Abstract
Background & objectives:
Safe blood and blood products should be offered to all patients in need for blood transfusion. The objectives of the present study were to establish prevalence estimates for hepatitis B and hepatitis C virus infections as a foundation for safe blood transfusion in rural Vietnam, and to check the accuracy of the laboratory analysis used for hepatitis testing of blood donors in Vietnam.
Methods:
A cross-sectional study was conducted in two rural communities in Quang Tri, Vietnam. A total of 1,200 blood samples collected from potential blood donors were tested by an enzyme immunoassay technique (EIA) for detection of hepatitis surface antigen (HBsAg), antibodies to hepatitis B core antigen (anti-HBc), and antibodies to hepatitis C antigen (anti-HCV). The EIA test outcome was validated by a chemiluminescent micro particle immunoassay technique (CMIA).
Results:
The prevalence of HBsAg and anti-HBc in the study population was 11.4 per cent (95% CI 9.6 - 13.2) and 51.7 per cent (95% CI 48.8 - 54.5), respectively, the prevalences being higher in males than females. The prevalence of anti-HCV was 0.17 per cent. The test agreement between the EIA and CMIA techniques was high both for HBsAg detection (κ = 0.91; 95% CI: 0.83 - 0.99) and for anti-HBc detection (κ = 0.89; 95% CI 0.81 - 0.97). Compared to CMIA results, the positive and negative predictive values of the EIA tests were found to be 94.9 per cent (95% CI 87.5 - 98.6) and 97.5 per cent (95% CI 86.8 - 99.9) for HBsAg, and 92.4 per cent (95% CI 84.2 - 97.2) and 100 per cent (95% CI 91.2 - 100) for anti-HBc.
Interpretation & conclusions:
The study shows that hepatitis B virus infection is endemic in rural areas of Vietnam and that almost half of the population is or has been infected. Hepatitis C infection is rare, but false negative test results cannot be ruled out. Also, the results indicate that the EIA performance in blood donor screening in Vietnam may be sub-optimal, missing 2.5 per cent of hepatitis B virus carriers and falsely excluding more than 7 per cent of blood donors. As the prevalence of hepatitis B infection is high, occult hepatitis B infection may represent a threat to safe blood transfusion. Therefore, nucleic acid amplification testing for HBV should be considered for blood donor screening in Vietnam.
Keywords: Anti-HBc, blood donor screening, HBsAg, hepatitis B core antibody, hepatitis B surface antigen, hepatitis C virus, occult hepatitis B infection, safe blood transfusion, Vietnam
Safe blood transfusions remain a challenge in resource-limited settings where blood-transmitted diseases are endemic. The transmission risk of hepatitis B virus (HBV) infection depends on the actual disease prevalence rate; where the prevalence is low the transmission risk is estimated at approximately 1:60,000; where HBV infection is endemic, the transmission rate is probably much higher1–3. Testing for hepatitis surface antigen (HBsAg) is in place in most low-income countries. However, the sensitivity of HBsAg assays is genotype dependent; tests developed on populations where genotype A is predominant as in Western countries, may be inaccurate in populations of genotype B and C as in Asia. Also patients with HBV S gene mutants may escape detection by standard blood donor screening tests4. Besides, transmission can still occur during the initial window-period of an acute infection, or during late stages where virus is still present (HBV-DNA positive) though HBsAg is negative, so-called occult hepatitis B infection (OBI)2,5,6. OBI may originate from recovered infections with persistent low level viral replication, from escape mutants blocking export of antigen, or from a reduced HBV replication after co-infection with hepatitis C virus (HCV) infection2,7. The presence of antibodies to hepatitis B core antigen (anti-HBc) indicates previous infection with HBV. In many Western countries the presence of anti-HBc excludes blood donation. However, due to limited resources and the potential exclusion of many blood donors, this routine is seldom practiced in low-income countries where HBV infection is endemic. There has been an increased focus on OBI lately, with several studies published both on the clinical significance of anti-HBc and OBI, and as a starting point for discussing the matter of safe blood transfusion. The infectiousness of OBI cases is not clear although several studies report that exclusion of anti-HBc positive blood probably decreases the rate of HBV transmission by blood transfusion5,8,9. The nucleic acid amplification (NAT) technology has greatly enhanced accuracy in identification of OBI cases, and NAT negative individuals are considered safe blood donors10. However, NAT testing is expensive, the NAT tests may not pick up mutated virus, cost-effectiveness may be low, and the technology may not be feasible in blood transfusion services where resources are scarce4.
