Abstract
Background & objectives:
Venous thromboembolism (VTE) is a major health problem with substantial morbidity and mortality. It is often underdiagnosed due to lack of information on VTE risk and prophylaxis. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study aimed to assess the prevalence of VTE risk in acute hospital care setting and proportion of at-risk patients receiving effective prophylaxis. We present here the risk factor profile and prophylaxis pattern of hospitalized patients who participated in ENDORSE study in India.
Methods:
In this cross-sectional study in India, all patients (surgical >18 yr, medical >40 yr) from 10 hospitals were retrospectively studied. Demographics, VTE risk factors and prophylaxis patterns were assessed according to the 2004 American College of Chest Physicians (ACCP) evidence-based consensus guidelines.
Results:
We recruited 2058 patients (1110 surgical, 948 medical) from 10 randomly selected hospitals in India between August 2006 and January 2007. According to the ACCP criteria, 1104 (53.6%) patients [surgical 680 (61.3%), medical 424 (44.7%)] were at-risk for VTE. Chronic pulmonary disease/heart failure and complete immobilization were the most common risk factors before and during hospitalization, respectively. In India, 16.3 per cent surgical and 19.1 per cent medical at-risk patients received ACCP-recommended thromboprophylaxis.
Interpretation & conclusions:
Despite a similar proportion of at-risk hospitalized patients in India and other participating countries, there was major underutilization of prophylaxis in India. It necessitates increasing awareness about VTE risk and ensuring appropriate thromboprophylaxis.
Keywords: India, thromboprophylaxis, venous thromboembolism (VTE), VTE risk
Venous thromboembolism (VTE) is a global health concern with substantial morbidity and mortality1. It is often asymptomatic and underdiagnosed, leading to long-term complications and reduced survival. Approximately 30 per cent of patients with symptomatic VTE manifest pulmonary embolism (PE), whereas others manifest deep vein thrombosis (DVT). Thus, it is a major health issue in western countries1. VTE events remain a relatively common cause of death in hospitalized patients and almost 75 per cent of all VTE-related deaths are from hospital-acquired VTE1. Earlier evidence suggests that hospital-acquired VTEs can be prevented given the availability of effective prophylaxis2,3.
Earlier studies have demonstrated the increasing incidence of VTE in Asian populations4. The incidence of post-operative DVT in Indian patients undergoing major lower limb surgery is as high (43.2 and 60% patients in the groups with and without prophylaxis, respectively) as seen in the Western world5. It has been also shown that PE occurs frequently in Indian patients with symptomatic DVT; 40 per cent patients with DVT had high probability lung scan showing high prevalence of PE6. In the Indian subset data of the global Prospective Registry On Venous thromboembolic Events (PROVE) registry, proximal plus calf DVTs were found in 54 per cent of Indian patients, proximal DVT in 17 per cent, and calf DVT in 13 per cent, compared with 52, 18, and 24 per cent in the global PROVE population, respectively7. VTE is no longer a rarity in India with general surgical operations being the most common causes of post-operative DVT. Also, morbidity and mortality from VTE is a significant problem in India8.
The Eighth American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy: Evidence-Based Guidelines (ACCP guidelines) help in the assessment of risk factors for VTE and recommend the appropriate use of prophylaxis to prevent VTE in patients at risk9. Although the incidence of DVT in India is comparable to that in the Western countries, the awareness level of VTE is particularly low among Indians8. The newly developed consensus/clinical practice guidelines provide recommendations for the continued management of patients with VTE, addressing specifically the risk stratification of VTE, and the appropriate use of low molecular weight heparins (LMWHs) in the prophylactic management of this condition10. Earlier studies have demonstrated that thromboprophylaxis reduces adverse outcomes along with reduction in overall costs11,12.
Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study is a multinational, cross-sectional survey in both medical as well as surgical patients in a large number of hospitals worldwide. The study aimed to assess the number of patients at risk for VTE and the proportion that received effective prophylaxis as recommended by the ACCP guidelines13. This study presents the risk factor profile and prophylaxis patterns in hospitalized Indian patients who participated in the ENDORSE study.
Material & Methods
The detailed methodology of the ENDORSE study has been previously published13. Briefly, the primary objective was to identify hospitalized patients at risk of VTE and to determine the proportion of patients receiving effective VTE prophylaxis. Patients ≥40 yr age in eligible medical wards (general medical, respiratory or cardiac wards) or ≥18 yr age in eligible surgical wards (general surgical and orthopaedic wards) were screened. The study excluded patients if their charts were unavailable, admitted to ineligible wards, were admitted solely for the treatment of VTE, were too young (age <18 yr for surgical, <40 yr for medical), admitted for diagnostic testing/minor operation (type of ward not specified), transfer patients, or their key information (birth year, surgery types, etc.) was missing. According to the national rules, the approval of the ethics committee at Nizam's Institute of Medical Sciences, Hyderabad, was obtained for the study in India.
The Indian component of the ENDORSE study was conducted according to the global protocol. In India, 2058 patients were enrolled from 10 hospitals between August 2006 and January 2007. These 10 hospitals were Nizam's Institute of Medical Sciences, Hyderabad; Southern Railway Hospital, Chennai; Chennai Port Trust Hospital, Chennai; Holy Family Hospital, New Delhi; Woodlands Medical Centre Ltd., Kolkata; Prince Aly Khan Hospital, Mumbai; MS Ramaiah Hospital, Bangalore; St John's Medical College Hospital, Bangalore; Rabindranath Tagore International Institute of Cardiac Science, Kolkata; and Gandhi Hospital, Hyderabad. Data were collected by reviewing the hospital charts and filling the relevant information in standardized case report forms. Patient demographics, admission and post-admission diagnoses, risk factors for VTE (as defined in ACCP guidelines)9, risk factors for bleeding, duration of hospitalization, and type of VTE prophylaxis (as defined in ACCP guidelines)9 were obtained.
Statistical analysis: A minimum of 450 patients were required per analysis group in order to assess the true occurrence of VTE risk at 25 per cent with a margin of error of 4 per cent. Quantitative data were expressed as median (inter-quartile range) and the number of non-missing data, while categorical data were expressed as number and percentage of the population. SAS version 9.1 (SAS Institute Inc., Cary, North Carolina, USA) was used for all statistical analyses.
Results
The number of beds assessed and reasons for exclusion from assessment are shown in the Fig The assessable surgical and medical patients were 1110 (53.9%) and 948 (46.1%), respectively. Of the total 2058 evaluable patients, 1104 (53.6%) were identified to be at-risk of VTE based on the ACCP risk criteria, including 680 (61.3%) surgical patients and 424 (44.7%) medical patients. A total of 1104 patients at risk of VTE were recruited, of which 68.9 per cent were males with median age 51 yr, median body mass index (BMI) 24.7 kg/m2, and median length of hospital stay 6 days (Table I). In patients from surgical wards, the major surgeries done during hospitalization were orthopaedic surgeries (19.1%) including hip/knee replacement, hip fracture, curative arthroscopy, and surgeries for other orthopaedic trauma. The major reasons for hospitalization were other medical conditions (22.4%) and cardiovascular disease including acute heart failure (17.8%). In patients from medical wards, the major reason for hospitalization was cardiovascular disease including acute heart failure (54.7%).
Fig.
