Abstract
Background & objectives:
Transmission of dengue virus depends on the presence of Aedes mosquito. Mosquito generation and development is known to be influenced by the climate. This study was carried out to examine whether the climatic factors data can be used to predict yearly dengue cases of Dhaka city, Bangladesh.
Methods:
Monthly reported dengue cases and climate data for the years 2000–2008 were obtained from the Directorate General of Health Services (DGHS) and Meteorological Department of Dhaka, Bangladesh, respectively. Data for the period 2000 to 2007 were used for development of a model through multiple linear regressions. Retrospective validation of the model was done with 2001, 2003, 2005 and 2008 data. Log transformation of the dependent variable was done to normalize data for linear regression. Average monthly humidity, rainfall, minimum and maximum temperature were used as independent variables and number of dengue cases reported monthly was used as dependent variable. Accuracy of the model for predicting outbreak was assessed through receiver operative characteristics (ROC) curve.
Results:
Climatic factors, i.e. rainfall, maximum temperature and relative humidity were significantly correlated with monthly reported dengue cases. The model incorporating climatic data of two-lag month explained 61 per cent of variation in number of reported dengue cases and this model was found to predict dengue outbreak (≥ 200 cases) with considerable accuracy [area under ROC curve = 0.89, 95%CI = (0.89-0.98)].
Interpretation & conclusions:
Our results showed that the climate had a major effect on the occurrence of dengue infection in Dhaka city. Though the prediction model had some limitations in predicting the monthly number of dengue cases, it could forecast possible outbreak two months in advance with considerable accuracy.
Keywords: Climate, dengue, humidity, outbreak, prediction model, rainfall, temperature
The dengue virus, one of the arboviruses causes classical dengue fever (DF) and dengue haemorrhagic fever (DHF) primarily in the tropical and subtropical regions. Bangladesh with the tropical climate has become an ideal land for dengue virus transmission. Though the first documented outbreak of DF from Bangladesh was in 19641 in Dhaka city followed by a few scattered cases of DF during 1977-1978 and 1996-1997, the first epidemic of DHF occurred in mid 2000, which was reported from all the major cities2. Presence of both the vectors, Aedes. aegypti and Ae. albopictus, was also identified during different outbreaks3,4.
The Aedes mosquito is a climate sensitive vector. It is the primary amplification and transmission host for dengue and yellow fever virus. The geographic distribution of the Aedes mosquito is largely determined by climate. Several studies found that the climate of a specific geographic region and number of dengue cases have a strong and consistent relationship5. Some found the climate likely to play a small but significant part6, others ruled out any significant role7. To predict a possible dengue outbreak, different models have been developed by relating dengue cases with climatic data8,9. In this study we aimed to develop a model to predict monthly dengue cases using eight years monthly data on climate factors and reported cases of dengue infection from the Dhaka city, Bangladesh.
Material & Methods
Data analysis, model development and validation: The number of monthly reported dengue cases admitted or treated at various government and private health facilities in Dhaka city was obtained from the Diseases Control room of Directorate General of Health Services (DGHS) and the climatic data from Bangladesh Meteorological Department at Dhaka.
The monthly climate data were correlated with the number of monthly reported dengue cases. Data of 2000 to 2007 were used for development of the model through multiple linear regression analysis and of 2001, 2003, 2005 and 2008 were used for retrospective validation of the proposed model. As the data were positively skewed (skeweness = 3.9 ± SE 0.246), log transformation of the dependent variable was done to normalize the data for linear regression. Assumption for independence of data points were checked through generation of Durbin-Watson estimate10. Average monthly humidity, rainfall, minimum and maximum temperature were used as independent variables and number of dengue cases reported monthly was used as dependent variable. A one-way analysis of variance (ANOVA) was used to see if each one of the climate variables differs significantly between seasons.