Prevalence studies of HBV and HCV in Vietnam have so far been conducted on relatively small study samples11–15. Consequently, the prevalence estimates are too imprecise to give evidence-based recommendations for blood donor screening. The aim of the present study was, therefore, to estimate accurate prevalence rates of HBV and HCV infections among potential blood donors in a rural area of Vietnam prior to setting up rural blood transfusion services. Another aim was to examine the accuracy of enzyme immunoassay (EIA) technique used for HBsAg, anti-HBc, anti-HCV testing of blood donors at a local laboratory in Vietnam.
Material & Methods
The study was designed as a cross-sectional study in a reference population of potential blood donors in rural areas of Vietnam. The study was carried out in February - March 2007 in the Quang Tri province of Vietnam.
Study population: For the purpose of establishing rural blood transfusion service, any healthy consenting adult between the age of 18 and 55 yr living permanently in the study area was considered a potential blood donor. Prior to the collection of blood samples, villagers were informed by local health authorities in media and community meetings that the study was linked with the introduction of safe blood transfusion service for the local population; that participation was voluntary and free of charge; that all participants would be informed of the test outcome and get medical advice and counselling accordingly. Individuals previously vaccinated for HBV were excluded from the study. After the informed consent forms were signed with the witness of blood collectors, participants were asked to fill in and sign registration forms before inclusion. A total of 1,200 persons who were willing to participate, and meeting selection criteria, were included in the study. Data on HBe antigen were not gathered.
The prevalence of HCV infection is reported to be very low in Vietnam (Dr NTT Thu and colleagues, personal communication) therefore, the HBV prevalence was used to estimate the sample size. The sample size of 600 in each region enabled us to detect a difference of HBV prevalence of at least 10 per cent with significance level 5 per cent with two-sided testing, test power at 90 per cent. To assess local prevalence variations the blood samples were collected with stratified sampling procedure: 600 samples were collected from the villages in one remote rural area with less developed health infrastructure (Trieu Trach community, TT, with a total population of 6,801 persons, 3,510 female), and 600 samples from all villages in an area with more developed infrastructure (Cam Thuy community, CT, with a total population of 4,994 persons, 2,557 female). Within each area, sampling sites at village level were established, and blood samples collected from the consenting individuals. With this sampling design, potential clustering of results at village level were considered and adjusted for the prevalence estimates.
To control the quality of sample processing in Vietnam, a subset of 321 serum samples was randomly selected for analysis by an automated chemiluminescent micro particle immunoassay (CMIA) technique at the Department of Microbiology, University Hospital North Norway (n=120 for each category, HBsAg, anti-HBc, and anti-HCV) (Fig. 1). The subset sample size (n = 120) was estimated to detect test indicator differences of more than 5 per cent with 95% confidence interval, the subset being selected to get at a balance of 2/3 assumed test-positive units versus 1/3 assumed test-negative units in Vietnam. Except for a slight under-representation of females in the HBsAg subset, the demographical composition of the three subsets corresponded well with the total study population (Table I).
Fig. 1.
