Abstract
The Visceral Leishmaniasis (VL) Elimination Initiative in the Indian subcontinent was launched in 2005 as a joint effort between the governments in the Region (India, Nepal and Bangladesh) and the World Health Organization (WHO). The objective is to reduce the annual VL incidence below 1/10,000 inhabitants by 2015 based on detection and treatment of VL cases and vector control. We present here a review of studies published in the period 2005-2010 on the efficacy of different tools to control Phlebotomus argentipes. The review indicates that the current indoor residual spraying (IRS) and novel vector control methods mainly insecticide treated nets (ITN) have low effectiveness for several reasons. Efforts to improve quality of IRS operations and further research on alternative and integrated vector control methods need to be promoted to reach the VL elimination target by 2015.
Keywords: Elimination, indoor residual spraying, insecticide treated nets, integrated vector control, Phlebotomus argentipes, visceral leishmaniasis
Introduction
Kala azar, also known as visceral leishmaniasis (VL), is the second largest parasitic killer in the world after malaria1. In May 2005, the governments of India, Nepal and Bangladesh committed themselves to mutual co-operation towards elimination of kala-azar from their countries by 2015 by signing a Memorandum of Understanding during the World Health Assembly in Geneva. Most of the estimated 200 to 400 thousand annual VL cases reported worldwide are located in South Asia (SA)2,3. In this Region the highest burden of the disease is concentrated in northern India (32813 and 25113 cases reported in 2005 and 2010, respectively), mainly Bihar State; eastern Bangladesh (6892 cases in 2005 and 2763 cases in 2010) and the south-eastern districts in Nepal (1463 and 418 cases in 2005 and 2010, respectively)4. However, these figures are based on official reports and are considered to be an underestimation of the real number of VL cases5.
Though isolated case reports on VL attributed to Leishmania tropica exist6, in India, Nepal and Bangladesh VL is mainly caused by the parasite species Leishmania donovani, which is transmitted from man to man by the sandfly Phlebotomus argentipes (Diptera; Psychodidae)7. The objective of the Visceral Leishmaniasis Elimination Initiative is to reduce VL incidence below 1 per 10 000 per year at district, subdistrict or upazilla level in India, Nepal and Bangladesh, respectively by 2015. Elimination was deemed feasible as man and P. argentipes are the only known host and vector for L. donovani, respectively in this Region. The elimination should be also favoured by the availability of a new oral drug (miltefosine) and a reliable rapid diagnostic test (rK39 dipstick). The fact that VL was limited to 96 districts and the positive experiences in controlling the disease using indoor residual spraying (IRS) were considered favourable factors. Furthermore, the initiative was strongly supported by the governments of the three affected countries and the WHO2,8. The strategy for VL elimination in SA is based on case detection and management as well as vector control. Due to the limited number of VL drugs available and the increasing number of unresponsive cases to pentavalent antimonials (the first line treatment for over 70 years) in Bihar (up to 60% in some areas) and emerging resistance in other regions9, the control of P. argentipes in endemic regions should be prioritized. In this paper, we review the evidence on vector control available in 2005 and the recent advances in the field over the period 2005 to 2010.
Situation in 2005
P. argentipes sandflies are described as perido-mestic insects, mainly endophilic and endophagic (mainly feed indoors) and most active when people are asleep10. During daytime these are not very active, but seek shelter in dark, cool places. The sandflies feed on plant sugars, and only the female fly needs a bloodmeal to acquire the necessary protein for egg production. Vector density peaks in March-May and October-November in India8 and is related to temperature and rainfall11.
Indoor residual spraying
There is historic evidence that insecticide spraying during the malaria eradication campaigns of the 1950s-1970s had a drastic impact on transmission of L. donovani in the Indian subcontinent12. This observation was supported by the fact that VL did not reappear in areas were IRS was continued in the 1970s-1980s such as Assam13. However, the information on the effect of IRS on P. argentipes density was limited to a few studies only14–16. IRS campaigns in India in the early 1990s (i.e. 2 annual blanket rounds in endemic districts) reduced the number of reported cases, but their effect was limited in time as the VL cases started to rise again early in the 21st century12,17,18. Nevertheless, IRS was recommended as the main vector control strategy in 20058. When the VL elimination programme was launched the spraying strategies (i.e. time and number of rounds, insecticide used) varied among countries. Despite some reports on DDT-resistance12 India decided to use DDT while Nepal used lambdacyhalothrin19. In Bangladesh, vector control activities for VL were rather limited or nonexistent20. The Regional Technical Advisory Group (RTAG) to the VL Elimination Initiative recommended that IRS should be standardized between countries (i.e. spraying calendar and strategies) and optimised (i.e. use of geographical information systems, GIS). It was also suggested that the efficacy of IRS to control VL and the development of insecticide resistance should be monitored in the Region. The effectiveness of these spraying programmes was not the only issue of concern, other problems are their side effects on health and environment and their sustainability. Several factors such as cost of the insecticides, the logistical constraints and the low acceptance by the community compromise the longer-term effectiveness and sustainability of this intervention21.