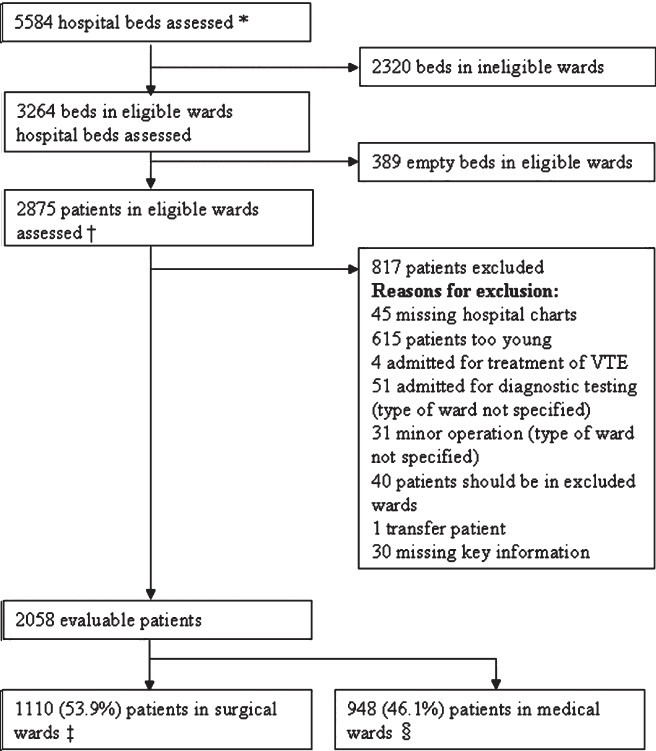
ENDORSE study: Indian patient population. VTE, venous thromboembolism. *Based on hospital enrolment forms; † Based on patient enrolment logs, includes patients who did not meet protocol requirements (e.g. age, type of condition, or missing hospital chart); ‡ Includes patients in general surgical units, surgical ICUs, neurosurgery, gynaecology, and orthopaedics; and § Includes patients in other surgical wards
Table I.
Patients’ characteristics and reasons for hospitalization
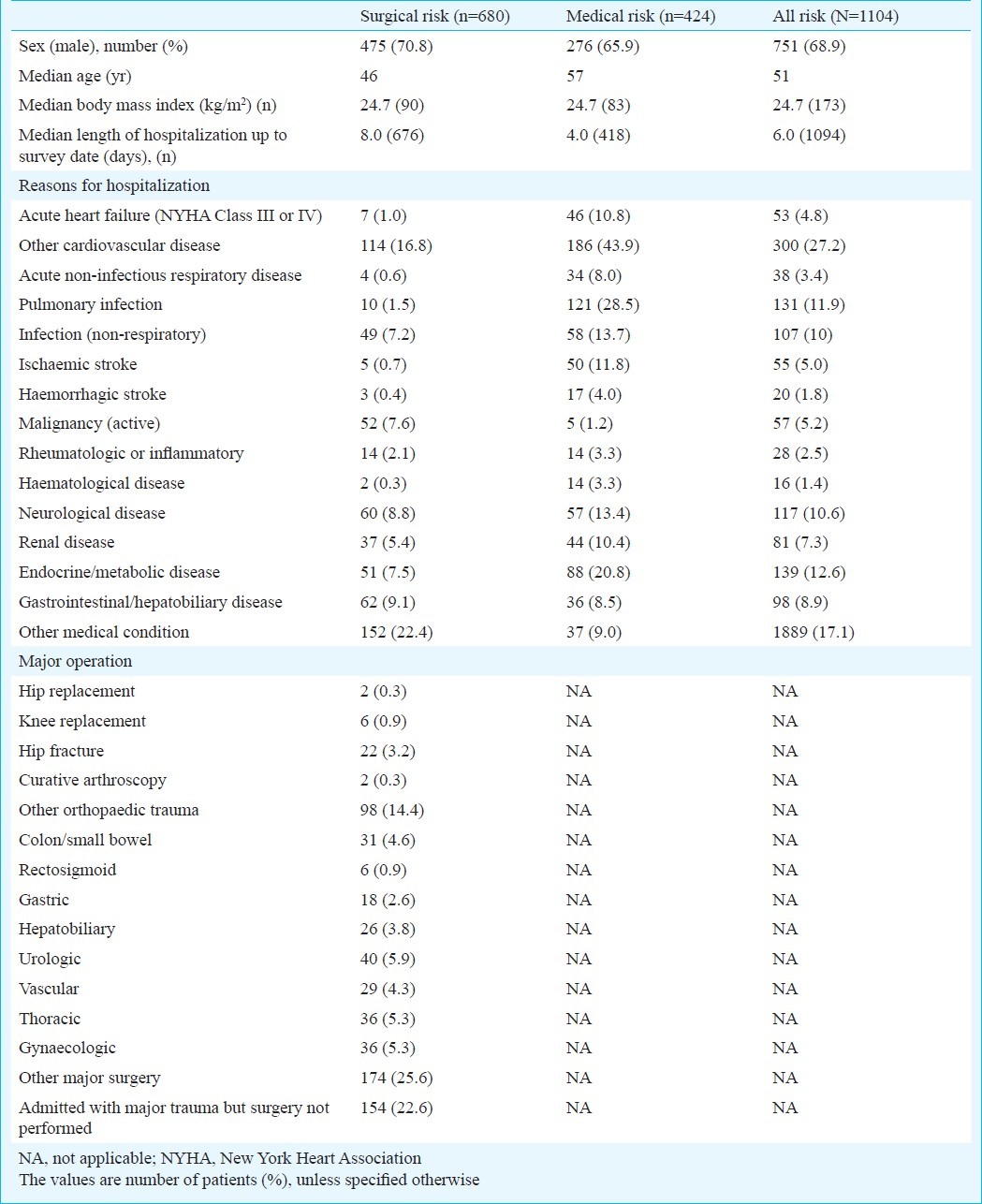
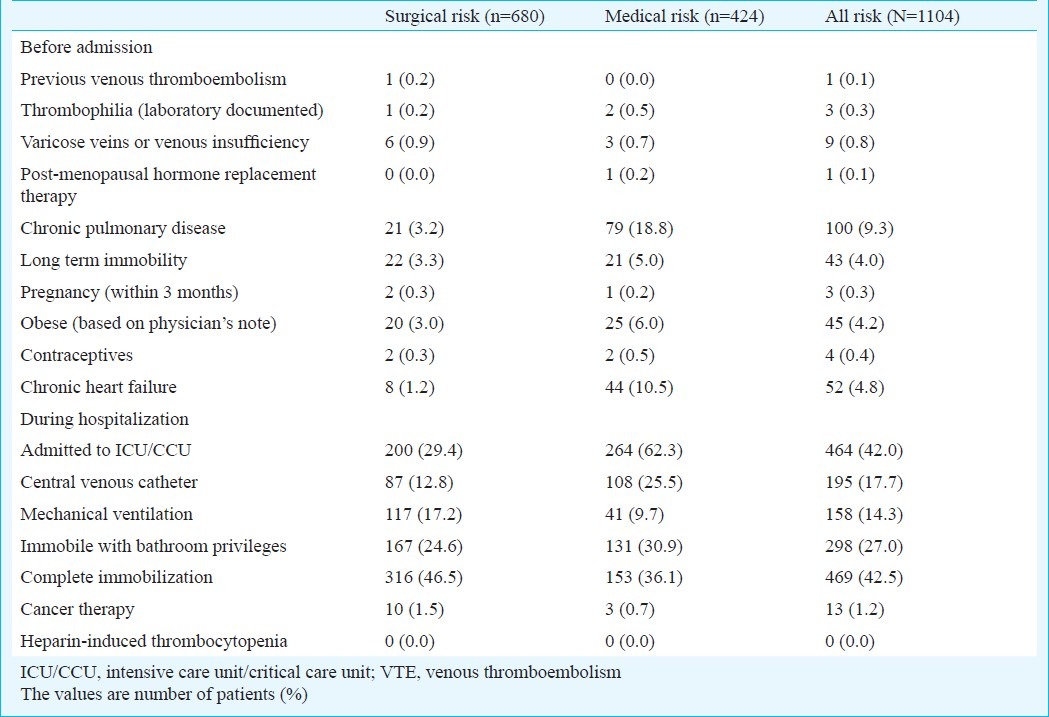
Table II presents the risk factors for VTE prior to and during hospitalization. Before hospitalization, long-term immobility (3.3%), chronic pulmonary disease (3.2%) and obesity (3.0%) were the most common risk factors for surgical patients, while chronic pulmonary disease (18.8%) and chronic heart failure (10.5%) were the major risk factors for medical patients. The most common VTE risk factors reported in patients in surgical and medical wards during hospital stay were complete immobilization (46.5 vs. 36.1%), admission to intensive or critical care unit (29.4 vs. 62.3%), and immobilization with bathroom privileges (24.6 vs. 30.9%).