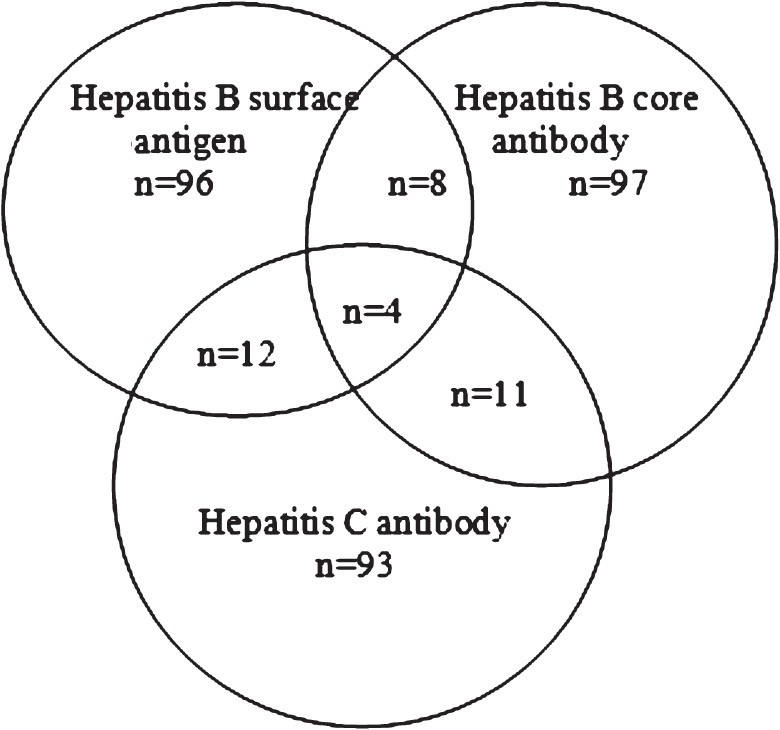
Venn diagram describing the composition and overlap of subsets for chemiluminescent micro particle immunoassay (CMIA) analysis. The numbers within each area indicate the number of study samples.
Table I.
Demographic factors in the study population and in chemiluminescent micro particle immunoassay (CMIA) subsets
Methods: The EIA tests were performed at the Quang Tri Preventive Medicine Centre, Vietnam, according to the manufacturer's instructions. Blood samples (5 ml) were drawn in field from each study person by trained laboratory technicians and set aside for spontaneous coagulation for 30 min before centrifugation. After centrifugation, the serum was pipetted into two new tubes, cooled to 4°C in a portable cooler and analysed within three days by an EIA technique (Monolisa® HBsAg ultra, no. 72348; Monolisa® anti-HBc plus, no. 72316; Monolisa® anti-HCV plus, no. 72318; BioRad Diagnostics, 92430 Marnes-la-Coquette, France) The frozen serum samples from all participants were stored for one year in case further analysis should be required. The Monolisa test has a claimed sensitivity at 100 and specificity at 99.9 per cent for HBsAg; for anti-HBc the claimed sensitivity is 99.5 per cent and specificity 99.5 per cent; for anti-HCV the respective performance is claimed to be at 100 and 99.8 per cent14. The following confirmation of positive tests is recommended by the manufacturers: A neutralization test for reactive/positive HBsAg samples; a recombinant immunoblot assay test or HCV-RNA polymerase chain reaction test for reactive/positive anti-HCV; alternatively anti-HBc assay for reactive/positive anti-HBc. These tests as well as services for NAT testing were not available at Quang Tri Preventive Medicine Centre at the time of study.
In the Department of Microbiology & Infectious Control, University Hospital North Norway, Norway, the CMIA testing of HBsAg was performed according to the manufacturers instructions (Architect® HBsAg, ref 6C36; Architect® anti-HBc, ref 7C17; Architect® anti-HCV, ref 6C37; Abbott GmbH & Co. KG, 65205 Wiesbaden-Delkenheim, Germany). The subset of 321 frozen blood samples was transported to Norway. Samples with concentration values less than 0.05 IU/ml were considered negative and those that had values ≥ to 0.05 IU/ml were considered positive. CMIA analysis of anti-HBc and anti-HCV assays is based on the ratio of signal to cut-off value (S/CO). The S/CO values <1.00 are classified as negative, and values ≥1.00 are classified as positive. Units with ratios in the range of 0.90 - 1.00 were classified as borderline and re-analysed twice16,17.
Statistical analysis: After establishing the database in Excel® (2003 for Windows), the data were transferred to the statistical software Stata (version 11/ SE for Windows, StataCorp Inc., College Station, TX). After initial tabulation and graphical examination of the prevalence data, adjusted prevalence estimates for HBsAg, anti-HBc, and anti-HCV were established using the survey (svy) commands in Stata with area as stratifying variable and village as primary sampling unit. To test if observed differences between areas were due to sampling biases, a multivariable logistic regression model with age, sex, and area as explanatory variables were established in Stata, still with village as a cluster variable. Model fit was assessed using the Hosmer-Lemeshow test and receiver operating statistics curves (ROC). The EIA methods used in Vietnam were assessed against the CMIA methods using the Kappa (κ) analysis and sensitivity/ specificity and positive/ negative predictive values for the EIA calculated using the diagt procedure in Stata. In accordance with common classification, κ-values between 0.4 and 0.6 is “acceptable or moderate agreement”, 0.6 to 0.8 is “good agreement” and 0.8 to 1 is “very good agreement”. Differences were considered significant if the P value were found <0.0518.