Insecticide treated nets
Insecticide treated nets (ITN) were suggested as a potential complement to IRS8. P. argentipes peridomestic behaviour suggests that ITN would be an appropriate tool to control L. donovani vector in the Indian subcontinent12. However, in 2005 there was no evidence on the effectiveness of ITN to prevent VL in the Region, apart from a few observational studies in Nepal and Bangladesh suggesting that untreated bednets provide some degree of personal protection against VL12.
Alternative vector control methods
Alternative vector control technologies have been proposed. For example, plastering of walls and floors using mud and lime plaster was associated with a decrease in P. argentipes indoor density compared to controls in a pilot study in India22. However, this and other environmental management (EVM) methods needed further evaluation.
Situation in 2010
Since 2005 a number of studies have been published covering different areas related to P. argentipes control in the Indian subcontinent. The results from recent studies on the impact of IRS (Table I), ITNs and EVM (Table II) on P. argentipes density and VL are discussed below.
Table I.
Studies on the effect of indoor residual spraying (IRS) on Phlebotomus argentipes and visceral leishmaniasis conducted recently in the Indian subcontinent
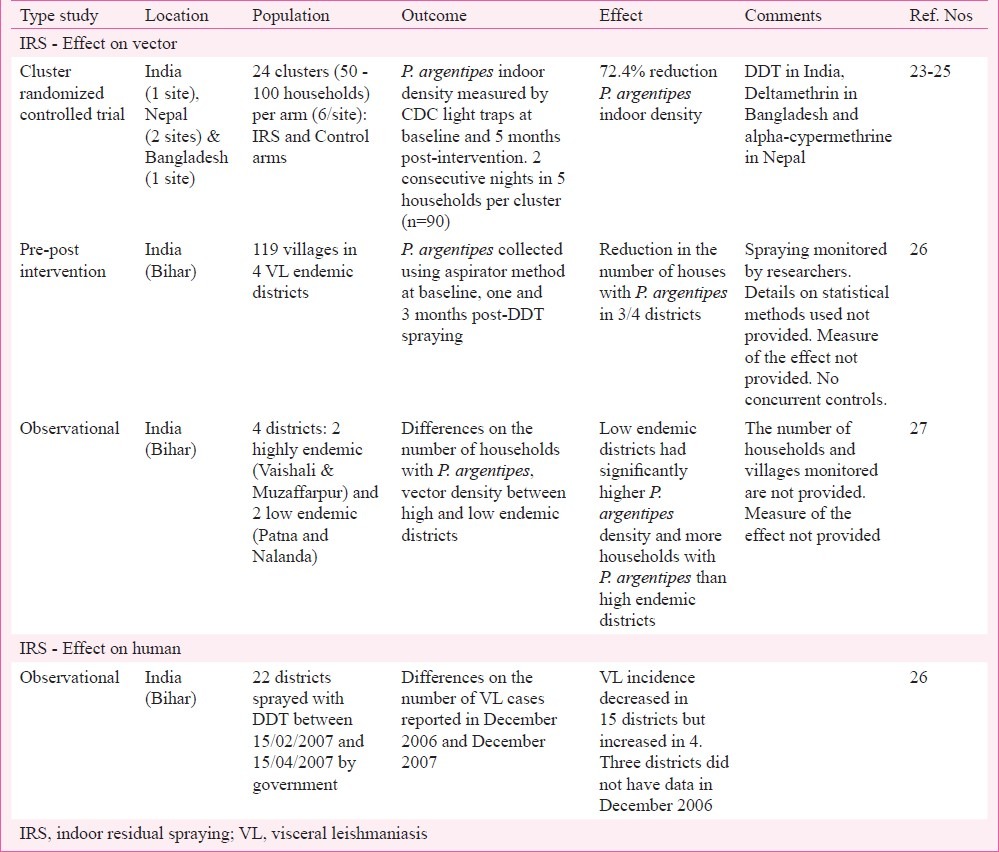
Table II.