The accuracy of model was judged based on explanatory capacity (coefficient of determination, adjusted R2). We tried to predict the number of cases that would occur (forecasted) in the years 2001, 2003, 2005 and 2008 with the regression equation obtained from our best-fit model and later on compared the number of cases that were actually reported in that year. Both the forecasted and observed dengue data were plotted for correlation. Pearson correlation coefficient was obtained to see the strength of correlation. Accuracy of the model for predicting outbreak (≥ 200 cases) was assessed through receiver operative characteristics (ROC) curve.
Results
Temporal distribution of dengue cases: Over the 9-year (2000-2008) period, a total of 22,705 cases of dengue were reported to the surveillance system. The number of reported dengue cases varied by year. Over the study period, highest cases of dengue were reported in 2002 (n=5851) and lowest in the following year (n=450) (Fig. 1). Interestingly every second year had an explosive number of cases, followed by lower number of cases in the following year.
Fig. 1.
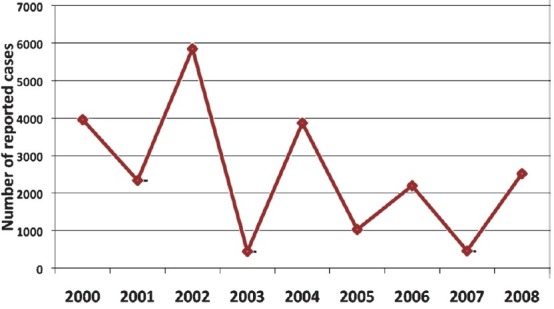
Total number of reported dengue cases, by year (2000-2008), Dhaka city, (n = 22,705).
In each year, dengue cases showed a specific pattern of occurrence. During pre-monsoon period barely any cases of dengue were reported (Table I). Most of the cases were reported during monsoon in each year except in the year 2003 where monthly dengue cases reported were only two during monsoon period, and interestingly in this year lowest number of dengue cases were reported (n=450) among all the years. In this particular year more cases were reported in the post-monsoon period. During the nine years, hardly any case was found to occur during January to June (Fig. 2). Significant number of cases were reported in the month of July and reaching a peak in August and gradually declined at the end of the year.
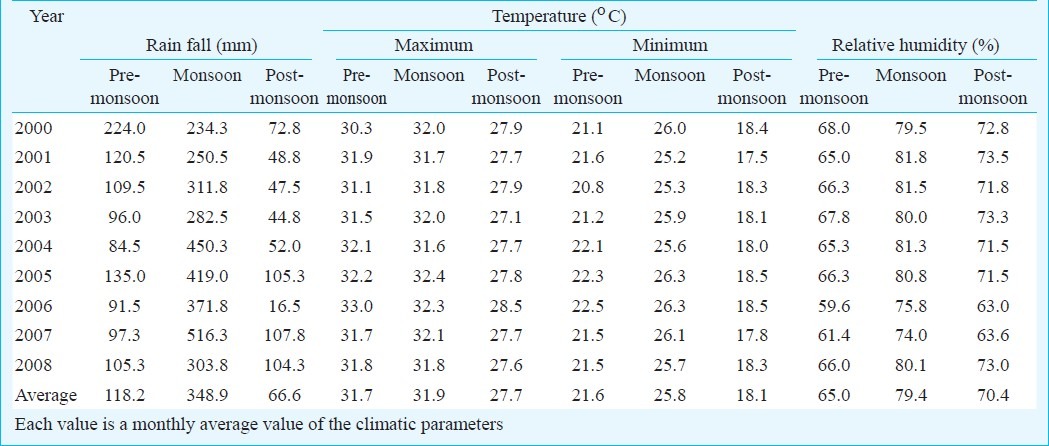
Table I.