Ethical considerations: The participants’ consent was based on both oral and written information by local health workers as well as the in-field investigators. Medical counselling based on test outcomes was granted for all study participants. Local health workers delivered the informed consent forms to potential participants so that the participants got a full understanding of the study aims and study process. The consent form was signed on-site at the time when the blood samples were drawn. Written files of demographical and laboratory data were stored at the Quang Tri Preventive Medicine Centre. Accesses to non-anonymous data were restricted to members of the research team. Frozen samples were destroyed one year after the in-field sampling. The ethical approval of the study protocol was granted by the Quang Tri Health Service, and by Quang Tri Provincial People's Committee, Vietnam (Decision No. 2472/QD-UBND, 20/12/2006). All data were stored and processed by permission from the Norwegian Social Science Data Service, Norway (ref. no. 13702).
Results
The demographic characteristics of the study sample are presented in Table I and Fig. 2. The sub-samples compared well to the main sample regarding age, and the subsamples were approximately equal for age and gender, while the main sample had a gender bias. The analyses showed that 11.4 per cent of study samples (137/1200, 95% CI 9.6 - 13.2) were positive for HBsAg, 51.7 per cent (620/1200, 95% CI 48.8 - 54.5) were anti-HBc-positive, while 9.5 per cent (114/1200, 95% CI 7.9 - 11.3) were positive for both HBsAg and anti-HBc. Further, 1.9 per cent (23/1200, 95% CI 1.2 -2.8) of the serum samples were positive for HBsAg and negative for anti-HBc; and 42.2 per cent (506/1200, 95% CI 39.4 - 45.0) negative for HBsAg and positive for anti-HBc. There were only two positive anti-HCV samples (0.17%). A substantial age gradient with more positives with increasing age was found for anti-HBc, but not for HBsAg (Fig. 3). Gender was found to be important, as more men than women were positive for HBsAg (16.8%; 95% CI 13.4 - 20.2 versus 7.9%, 95% CI 6.0 - 9.9) and also for anti-HBc (57.0%; 95% CI 52.5-61.5 versus 48.2%; 95% CI 44.6 - 51.8).
Fig. 2.
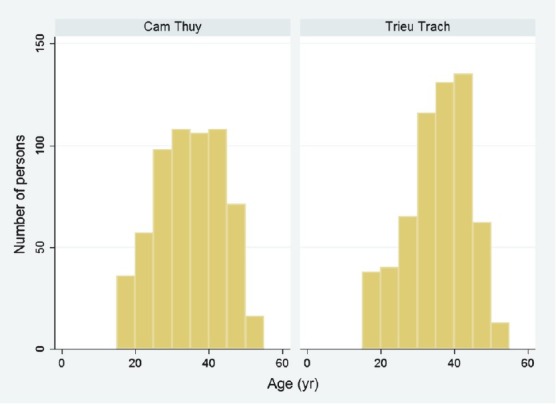
The age distribution in the two study areas, Cam Thuy (more developed community, n = 600) and Trieu Trach (less developed community, n = 600).
Fig. 3.
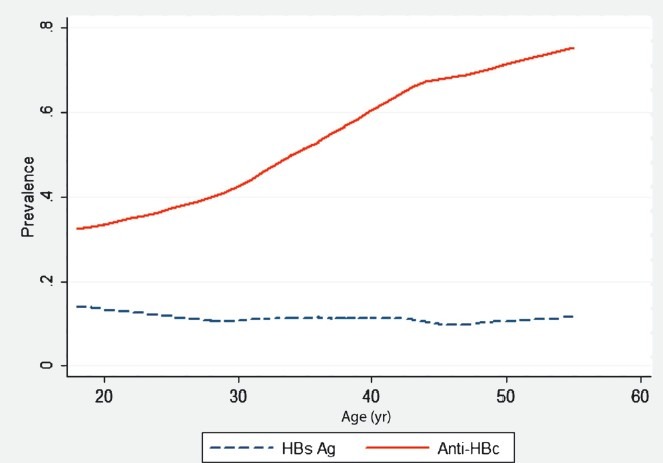
The age profile for HBsAg and anti-HBc positive individuals established using the lowess smoother function in Stata.