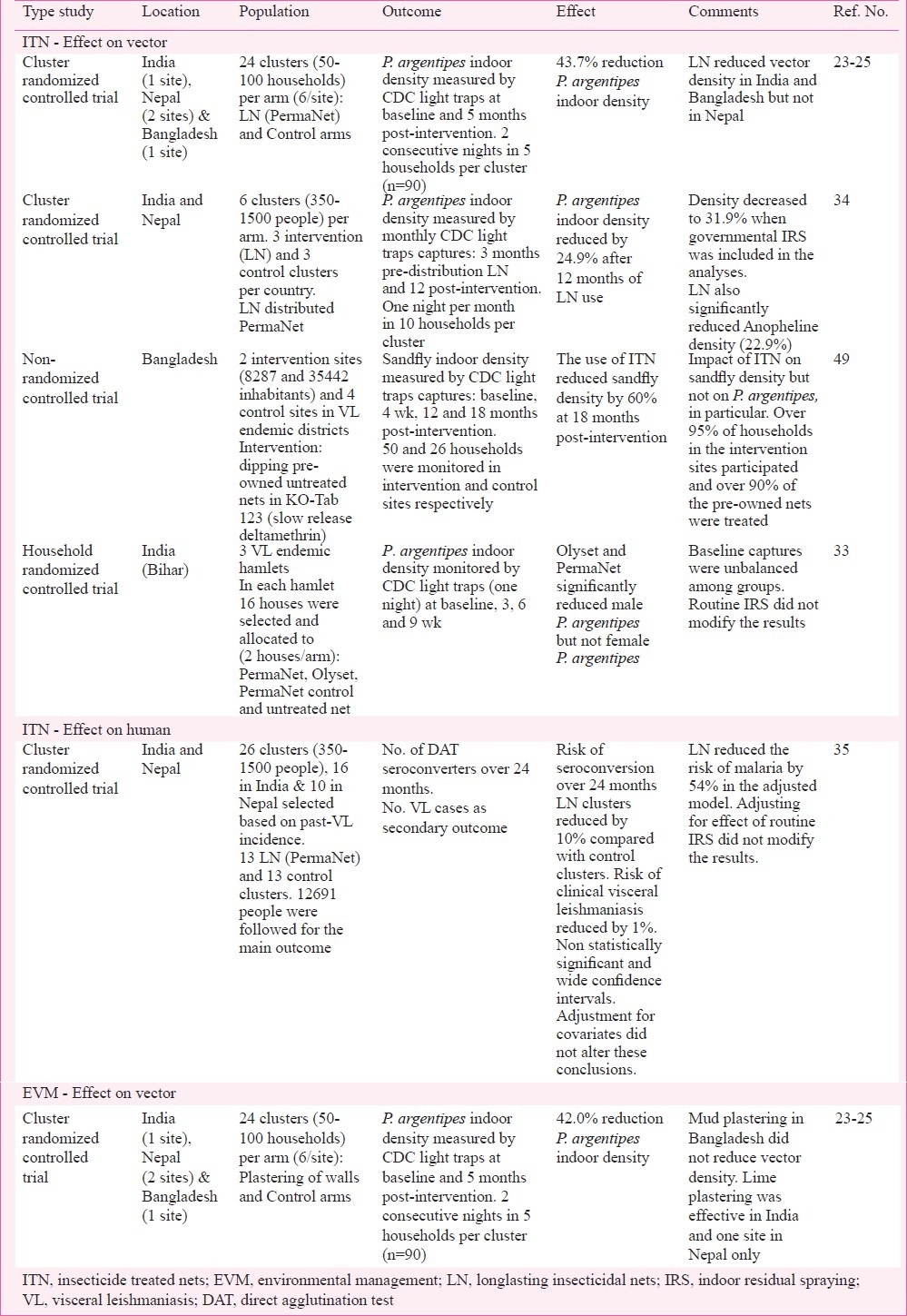
Studies on the effect of insecticide treated nets (ITN) and environmental management (EVM) on Phlebotomus argentipes and visceral leishmaniasis conducted recently in the Indian subcontinent.
Indoor residual spraying
The only cluster randomized controlled trial evaluating the impact of IRS on vector density was conducted in India, Nepal and Bangladesh in 2006-200723. The same study also assessed the effect of longlasting insecticidal nets (LN) and EVM on P. argentipes density. A total of 96 clusters each counting between 50 to 100 households were studied at 4 sites: one in India, one in Bangladesh and two in Nepal. These clusters were selected based on past VL incidence and matched with control by pre-intervention vector density. The trial had four arms (IRS, EVM, LN and Control) with 24 clusters in each (6 clusters per arm and site). The impact of the different vector control methods was assessed by comparing the P. argentipes captures in five households per cluster between intervention and control groups. The sandfly density was measured by CDC light traps, a tool that was found appropriate to measure P. argentipes density24, on two consecutive nights at baseline (November 2006) and 5 to 6 months post-intervention (April 2007). The results were analysed as a pool (4 sites together) and per site using a mixed Poisson regression model. IRS of houses and cattle sheds, supervised by the researchers, reduced the indoor P. argentipes density by 72.4 per cent in intervention clusters compared to control. This effect was consistent in all sites23 and confirmed in Nepal where additional entomological surveys were conducted25. However, the site specific results should be interpreted with caution as the number of replicas was limited (6 clusters per arm and site), the insecticides used were different (DDT in India, deltamethrin in Bangladesh and alpha-cypermethrine in Nepal) and exterior as well as interior walls were sprayed in Bangladesh but not in the other sites. In India, a similar effect of IRS on P. argentipes density was also observed in a “before-after comparison” study in 119 villages in 4 endemic districts in Bihar26. The IRS activities conducted from February 15 to April 15, 2007 as part of the national programme were supervised by the researchers. P. argentipes indoor density was evaluated by aspirator method at baseline and at one and three months post-spraying. The percentage of houses with P. argentipes significantly decreased after IRS in 3 of the 4 districts. Unfortunately the effect was only assessed for a maximum of 3 months and no details on the statistical methods or the magnitude of the effect were provided. In the same study, the number of reported VL cases in December 2006 and 2007 in 19 districts in Bihar were compared. The incidence decreased in 15 districts but increased in four. The latter increase was associated with a rise in the number of cases reporting to public health facilities where miltefosine was provided. No formal statistical analyses were conducted to study the association between IRS and reported VL26, and no controls were included in the study. The same authors reported an observational study (1998-2008) on the association between VL cases and vector density in four districts in Bihar: two highly endemic (Vaishali and Muzaffarpur) and two low endemic (Patna and Nalanda)27. Low endemic districts had higher P. argentipes density but lower VL incidence than high endemic districts. The authors hypothesised that higher level of P. argentipes exposure in Patna and Nalanda, where IRS was occasional and on focal basis, would confer some degree of protection against L. donovani infection. This protection would be provided by the regular exposure to sandfly saliva27. However, this may be an oversimplification as the risk factors associated to L. donovani infection and VL are quite complex28. Further, this hypothesis was not supported by the observations in Nepal where focal and occasional spraying had little impact on VL incidence29. Moreover, in Bangladesh where vector control activities were almost inexistent since the early 1980s the VL incidence has been rising since then20.