Table II.
Risk factors for venous thromboembolism
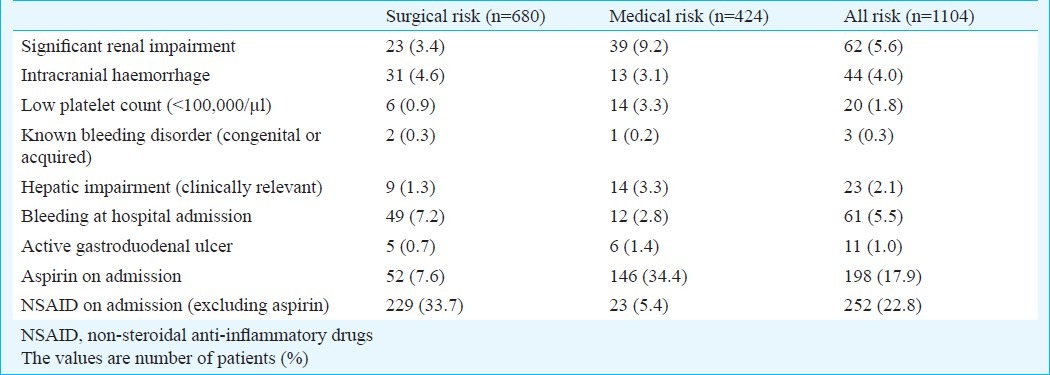
In patients from surgical wards, the most common contraindications to pharmacological prophylaxis were bleeding on hospital admission (7.2%) and intracranial bleeding (4.6%), while in patients from medical wards, clinically relevant renal impairment (9.2%) and hepatic impairment (3.3%), and low platelets count (3.3%) were the most common contraindications (Table III). According to the ACCP criteria for risk factors for bleeding, from the 1104 patients at risk for VTE, 79 patients (11.6%) from surgical wards and 42 patients (9.9%) from medical wards were considered to have contraindications to anticoagulant prophylaxis.
Table III.
Contraindication to pharmacological prophylaxis
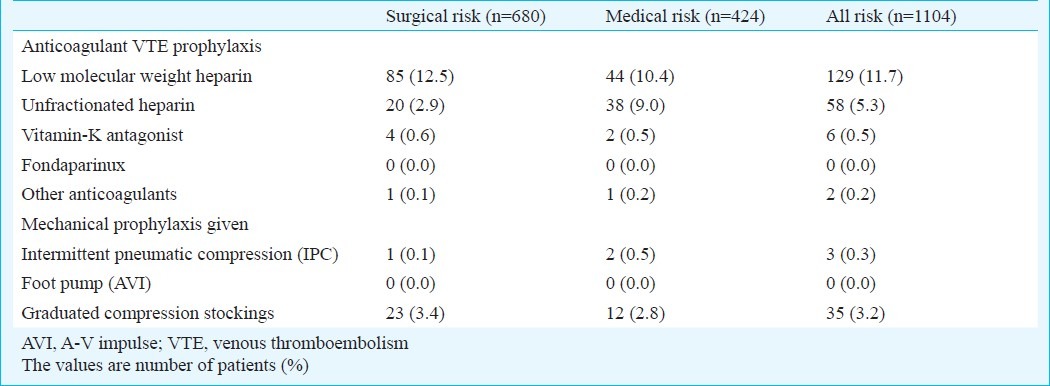
Of the 1104 patients at-risk for VTE, only 192 (17.4%) received the ACCP-recommended prophylaxis. Of the 680 patients from surgical wards, only 126 (18.5%) received any VTE prophylaxis, and 111 (16.3%) received ACCP-recommended VTE prophylaxis. Of the 424 patients from medical wards, 95 (22.4%) received any VTE prophylaxis and 81 (19.1%) received ACCP-recommended VTE prophylaxis.