Seasonal variation in number of reported dengue cases in each year (2000-2008)
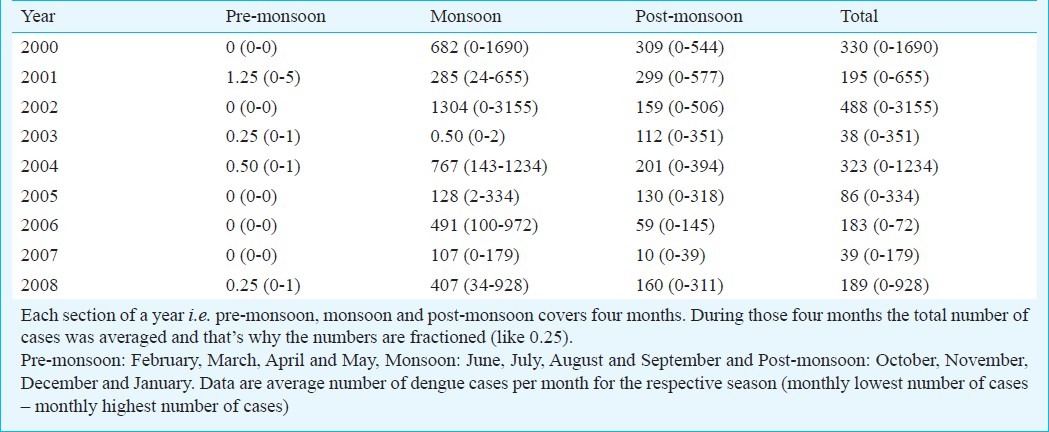
Fig. 2.
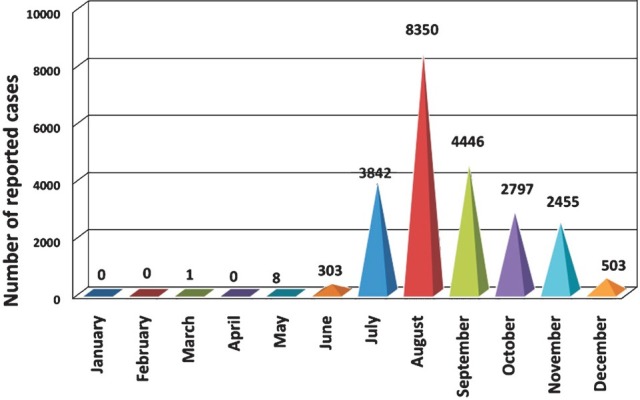
Total number of reported dengue cases, by month (January to December), Dhaka city, 2000-2008 (n = 22,705)
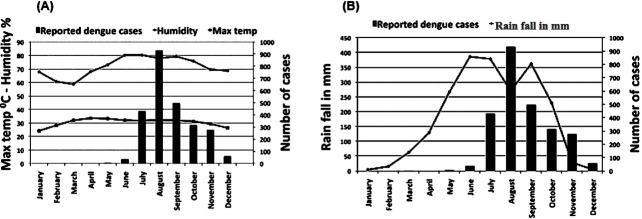
Climatic influence: The dengue outbreak in Dhaka city coincided mainly with the monsoon period with heavy rainfall started from June and lasted up to September, followed by relatively less rainfall during the post-monsoon period from October to January. The period from February to May is considered as pre-monsoon period. Temperature, rain and humidity varied significantly over three distinct periods in each year (Table II). Average monthly pre-monsoon rain was recorded 118.2 mm; during monsoon, which raised to 349 mm and during post-monsoon the amount sharply falls to 67 mm only. The overall variation was significant (ANOVA P<0.001). Rainfall during monsoon was significantly higher than the other two periods (P<0.01) but was not significantly different between pre- and post-monsoon period. The average relative humidity recorded during the pre-monsoon period was 65.0 per cent, which was found to be 79.4 per cent during the monsoon period and 70.04 per cent during the post-monsoon period (Table II). The overall variation was statistically significant (ANOVA P<0.001). The relative humidity during monsoon was significantly higher than the other two periods (P<0.001). Average highest and lowest temperatures were 31.7°C and 21.6°C, respectively during the pre- monsoon period. During monsoon and post-monsoon periods the mercury rose to 31.9 and 25.8°C and fell to 27.7°C and 18.1°C, respectively. The overall variation of both maximum and minimum temperatures across the three seasons was found to be significant (P<0.001). The minimum temperature was higher in the monsoon than the other two periods (P<0.001) but the maximum temperature during monsoon was significantly higher only from the post-monsoon period (P<0.01).