Without doubt, when applied properly IRS significantly reduces the P. argentipes indoor density23. Intensive and correct IRS combined with active detection and treatment of VL cases seems to effectively control L. donovani transmission in endemic communities18,30. However, the impact of IRS on VL has not been properly evaluated yet and it seems that the current IRS strategies are failing to control VL in India12,17,18, Nepal13 and Bangladesh20. Mondal et al31 reported that, despite the recommendations from the VL elimination programme8, vector control activities are almost nonexistent in VL endemic districts in Bangladesh. In India and Nepal the routine IRS activities conducted as part of the national VL elimination programme were suboptimal in terms of training, equipment, insecticide concentration and application32. It should be noted that even under these suboptimal conditions, IRS reduced the vector density for at least four weeks32. However, other groups reported that governmental IRS campaigns did not have a significant effect on P. argentipes indoor density33,34 or L. donovani infection and VL35. Recently, Dinesh et al36 reported decreased susceptibility of P.argentipes to DDT in India. Efforts should be made to ensure that adequate training is provided to local and managerial staff and that IRS activities are properly monitored. WHO/TDR has recently released specific guidelines to monitor IRS in the context of the VL elimination programme37.
Insecticide treated nets
Two cluster randomised trials have been conducted since 2005 in the Indian subcontinent to assess the impact of village-wide distribution of ITNs on P. argentipes density23,34 though only one included clinical endpoints35. Both trials used the same long-lasting insecticidal nets (PermaNet 2.0 - 55 mg/m2, Deltamethrin coated net) in the intervention clusters.
Joshi et al23 conducted a study in VL endemic villages in India, Nepal and Bangladesh. Households in clusters where LNs were distributed had 43.7 per cent less P. argentipes compared to control 5 months post-intervention. However, when the impact was analysed per site, the reduction was significant in the Indian and Bangladeshi sites but not in Nepal23. As part of the KALANET project (ClinicalTrials.gov NCT00318721) the effectiveness of LN to prevent VL was assessed in 26 clusters (16 in India and 10 in Nepal) matched by country, population and past VL incidence. All household in intervention clusters (8 in India and 5 in Nepal) received LN in November-December 2006. The control clusters did not receive any treated nets but were allowed to use their own untreated nets. The research team did not interfere with the governmental IRS activities but collected detailed information on the houses sprayed during the trial and considered this in the analysis. The impact of LN on indoor P. argentipes density was assessed in a subset of clusters (n=12), six clusters (3 intervention and 3 control) per country. The sandfly indoor density was evaluated in 10 sentinel households and cattle sheds per cluster selected based on their P. argentipes density in September 2006. Monthly CDC light trap captures and mouth aspiration collections were conducted before intervention (September-November 2006) and 12 months post-distribution of LN in intervention clusters. The intervention and control clusters had similar pre-intervention P. argentipes indoor density. After 12 months of LN use, households in intervention clusters had 24.9 per cent less P. argentipes compared to controls measured using CDC light traps. A similar effect was observed in India and Nepal. The effect of the governmental IRS campaign on the sandfly density was limited. When house spraying was accounted for in the analysis, the reduction of P. argentipes density due to LN was 31.9 per cent. The distribution of LN did not have an effect on the number of P. argentipes captured in cattle sheds located close to the sentinel households34. The slightly stronger reduction of vector density observed in the trial reported by Joshi et al23. may be related to the study design (i.e. inclusion of clusters from Bangladesh), the shorter follow up time (i.e. 5 vs 12 months), timing of the post-intervention surveys or the statistical models used to analyse the data, but both trials consistently showed that LN reduced the P. argentipes indoor density.
The KALANET project was the only trial that evaluated the impact of LN on L. donovani infection. The direct agglutination test (DAT) was used to compare the risk of L. donovani infection over 24 months in 13 clusters where LN were distributed to 13 control clusters. The impact of LN on the number of VL cases was assessed as a secondary outcome. The 26 clusters were selected based on their past VL incidence38,39. Almost 20,000 people were included in the trial and 12,261 of them were enrolled for the main outcome (L. donovani infection). Over 24 months the incidence of L. donovani infection (5.4 vs 5.5%) and clinical visceral leishmaniasis (0.38 vs 0.40%) were similar in intervention and control groups. The cluster analysis indicated that LN reduced the risk of L. donovani infection and VL by 10 and 1 per cent, respectively. These conclusions were not altered when the statistical models were adjusted for IRS, socio-economic status, age and sex. The results of the trial, which were not statistically significant and had large confidence intervals, indicate that large-scale distribution of long-lasting insecticidal nets would not provide much additional protection against VL at village level compared to current control measures in India and Nepal35. LN and untreated nets seem to provide some degree of personal protection because the risk for infection was lower in people using untreated nets or LN compared to people not using any net35. The number of bloodfed P. argentipes collected in houses was also lower after untreated nets were used40. The investigators of the KALANET trial concluded that LNs have beneficial effects as these they provided some degree of personal protection against L. donovani infection and reduced the risk of malaria, and therefore, the use of LN should be promoted in the region. However, the authors warned as well that large-scale distribution of LNs in VL endemic communities in India and Nepal in the current context (i.e. suboptimal IRS and widespread use of untreated nets) would not be sufficient to reduce significantly the L. donovani transmission in the community. So vector control activities to prevent VL in endemic communities in India and Nepal cannot rely on LN distribution only.