Overall prophylaxis in at-risk VTE patients was low. Anticoagulants were the commonly used type of prophylaxis (Table IV). Low molecular weight heparin (LMWH, 11.7%) was the most preferred choice of anticoagulant in surgical as well as medical wards patients, followed by unfractionated heparin (UFH, 5.3%). Among mechanical prophylaxis available, graduated compression stockings were used only in 35 (3.2%) patients.
Table IV.
Types of prophylaxis prescribed for the patients
Discussion
In the present study, we assessed the number of hospitalized patients with VTE risk in 10 selected hospitals in India. The study demonstrated high occurrence of patients at-risk for VTE in India. The proportion of Indian patients considered at risk for VTE (53.6%) was similar to that of the global patients at risk for VTE (51.8%)13. We also assessed the current thromboprophylaxis practice in India in order to optimize practice patterns for appropriate use of thromboprophylaxis. The global ENDORSE data showed that 50.2 per cent at-risk patients received ACCP-recommended prophylaxis13, while in India, very low proportion of at-risk patients (17.4%) received ACCP-recommended prophylaxis. We observed that among at-risk patients, 18.5 per cent surgical and 22.4 per cent medical patients received any VTE prophylaxis. Similarly 16.3 per cent surgical and 19.1 per cent medical patients received ACCP-recommended thromboprophylaxis.
The Indian data from ENDORSE study revealed that despite a similar proportion of patients at risk in India and other participating countries, there is major underutilization of prophylaxis (17.4%) in India as compared to higher usage of prophylaxis globally (50.2%)13. According to the ENDORSE global study, higher percentage of at-risk medical patients received ACCP-recommended prophylaxis countries such as Germany (70%), Colombia (64%), Spain (64%) and Switzerland (61%)14–17. Also, in case of at-risk surgical patients, Germany (92%), Hungary (87%), Spain (82%) and Switzerland (81%) showed high usage of ACCP-recommended prophylaxis14,16–18. ENDORSE global data in hospitalized medically ill patients demonstrated that the prophylaxis use varied between the participating countries, and was associated with disease severity rather than medical diagnosis19. Among the hospitalized surgical patients in ENDORSE global study, the rates of prophylaxis use varied according to surgery type and presence of comor-bidities20. The Indian data are in agreement with the results of earlier studies from India and emphasize the underutilization of prophylaxis7. The Prospective Registry on Venous thromboembolic Events (PROVE) study showed that only 7 per cent of patients with confirmed symptomatic DVT received appropriate thromboprophylaxis7. Although critically ill patients require more intensive and prolonged thromboprophylaxis, earlier studies have demonstrated that only half the patients in multidisciplinary critical care units (44-47%) had received thrombo-prophylaxis21,22.
The most common reasons for the underutilization might be bleeding complications as contraindications to anticoagulants23. However, the inadequacy of thromboprophylaxis cannot be explained only by contraindications to anticoagulant use, since mechanical thromboprophylaxis was also underutilized. Earlier evidence has shown that LMWH is as effective and safe as UFH for treatment of VTE24,25. However, since LMWH is associated with lower incidence of thrombocytopenia and osteoporosis during long-term use, it is generally preferred over UFH despite its high cost26. In the present study also, LMWH (11.7%) was used more frequently than UFH (5.3%).