Table II.
Distribution of climatic parameters of Dhaka city, by season (2000 - 2008)
The number of dengue cases was plotted against the climatic factors (rain, temperature and humidity) to assess their contribution across the years (Fig. 3). As the rainfall increased, the cases of dengue also started rising from June. With declining rainfall from October to December, dengue cases also showed gradual decline. Over the years, the average relative humidity showed increasing trend during the dengue outbreak period and the concavity started at least two months before the starting of the increase in the number of dengue cases and lasted two months ahead of outbreak period. There was narrowing of the gap between the average maximum and minimum temperature for a period of time, which corresponded with the monsoon period and highest number of dengue infection of a year.
Fig. 3.
Monthly average rainfall (A), humidity and maximum temperature (B) was plotted against average monthly dengue cases reported during 2000 and 2008.
Development of prediction model: The notification of dengue cases increased in a particular season i.e. monsoon, indicating a positive relation of dengue infection and climate. This correlation provided us a basis for a possible dengue prediction model. For prediction of number of dengue cases we initially pooled average monthly rainfall in mm, average maximum temperature, average minimum temperature, temperature fluctuation and average humidity in percentage in a particular month. Minimum temperature and temperature fluctuation were excluded from the initial set of variables due to their collinearity with maximum temperature. Automated model, selection through sequential entry of factors also reached similar conclusion on set of factors to be included. For the final model, average monthly rain, average maximum temperature and humidity were considered. We used tolerance measure to see collinearity between final set of factors included in the model. The tolerance value of collinearity diagnostics for humidity (0.584), rain (0.490) and maximum temperature (0.783) lied clearly above the acceptable value of 0.4. We also checked the independence of the data points through Durbin Watson estimates. The value (0.759) was clearly away from zero suggesting the considerable independence of the data points.
Three models were developed using the multiple regression analysis (Table III). In the first model, we incorporated data of all the seasons of eight consecutive years (2000-2007) and developed a model considering data of the present month (pm) and found the model to demonstrate capacity of explaining only 26.4 per cent of the total variations in the occurrence of dengue case. Due to direct but variable influence of the climatic factors, it takes 7 to 45 days to become an adult mosquito from an egg8. Therefore the influence of climate is expected to be visible 1 or 2 months later. In the next model (Model-2) we incorporated the data of one lag month as a lag phase allowing time for a new generation of mosquitoes to appear. With this model the explanatory capability seems to be increased to around 50.9 per cent. We tried another model (Model-3) with the assumption of average 2 months lag phase. In this model the explanatory capability further increased over the model-2 and could explain 61 per cent of the variation in dengue case occurrence. We tried another model with even higher lag period, however, the models failed to add to explanatory capacity, rather showed sharp decline. Hence model-3 was chosen as final model.
Table III.
