence of honeybee prepromelittin synthesized in vitro. Proc Natl Acad Sci U S A. 1978 Feb;75(2):701–704. doi: 10.1073/pnas.75.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchanek G., Kreil G. Translation of melittin messenger RNA in vitro yields a product terminating with glutaminylglycine rather than with glutaminamide. Proc Natl Acad Sci U S A. 1977 Mar;74(3):975–978. doi: 10.1073/pnas.74.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau S. N., Walsh K. A. Processing of precursor proteins by preparations of oviduct microsomes. Ann N Y Acad Sci. 1980;343:180–191. doi: 10.1111/j.1749-6632.1980.tb47251.x. [DOI] [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]