The main limitation of the KALANET trial was that it used L. donovani infection and not clinical VL as the main outcome to assess the impact of LN. Unfortunately the latter would require following a larger number of people for a long period of time because of the long and variable incubation period of VL and the relatively low VL incidence in the region. Bern et al28 suggest that using seroconversion (L. donovani infection) as the major outcome in intervention trials may lead to erroneous conclusions about the risk of disease. The results of the KALANET trial challenge this theory as (i) the impact of LN on DAT seroconversion and clinical VL were consistent35, and (ii) DAT seroconverters were at higher risk to develop VL than non-seroconverters (Relative Risk=11.5) in the study clusters41. Similar results were obtained when the impact of LN was assessed using a different serological marker (i.e. rK39 ELISA) (J Menten, personal communication), and when a proxy marker of exposure (sandfly saliva antibody) was used42.
Several other factors may explain the lack of effect (non-compliance, insecticide resistance, quality of nets, etc.), and many of these were studied in the trial. As the use of LN in the intervention clusters was high35 and correct (Vanlerberghe, personal communication) throughout the trial, LN deltamethrin concentration and effectiveness after 24 months of household use were acceptable43 and P. argentipes were susceptible to deltamethrin36.; the authors suggested that the lack of effect observed may be related to a substantial fraction of L. donovani transmission occurring outdoors where LNs would have less impact on preventing sandfly-human contact35. This theory is supported by entomological findings that P. argentipes breed preferably in cattle sheds compared to households44. Similarly, the fact that LN distributed to some households in VL endemic villages in Bihar failed to reduce indoor female P. argentipes suggests that most of the sandflies are breeding outdoors33. Further, recent studies have captured significant numbers of adult P. argentipes outdoors using CDC light traps45,46 and a large proportion of these (i.e. 90% of female P. argentipes collected from palm tree) had fed on humans, suggesting that P. argentipes are exophagic46,47. These findings are also supported by epidemiological findings. Using a recently developed sandfly saliva ELISA to assess the exposure to P. argentipes48, LN reduced P. argentipes exposure only by 9 to 12 per cent and a significant number of individuals had high levels of anti-P. argentipes saliva antibodies (i.e. 43.5% were ELISA positive) after 24 months of LN use42.
These results do not dismiss the use of LN, or insecticide treated nets in general, as a tool for P. argentipes control, especially in Bangladesh where no IRS programme was operating until recently20. A non-randomized controlled trial in two VL endemic districts in Bangladesh found that villages where the bed nets owned by the households were treated with KO-Tab 123 (slow release deltamethrin dipping tablets) had a 60 per cent reduction in indoor sandfly density compared to control after 18 months49. Over 95 per cent of households in the intervention sites participated and over 90 per cent of the households’ nets were treated. The impact of ITN was measured comparing the sandfly indoor density at 4 wk, 12 and 18 months between 50 and 26 households in intervention and control sites, respectively. The trial results were similar at the different time points and they were not altered when the model was adjusted for presence of cattle sheds and the number of trees around the house49. The study was part of an operational research programme so study sites could not be randomized and there were only two intervention areas which had different population sizes (8287 vs 35,442 inhabitants). Baseline characteristics and control sites were not described in detail. The authors analysed the impact of ITN on sandflies in general but not on P. argentipes specifically49. The authors suggest that higher susceptibility of the vector to insecticide in Bangladesh may explain the differences with the results obtained in previous trials in India and Nepal49. Recent studies showed that wild caught P. argentipes are fully susceptible to deltamethrin in VL endemic areas in India and Nepal36.
Alternative and integrated vector control methods
The fact the current IRS and LN (or ITN) had an inconclusive impact on P. argentipes population and L. donovani transmission urges the need to develop alternative vector control methods and assess the impact of integrated vector management (IVM). The impact of plastering household walls on P. argentipes density was assessed in 24 intervention sites compared to 24 control clusters in India, Nepal and Bangladesh23. The overall effect was a 42 per cent reduction in sandfly counts in households in the intervention clusters. However, there were important differences among sites. In Bangladesh (one site) walls plastered with mud did not reduce P. argentipes density. In India (one site) and Nepal (two sites) walls were plastered with lime. Vector densities were significantly reduced in two sites only (one in India and one in Nepal). The authors associated the failure in the second Nepalese site to random error, the quality of the lime applied or the acidity of the soil23. Currently Genesis laboratories (http://www.genesislabs.com) are testing the impact of oral delivery of insecticides to cattle on P. argentipes density in Bihar. The preliminary results of this study showed an important reduction in vector density (over 90%) when oral insecticides and IRS were used in VL endemic villages in Bihar50. The efficacy of this strategy will need to be tested in a larger trial.