The ENDORSE study had some potential limitations which should be considered while interpreting the results. Due to the cross-sectional design of the study, we could capture the data only during the hospital stay. Since there were no follow up visits, we could not evaluate the prophylaxis duration compliance with the recommendations of the ACCP guidelines. The data were collected from the patient charts and there was a possibility to include inaccurate data. Another limitation of the study was that some excluded younger patients also might have been at-risk for VTE. there was also a possibility of excluding at-risk patients who did not meet the ACCP criteria for VTE risk and recommendations for prophylaxis. Additionally, though we have collected reports of patients with standard bleeding risks, some physicians might have applied broader interpretation for not prescribing the anticoagulants.
In conclusion, our results showed a high occurrence of VTE risk in hospitalized Indian patients and underutilization of effective prophylaxis to a large extent. This confirms the need for increasing awareness about VTE risk, optimum risk assessment, and improved implementation of appropriate thromboprophylaxis in at-risk hospitalized patients. This will help in successful management of VTE and prevent the morbidity and mortality due to VTE.
Acknowledgment
We thank the staff at the Center for Outcomes Research, University of Massachusetts Medical School, Worcester, MA, USA, for conducting the study and analyzing the results of Indian data. We would also like to thank Dr (Ms) Jai Tilak-Jain from Sanofi, India, for writing assistance.
References
- 1.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. for the VTE Impact Assessment Group in Europe (VITAE) Venous thromboembolism (VTE) in Europe: The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–64. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 2.Leizorovicz A, Cohen AT, Turpie AGG, Olsson C-G, Vaitkus PT, Goldhaber SZ for the PREVENT Medical Thromboprophylaxis Study Group. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–9. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, et al. ARTEMIS investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomized placebo controlled trial. BMJ. 2006;332:325–9. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leizorovicz A, Turpie AGG, Cohen AT, Wong L, Yoo MC, Dans A, et al. for the SMART Study Group. Epidemiology of venous throboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART Study. J Thromb Haemost. 2005;3:28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 5.Agarwala S, Bhagwat AS, Modhe J. Deep vein thrombosis in Indian patients undergoing major lower limb surgery. Indian J Surg. 2003;65:159–62. [Google Scholar]
- 6.Parakh R, Kapadia S, Agarwal S, Grover T, Bukhari S, Yadav A, et al. Assessment of total thrombus load in symptomatic patients with venous thromboembolism. Clin Appl Thromb Hemost. 2006;12:369–72. doi: 10.1177/1076029606291408. [DOI] [PubMed] [Google Scholar]
- 7.Pinjala RK, Agarwal MB, Turpie AGG for PROVE investigators. A Characterization of Patients with Symptomatic DVT in India. J Thromb Haemost. 2005;3(Suppl 1) abstract no. P1043. [Google Scholar]
- 8.Lee AD, Stephen E, Agarwal S, Premkumar P. Venous Thrombo-embolism in India. Eur J Vasc Endovasc Surg. 2009;37:482–5. doi: 10.1016/j.ejvs.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 10.Parakh R, Somaya A, Todi SK, Iyengar SS for Consensus Development Guidelines Panel. Consensus development recommendations for the role of LMWHs in prophylaxis of venous thromboembolism: An Indian perspective. J Assoc Physician India. 2007;55:5–30. [Google Scholar]
- 11.Bergqvist D, Lindgren B, Matzsch T. Cost-effectiveness of preventing postoperative deep vein thrombosis. In: Hull RD, Pineo GF, editors. Disorders of thrombosis. Philadelphia PA: WB Saunders; 1996. pp. 228–33. [Google Scholar]