Estimates and their standard errors (SE) based on the fitting of a multivarite linear regression model relating the monthly number of dengue cases with the climate variables
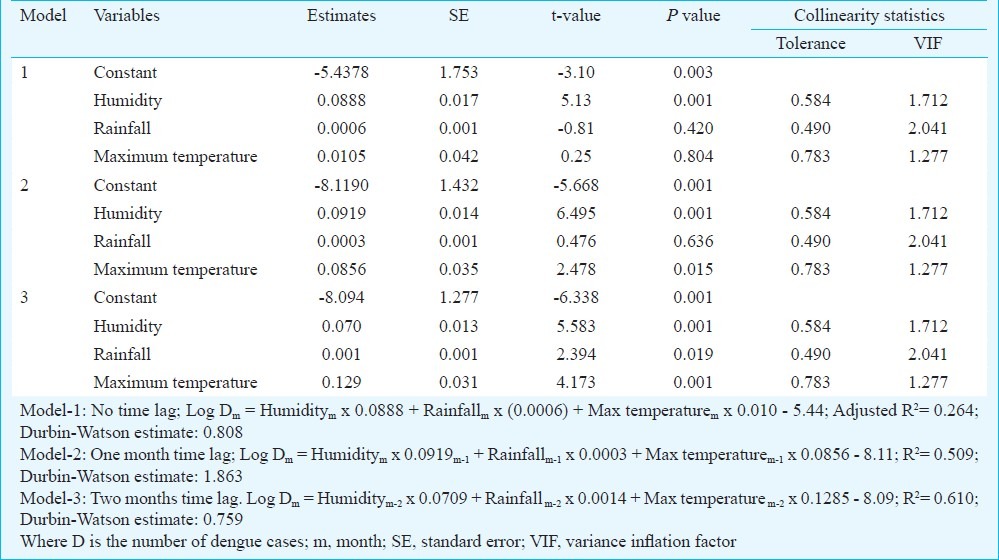
While generating the model all the relevant assumptions were checked for assuring best possible model to be selected. For model-3 standardized residual of the model (ranged between -3.0 and 2.2) did not exceed the tolerable limit suggesting minimal outlier in the data points. Normal probability plot of residuals also satisfied the normality assumption. Besides scatter plot of standardized residual against standardized predicted value depicted no clear pattern suggesting a desirable constant variance for the model. Among the variables included in the final model humidity (β = 0.591) demonstrated highest influence on number of dengue cases reported followed by maximum temperature (β = 0.025) and the average rainfall (β = -0.102).
Considering the fact that higher dengue cases were notified during the monsoon, we attempted to develop a prediction model with only monsoon period data (data not shown). The model demonstrated very poor explanatory capability (8.2%). The explanatory capacity of this model was not improved when we tried to generate models using the data of both 1 and 2 - lag month from the monsoon period.
Retrospective model validation: We retrospectively attempted to evaluate the prediction capability of our proposed model (Model-3) for predicting the number of dengue cases that occurred in 2001, 2003, 2005 and 2008 in Dhaka city. The forecasted notified dengue cases were plotted against the actually reported cases of the respective year. This model showed significant correlation between predicted and observed number of cases in 2001 (r =0.706 P=0.010), 2005 (r=0.837 P=0.001) and in 2008 (r=.627 P=0.029), although in 2003 (r=0.396 P=0.202) the correlation was not significant (Fig. 4).
Fig. 4.
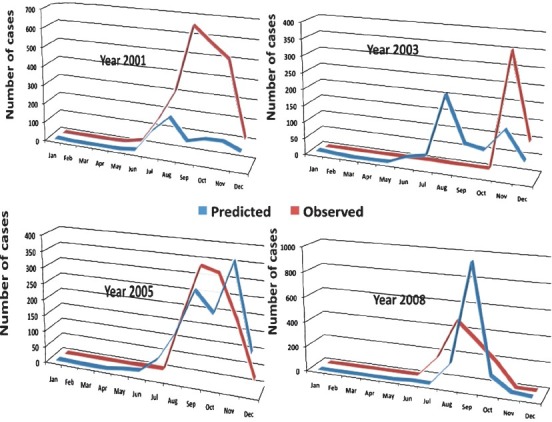
Corelation of observed and predicted dengue cases in 2001, 2003, 2005 and 2008 by Model-3.
Accuracy of the model for predicting outbreak (≥ 200 cases) was assessed through ROC analysis. Area under ROC = 0.89, 95%CI = 0.89-0.98 suggested high accuracy of the model.
Discussion
The growth and development of dengue vector is climate dependent. Being a vector borne disease, transmission of dengue virus is greatly dependent on the availability, density and extension of its vector. Worldwide studies have proposed that ecological and climate factors influence the seasonal prevalence of both the vector Ae. aegypti and dengue virus11,12.