To date there are no studies assessing the effect of combining different vector control methods on P. argentipes density or VL. Based on the results described above, an integrated approach using different control methods targeting adult sandflies at different location (i.e. IRS in cattle sheds and LN in households) and environmental changes reducing the P. argentipes breeding sites may have a greater impact on L. donovani transmission than any of these methods alone. Similarly, future trials on P. argentipes control methods in the Indian subcontinent should assess the impact of these on other relevant vector borne diseases in the Region, specially malaria51. As an example, IRS was effectively used to control malaria in the Region until 1970s12 and cluster-wide provision of LNs in the KALANET trial significantly reduced the indoor density of anophelines by 22.9 per cent34 and the risk of malaria by 54 per cent in VL endemic villages in India and Nepal35. The WHO is promoting integrated vector management to increase cost-effectiveness and sustainability of vector control programmes by optimising the available resources and targeting more than one disease52.
Conclusions
The following conclusions can be drawn based on studies described in this review: (i) indoor residual spraying has an important impact on P. argentipes density when it is conducted properly, (ii) governmental IRS campaigns were non-existent in Bangladesh up to 2010 and suboptimal in India and Nepal over the period 2005-2010, (iii) to date, there are no prospective studies assessing the impact of IRS on L. donovani infection or VL in the Region, (iv) LNs may provide some degree of personal protection against VL but these had a limited impact on L. donovani transmission in VL endemic communities in India and Nepal, (v) entomological studies should be designed to quantify the L. donovani transmission outdoors in VL endemic villages in India and Nepal, (vi) ITN seem to have greater effect on P. argentipes density in Bangladesh than in India and Nepal, and (vii) impact of integrated vector management on VL and other vector borne diseases in the Region should be tested in prospective studies.
References
- 1.Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis. 2007;1:e114. doi: 10.1371/journal.pntd.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narain JP, Dash AP, Parnell B, Bhattacharya SK, Barua S, Bhatia R, et al. Elimination of neglected tropical diseases in the South-East Asia Region of the World Health Organization. Bull World Health Organ. 2010;88:206–10. doi: 10.2471/BLT.09.072322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Update on the status of visceral leishmaniasis (kala azar) in the SEA region. New Delhi: WHO SEARO; 2010. WHO. [Google Scholar]
- 5.Singh SP, Reddy DC, Rai M, Sundar S. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop Med Int Health. 2006;11:899–905. doi: 10.1111/j.1365-3156.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 6.Sacks DL, Kenney RT, Kreutzer RD, Jaffe CL, Gupta AK, Sharma MC, et al. Indian kala-azar caused by Leishmania tropica. Lancet. 1995;345:959–61. doi: 10.1016/s0140-6736(95)90703-3. [DOI] [PubMed] [Google Scholar]
- 7.Swaminath CS, Shortt HE, Anderson LA. Transmission of Indian kala-azar to man by bites of Phlebotomus argentipes Annandale & Brunetti. Indian J Med Res. 1942;30:473–7. [PubMed] [Google Scholar]
- 8.Regional strategic framework for elimination of kalaazar from the South-East Asia region (2005-2015) New Delhi: Regional Office for South-East Asia SEA-VBC-85 (Rev-1). World Health Organization; 2005. WHO. [Google Scholar]
- 9.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–26. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK. Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes (Diptera: Psychodidae) Ann Trop Med Parasitol. 2001;95:197–202. doi: 10.1080/00034980120041071. [DOI] [PubMed] [Google Scholar]
- 11.Picado A, Das ML, Kumar V, Dinesh DS, Rijal S, Singh SP, et al. Phlebotomus argentipes seasonal patterns in India and Nepal. J Med Entomol. 2010;47:283–6. doi: 10.1603/me09175. [DOI] [PubMed] [Google Scholar]
- 12.Ostyn B, Vanlerberghe V, Picado A, Dinesh DS, Sundar S, Chappuis F, et al. Vector control by insecticide-treated nets in the fight against visceral leishmaniasis in the Indian subcontinent, what is the evidence? Trop Med Int Health. 2008;13:1073–85. doi: 10.1111/j.1365-3156.2008.02110.x. [DOI] [PubMed] [Google Scholar]
- 13.Joshi AB, Banjara MR, Pokhrel S, Jimba M, Singhasivanon P, Ashford RW. Elimination of visceral leishmaniasis in Nepal: pipe-dreams and possibilities. Kathmandu Univ Med J (KUMJ) 2006;4:488–96. [PubMed] [Google Scholar]
- 14.Joshi RD, Rai RN. Impact of DDT spraying on populations of P. argentipes and P. papatasi in Varanasi district, Uttar Pradesh. J Commun Dis. 1994;26:56–8. [PubMed] [Google Scholar]