- 12.Bick RL. Proficient and cost-effective approaches for the prevention and treatment of venous thrombosis and thromboembolism. Drugs. 2000;60:575–95. doi: 10.2165/00003495-200060030-00005. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, et al. for the ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–94. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 14.Zotz RB, Kauschat-Brüning D, Bramlage P ENDORSE Investigators. Thromboembolic risk and prophylaxis in hospitalized surgical and internal medicine patients. German results of the international ENDORSE study. Dtsch Med Wochenschr. 2009;134:2163–9. doi: 10.1055/s-0029-1241924. [DOI] [PubMed] [Google Scholar]
- 15.Dennis RJ, Roa JH, Villadiego J, Méndez F, Vieda E, Restrepo H. Venous thromboembolism prophylaxis in Colombian surgical and medical patients: results for Colombia of the ENDORSE study. Biomedica. 2011;31:200–8. doi: 10.1590/S0120-41572011000200007. [DOI] [PubMed] [Google Scholar]
- 16.Nieto Rodríguez JA ENDORSE. Venous thromboembolism risk and antithrombotic prophylaxis among patients admitted to Spanish hospitals (ENDORSE study) Med Clin (Barc) 2009;133:1–7. doi: 10.1016/j.medcli.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Chopard P, Spirk D, Beer HJ, Peter J, Brunner B, Bounameaux H, et al. Swiss results from a global observational study of venous thromboembolism risk and prophylaxis use in the acute care hospital setting: analysis from the ENDORSE study. Swiss Med Wkly. 2009;139:630–5. doi: 10.4414/smw.2009.12746. [DOI] [PubMed] [Google Scholar]
- 18.Losonczy H, Tar A ENDORSE investigators. Results of ENDORSE study in Hungary. Multinational, cross-sectional study to assess the prevalence of venous thromboembolism risk and prophylaxis in acute hospital care setting. Orv Hetil. 2008;149:2069–76. doi: 10.1556/OH.2008.28488. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann JF, Cohen AT, Tapson VF, Goldhaber SZ, Kakkar AK, Deslandes B, et al. ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients.The ENDORSE Global Survey. Thromb Haemost. 2010;103:736–48. doi: 10.1160/TH09-09-0667. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar AK, Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Deslandes B, et al. ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330–8. doi: 10.1097/SLA.0b013e3181c0e58f. [DOI] [PubMed] [Google Scholar]
- 21.Todi Sk, Sinha S, Chakraborty A, Sarkar A, Gupta S, Das T, et al. Utilization of deep venous thrombosis prophylaxis in medical / surgical intensive care units. Indian J Critical Care Med. 2003;7:103–5. [Google Scholar]
- 22.Ansari K, Dalal K, Patel M. Risk stratification and utilisation of thrombo-embolism prophylaxis in a medical-surgical ICU: a hospital-based study. J Indian Med Assoc. 2007;105:536–8. 540. [PubMed] [Google Scholar]
- 23.Mavalankar AP, Majmundar D, Rani S. Routine chemoprophylaxis for deep venous thrombosis in Indian patients: Is it really justified? Indian J Orthoped. 2007;41:188–93. doi: 10.4103/0019-5413.33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parakh R, Kakkar VV, Kakkar AK for the Venous Thromboembolism (VTE) Core Group. Management of venous thromboembolism. J Assoc Physician India. 2007;55:49–70. [PubMed] [Google Scholar]
- 25.Agarwal S, Lee AD, Raju RS, Stephen E. Venous thromboembolism: A problem in the Indian/Asian population? Indian J Urol. 2009;25:11–6. doi: 10.4103/0970-1591.45531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindhoff-Last E, Nakov R, Misselwitz F, Breddin HK, Bauersachs R. Incidence and Clinical relevance of Heparin Induced Antibodies in patients with DVT treated with UFH or LMWH. Br J Haematol. 2002;118:1137–42. doi: 10.1046/j.1365-2141.2002.03687.x. [DOI] [PubMed] [Google Scholar]