The climate of Bangladesh, which is a part of tropical Asia, is mainly dominated by two monsoons (wet and dry monsoon) with year-to-year time variation. In the present study, three periods in the year considered for the analysis were the monsoon (June–September) with heavy rain fall of average 348.9 mm, the post – monsoon (October– January) with average rainfall of 66.6 mm and pre-monsoon (February–May) with an average rain fall of 118.2 mm. There was a significant difference not only in the amount of rainfall but also in the maximum and minimum temperature and relative humidity between these periods. Anecdotal evidences show significant increase in dengue infection during the monsoon season starting from the month of June, reaching the peak in August and gradually coming down to about zero during the post-monsoon time. Season-specific pattern of dengue cases in Southeast Asia has been reported by others also13. Our observations matched with other long-term studies from the same geographical region, Myanmar and India14,15, as also studies from the region of the Gulf of Thailand and from Puerto Rico5,16. The increase in vector density due to rainfall and subsequent increase in dengue cases has been reported before17,18.
It is considered that increase in global temperature may lead to expansion of area of involvement and number of cases of vector-borne diseases19,20. This will increase the proportion of mosquitoes that become infectious at a given time21,22 leading to an exponential increase of dengue cases. In our study, temperature was found to be closely related with the rise of dengue infection. The minimum temperature was significantly different between the study periods, number of dengue cases also varied in accordance with temperature. It has been shown that increase of temperature from 26-28 to 30°C decreases the extrinsic incubation of the dengue virus (serotype 2 & 4) which may facilitate the transmission of virus22,23. Dengue cases were found to be reported more during the months of monsoon in the present study when relative humidity was higher similar to earlier reports16. Higher humidity during rainy season facilitates the growth and survival of mosquitoes24,25 leading to an increase in the number of infected mosquitoes for successful propagation of virus. According to Barbazan et al25, an increase in mosquito longevity disproportionately enhances the number of potential transmissions by as much as five times when the survival rate rises from 0.80 to 0.9525. Though availability of dengue vectors was reported during our study period from Bangladesh3,4, we could not verify our finding due to unavailability of information regarding the seasonal variation of mosquito population.
During the study period every second year had an explosive nature of outbreak followed by lower number of cases in the following year. This type of fluctuation of dengue outbreaks was also observed in Thailand26,27. In a massive urban dengue outbreak 70-80 per cent of a population can be involved28 which may lead to development of herd immunity to the circulating dengue serotype/serotypes and thus it may prevent another outbreak in the following year. Molecular studies showed that the 2000 outbreak in Dhaka city was due to DEN-329 and during the 2002 outbreak, both serotypes DEN-3 and DEN-2 were circulating30. This indicates that besides climatic factors, introduction of new serotype or new virulent strains of existing serotypes in a naïve population is one of the factors that could contribute to new outbreak31.
This study had two interesting findings. First, the years with explosive nature of outbreak coincided with the monsoon period with 2-8 times added number of dengue cases than the post-monsoon period. On the other hand, the years which had comparatively lower number of cases, both the periods i.e. monsoon and post-monsoon, either had almost equal number of infections or more number of infections occurred in post-monsoon period. The second finding is that the significant difference between the minimum and maximum temperature, rainfall and humidity of monsoon and post-monsoon period remained almost the same in every year but the number of dengue cases varied. It was observed that the seasonal dengue infection rate of a particular period was related with climatic factor changes when we compared within that year, but when we checked with the same period of time in different years, it did not correlate with the number of infections. Both these points signify the attribution of other factors which in co-occurrence with climate influence dengue infection outcome in a given geographical area. However, in a few studies from different parts of the world, it has been reported that individually or collectively climatic factors had no role in dengue infection6,7.