- 15.Kaul SM, Sharma RS, Dey KP, Rai RN, Verghese T. Impact of DDT indoor residual spraying on Phlebotomus argentipes in a kala-azar endemic village in eastern Uttar Pradesh. Bull World Health Organ. 1994;72:79–81. [PMC free article] [PubMed] [Google Scholar]
- 16.Mukhopadhyay AK, Hati AK, Chakraborty S, Saxena NB. Effect of DDT on Phlebotomus sandflies in Kala-Azar endemic foci in West Bengal. J Commun Dis. 1996;28:171–5. [PubMed] [Google Scholar]
- 17.Kishore K, Kumar V, Kesari S, Dinesh DS, Kumar AJ, Das P, et al. Vector control in leishmaniasis. Indian J Med Res. 2006;123:467–72. [PubMed] [Google Scholar]
- 18.Thakur CP. A new strategy for elimination of kala-azar from rural Bihar. Indian J Med Res. 2007;126:447–51. [PubMed] [Google Scholar]
- 19.Joshi AB, Bhatt LR, Regmi S, Ashford RW. An effectiveness of insecticide spray in the control of visceral leishmaniasis in Nepal. J Nepal Health Res Council. 2003;1:1–6. [Google Scholar]
- 20.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: prospects for improved control. Indian J Med Res. 2006;123:275–88. [PubMed] [Google Scholar]
- 21.Regional Technical Advisory Group on Kala-azar Elimination Report of the first meeting, Manesar. New Delhi: Regional Office for South-East Asia, World Health Organization; 2005. WHO. [Google Scholar]
- 22.Kumar V, Kesari SK, Sinha NK, Palit A, Ranjan A, Kishore K, et al. Field trial of an ecological approach for the control of Phlebotomus argentipes using mud & lime plaster. Indian J Med Res. 1995;101:154–6. [PubMed] [Google Scholar]
- 23.Joshi AB, Das ML, Akhter S, Chowdhury R, Mondal D, Kumar V, et al. Chemical and environmental vector control as a contribution to the elimination of visceral leishmaniasis on the Indian subcontinent: cluster randomized controlled trials in Bangladesh, India and Nepal. BMC Med. 2009;7:54. doi: 10.1186/1741-7015-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinesh DS, Das P, Picado A, Davies C, Speybroeck N, Boelaert M, et al. The efficacy of indoor CDC light traps for collecting the sandfly Phlebotomus argentipes, vector of Leishmania donovani. Med Vet Entomol. 2008;22:120–3. doi: 10.1111/j.1365-2915.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 25.Das ML, Roy L, Rijal S, Paudel IS, Picado A, Kroeger A, et al. Comparative study of kala-azar vector control measures in eastern Nepal. Acta Trop. 2010;113:162–6. doi: 10.1016/j.actatropica.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Kumar V, Kesari S, Dinesh DS, Tiwari AK, Kumar AJ, Kumar R, et al. A report on the indoor residual spraying (IRS) in the control of Phlebotomus argentipes, the vector of visceral leishmaniasis in Bihar (India): an initiative towards total elimination targeting 2015 (Series-1) J Vector Borne Dis. 2009;46:225–9. [PubMed] [Google Scholar]
- 27.Kumar V, Kesari S, Kumar AJ, Dinesh DS, Ranjan A, Prasad M, et al. Vector density and the control of kala-azar in Bihar, India. Mem Inst Oswaldo Cruz. 2009;104:1019–22. doi: 10.1590/s0074-02762009000700014. [DOI] [PubMed] [Google Scholar]
- 28.Bern C, Courtenay O, Alvar J. Of cattle, sand flies and men: a systematic review of risk factor analyses for South Asian visceral leishmaniasis and implications for elimination. PLoS Negl Trop Dis. 2010;4:e599. doi: 10.1371/journal.pntd.0000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi DD, Sharma M, Bhandari S. Visceral leishmaniasis in Nepal during 1980-2006. J Commun Dis. 2006;38:139–48. [PubMed] [Google Scholar]
- 30.Thakur CP, Meenakshi Thakur AK, Thakur S. Newer strategies for the kala-azar elimination programme in India. Indian J Med Res. 2009;129:102–4. [PubMed] [Google Scholar]
- 31.Mondal D, Alam MS, Karim Z, Haque R, Boelaert M, Kroeger A. Present situation of vector-control management in Bangladesh: a wake up call. Health Policy. 2008;87:369–76. doi: 10.1016/j.healthpol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury R, Huda MM, Kumar V, Das P, Joshi AB, Banjara MR, et al. The Indian and Nepalese programmes of indoor residual spraying for the elimination of visceral leishmaniasis: performance and effectiveness. Ann Trop Med Parasitol. 2011;105:31–5. doi: 10.1179/136485911X12899838683124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinesh DS, Das P, Picado A, Davies C, Speybroeck N, Ostyn B, et al. Long-lasting insecticidal nets fail at household level to reduce abundance of sandfly vector Phlebotomus argentipes in treated houses in Bihar (India) Trop Med Int Health. 2008;13:953–8. doi: 10.1111/j.1365-3156.