Since our findings indicated that dengue outbreaks in the Dhaka city had to some extent, a linear relationship with the climatic factors, we attempted to convert the relationship in to a mathematical equation for developing a model suitable for prediction of possible toll of dengue cases in Dhaka city in a given month. Our predictive model-3 with climate factors as predictors could explain only 61 per cent of the variation in the occurrence of dengue cases. The remaining 39 per cent unexplained variation could be due to the influence of other factors. The other probable influencing factors of dengue infection include multiple serotypes and strain polymorphism, immune-mediated serotype interactions and environmental variables. Therefore, future attempt to develop a model to predict the total number of dengue case in each month should include data on immunological, entomological and demographical factors along with climatic factors. Otherwise, oversimplification of prediction model without establishing significant correlations with other factors may lead to wrong forecast of dengue outbreaks and might hamper the dengue prevention and control programme.
References
- 1.Russel PK, Busescher EL, McCown JM, Ordonez J. Recovery of dengue viruses from patients during epidemics in Puerto Rico and East Pakistan. Am J Trop Med Hyg. 1966;15:573–9. doi: 10.4269/ajtmh.1966.15.573. [DOI] [PubMed] [Google Scholar]
- 2.Amin MMM, Hussain AMZ, Nahar K, Chowdhury IA, Murshed M, Chowdhury SA. Sero-diagnosis of dengue infections in four metropolitan cities of Bangladesh. Dengue Bull. 2000;24:29–33. [Google Scholar]
- 3.Chowdhury MA, Wagatsuma Y, Hossain MI, Ahmed TU, Uddin MA, Kittayapong P. The First International Conference on dengue and dengue haemorrhagic fever. Chiang Mai, Thailand: 2000. Nov 20-24, Entomological assessment during the dengue outbreak in Dhaka city. Abstract; p. 110. [Google Scholar]
- 4.Ali M, Wagatsuma Y, Emch M, Breiman RF. Use of a geographic information system for defining spatial risk for dengue transmission in Bangladesh: Role for Aedes albopictus in an urban outbreak. Am J Trop Med Hyg. 2003;69:634–40. [PubMed] [Google Scholar]
- 5.Johansson MA, Dominici F, Glass GE. Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Negl Trop Dis. 2009;3:e382. doi: 10.1371/journal.pntd.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunkard J JM, Cifuentes E, Rothenberg SJ. Assessing the roles of temperature, precipitation and ENSO in dengue re-emergence on the Texas -Mexico border region. Salud publica Mex. 2008;50:227–34. doi: 10.1590/s0036-36342008000300006. [DOI] [PubMed] [Google Scholar]
- 7.Rigau-Pérez JG, Ayuso-Lamadrid A, Wolff DR, Reiter P, Kuno G. Dengue severity throughout seasonal changes in incidence in Puerto Rico, 1989-1992. Am J Trop Med Hvg. 1994;51:408–15. [PubMed] [Google Scholar]
- 8.Nakhapakorn K, Tripathi NK. An information value based analysis of physical and climatic factors affecting dengue fever and dengue haemorrhagic fever incidence. Int J Health Geogr. 2005;4:13. doi: 10.1186/1476-072X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su GL. Correlation of climatic factors and dengue incidence in Metro Manila, Philippines. Ambio. 2008;37:292–4. doi: 10.1579/0044-7447(2008)37[292:cocfad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear regression models. 3rd ed. Chicago: Irwin; 1996. [Google Scholar]
- 11.Scott TW, Morrison AC. Aedes aegypti density and the risk of dengue virus transmission. In: Takken W, Scott TW, editors. Ecological aspects for application of genetically modified mosquitoes. Dordrecht The Netherlands: Frontis; 2003. pp. 187–206. [Google Scholar]
- 12.Hopp MJ, Foley JA. Global-scale relationships between climate and the dengue fever vector, Aedes Aegypti. Climatic Change. 2001;48:441–63. [Google Scholar]
- 13.Schwartz E, Weld LH, Wilder-Smith A, Sonnenburg FV, Keystone JS, Kain KC, et al. Seasonality, annual trends, and characteristics of dengue among returned travelers, 1997-2006. Emerg Infect Dis. 2008;14:1081–8. doi: 10.3201/eid1407.071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naing CM, Lertmaharit S, Naing KS. Time-series analysis of dengue fever/dengue haemorrhagic fever in Myanmar since 1991. Dengue Bull. 2002;26:24–32. [Google Scholar]
- 15.Victor TJ, Malathi M, Asokan R, Padmanaban P. Laboratory-based dengue fever surveillance in Tamil Nadu India. Indian J Med Res. 2007;126:112–5. [PubMed] [Google Scholar]
- 16.Promprou S, Jaroensutasinee M, Jaroensutasinee K. Climatic factors affecting dengue haemorrhagic fever incidence in Southern Thailand. Dengue Bull. 2005;29:41–8. [Google Scholar]
- 17.Sharma RS, Panigrahi N, Kaul SM, Shivlal, Barua K, Bhardwaj M. Status report of DF/DHF during 1998 in the National Capital Territory of Delhi, India. Dengue Bull. 1999;23:109–112. [Google Scholar]
- 18.Focks D, Barrera R. Report of the Scientific Working Group meeting on dengue. Geneva: WHO; 2007. Dengue transmission dynamics: assessment and implications for control; pp. 92–108. [Google Scholar]
- 19.Schnoor JL. The IPC fourth assessment. Environ Sci Technol. 2007;41:1503. doi: 10.1021/es072475x. [DOI] [PubMed] [Google Scholar]
- 20.Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 2004;17:136–73. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter P. Weather.Vector biology and arboviral recrudescence. In: Monath TP, editor. The arboviruses: epidemiology and ecology. Vol. 1. Boca Raton: FLCRC Press; 1988. pp. 245–55. [Google Scholar]
- 22.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–52. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 23.Rohani A, Wong YC, Zamre I, Lee HL, Zurainee MN. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in aedes aegypti (L.) Southeast Asian J Trop Med Public Health. 2009;40:942–50. [PubMed] [Google Scholar]
- 24.Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- 25.Barbazan P, Guiserix M, Boonyuan W, Tuntaprasart W, Pontier D, Gonzalez JP. Modelling the effect of temperature on transmission of dengue. Med Vet Entomol. 2010;24:66–73. doi: 10.1111/j.1365-2915.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 26.Hay SI, Myers MF, Burke DS, Vaughn DW, Endy T, Nisalak A, et al. Etiology of interepidemic periods of mosquito-borne disease. Proc Natl Acad Sci. 2000;97:9335–9. doi: 10.1073/pnas.97.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings DAT, Irizarry RA, Huang NE, Endy TP, Nisalak A, Ungchusak K, et al. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature. 2004;427:344–7. doi: 10.1038/nature02225. [DOI] [PubMed] [Google Scholar]
- 28.Gubler DJ, Trent DW. Emergence of epidemic dengue/dengue hemorrhagic fever as a public health problem in the Americas. Infect Agents Dis. 1994;2:383–93. [PubMed] [Google Scholar]
- 29.Rahman M, Rahman K, Siddque AK, Shoma S, Kamal AHM, Ali KS, et al. First outbreak of dengue hemorrhagic fever, Bangladesh. Emerg Infect Dis. 2002;8:738–40. doi: 10.3201/eid0807.010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aziz MM, Hasan KN, Hasanat MA, Siddiqui MA, Salimullah M, Chowdhury AK, et al. Predominance of the DEN 3 genotype during the recent dengue outbreak in Bangladesh. Southeast Asian J Trop Med Public Health. 2002;33:42–8. [PubMed] [Google Scholar]
- 31.Rico-Hessea R, Harrisona LM, Salasb RA, Tovarb D, Nisalak A, Ramosd C, et al. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–51. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]