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 34.Picado A, Das ML, Kumar V, Kesari S, Dinesh DS, Roy L, et al. Effect of village-wide use of long-lasting insecticidal nets on visceral leishmaniasis vectors in India and Nepal: a cluster randomized trial. PLoS Negl Trop Dis. 2010;4:e587. doi: 10.1371/journal.pntd.0000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picado A, Singh SP, Rijal S, Sundar S, Ostyn B, Chappuis F, et al. Longlasting insecticidal nets for prevention of Leishmania donovani infection in India and Nepal: paired cluster randomized trial. BMJ. 2010;341:c6760. doi: 10.1136/bmj.c6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinesh DS, Das ML, Picado A, Roy L, Rijal S, Singh SP, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl Trop Dis. 2010;4:e859. doi: 10.1371/journal.pntd.0000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monitoring and evaluation tool kit for indoor residual spraying. Kala-azar elilmination in Bangladesh, India and Nepal. New Delhi: Regional Office for South-East Asia, World Health Organization; 2010. WHO. [Google Scholar]
- 38.Rijal S, Uranw S, Chappuis F, Picado A, Khanal B, Paudel IS, et al. Epidemiology of Leishmania donovani infection in high-transmission foci in Nepal. Trop Med Int Health. 2010;15(Suppl 2):21–8. doi: 10.1111/j.1365-3156.2010.02518.x. [DOI] [PubMed] [Google Scholar]
- 39.Singh SP, Picado A, Boelaert M, Gidwani K, Andersen EW, Ostyn B, et al. The epidemiology of Leishmania donovani infection in high transmission foci in India. Trop Med Int Health. 2010;15(Suppl 2):12–20. doi: 10.1111/j.1365-3156.2010.02519.x. [DOI] [PubMed] [Google Scholar]
- 40.Picado A, Kumar V, Das M, Burniston I, Roy L, Suman R, et al. Effect of untreated bed nets on blood-fed Phlebotomus argentipes in kala-azar endemic foci in Nepal and India. Mem Inst Oswaldo Cruz. 2009;104:1183–6. doi: 10.1590/s0074-02762009000800018. [DOI] [PubMed] [Google Scholar]
- 41.Ostyn B, Gidwani K, Khanal B, Picado A, Chappuis F, Singh SP, et al. Incidence of symptomatic and asymptomatic Leishmania donovani infections in high-endemic foci in India and Nepal: a prospective study. PLoS Negl Trop Dis. 2011;5:e1284. doi: 10.1371/journal.pntd.0001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gidwani K, Picado A, Rijal S, Singh SP, Roy L, Volfova V, et al. Serological markers of sand fly exposure to evaluate insecticidal nets against visceral leishmaniasis in India and Nepal: a cluster-randomized trial. PLoS Negl Trop Dis. 2011;5:e1296. doi: 10.1371/journal.pntd.0001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picado A, Singh SP, Vanlerberghe V, Uranw S, Ostyn B, Kaur H, et al. Residual activity and integrity of PermaNet® 2.0 after 24 months of household use in a community randomised trial of long lasting insecticidal nets against visceral leishmaniasis in India and Nepal. Trans R Soc Trop Med Hyg. 2012;106:150–9. doi: 10.1016/j.trstmh.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Singh R, Lal S, Saxena VK. Breeding ecology of visceral leishmaniasis vector sandfly in Bihar state of India. Acta Trop. 2008;107:117–20. doi: 10.1016/j.actatropica.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Dinesh DS, Bhattacharya SK, Das P. Peridomestic breeding and resting sites of sandflies (Diptera: Psychodidae) in Bihar, India. Entomol News. 2009;120:496–501. [Google Scholar]
- 46.Poche D, Garlapati R, Ingenloff K, Remmers J, Poche R. Bionomics of phlebotomine sand flies from three villages in Bihar, India. J Vector Ecol. 2011;36(Suppl 1):S106–17. doi: 10.1111/j.1948-7134.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 47.Poche RM, Garlapati R, Elnaiem DE, Perry D, Poche D. The role of Palmyra palm trees (Borassus flabellifer) and sand fly distribution in northeastern India. J Vector Ecol. 2012;37:148–53. doi: 10.1111/j.1948-7134.2012.00211.x. [DOI] [PubMed] [Google Scholar]
- 48.Clements MF, Gidwani K, Kumar R, Hostomska J, Dinesh DS, Kumar V, et al. Measurement of recent exposure to Phlebotomus argentipes, the vector of Indian visceral leishmaniasis, by using human antibody responses to sand fly saliva. Am J Trop Med Hyg. 2010;82:801–7. doi: 10.4269/ajtmh.2010.09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mondal D, Chowdhury R, Huda MM, Maheswary NP, Akther S, Petzold M, et al. Insecticide-treated bed nets in rural Bangladesh: their potential role in the visceral leishmaniasis elimination programme. Trop Med Int Health. 2010;15:1382–9. doi: 10.1111/j.1365-3156.2010.02635.x. [DOI] [PubMed] [Google Scholar]
- 50.Poche D. 7th International Symposium on Phlebotomine Sand flies (ISOPS) 2011. Kusadasi (Turkey): 2011. Apr 25-30, Control of adult and larval Phlebotomus argentipes by treating cattle with oral insecticides and insect growth regulators in India; p. 111. 0-099. [Google Scholar]
- 51.Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, et al. Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet. 2010;376:1768–74. doi: 10.1016/S0140-6736(10)60831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Global strategic framework for integrated vector management. WHO/CDS/CPE/PUC/2004.10. Geneva: World Health Organization; 2004. WHO. [Google Scholar]