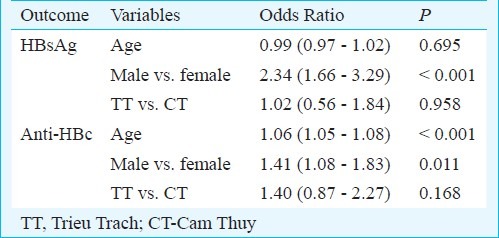
Higher prevalence levels for both HBsAg and anti-HBc were found in remote rural area compared with the less remote area. However, these differences were mainly due to sampling bias (age, gender), as the area variable was without significant importance in the logistic models (Table II). As shown in Table III, that age and gender were substantial factors for anti-HBc variations while only gender was important for HBsAg. For both HBsAg and anti-HBc, higher prevalence was found for males versus females. Model fit was acceptable with a ROC area of 66 per cent for anti-HBc and 62 per cent for HBsAg.
Table II.
Adjusted prevalence estimates (%) of HBsAg, anti-HBc, and anti-HCV with 95% confidence intervals, established with the svy procedure in Stata, with village as primary sampling unit and area as stratifying variable, stratified over gender
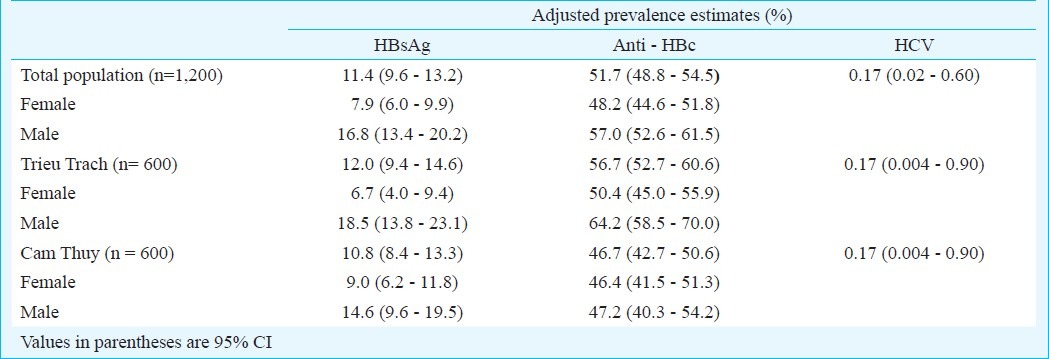
Table III.
A multivariable logistic model for HBsAg and anti-HBc expressing the influence of possible confounders on prevalence estimates, the results being expressed with 95% confidence intervals
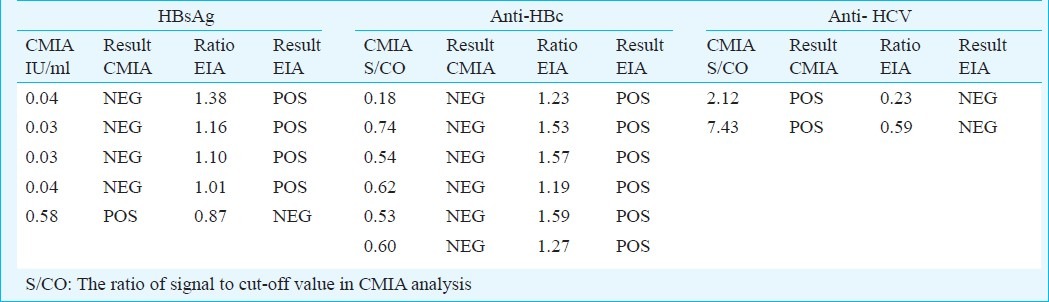
The test agreement (Kappa) between the EIA and CMIA was 0.91 for HBsAg (95% CI: 0.83 - 0.99), and 0.89 for anti-HBc (95% CI: 0.81 - 0.97) (Table IV). Using CMIA test results as reference, the positive and negative predictive values were found to be 94.9 per cent (95% CI 87.5 - 98.6) and 97.5 per cent (95% CI 86.8 - 99.9) for HBsAg; and 92.4 per cent (95% CI 84.2 - 97.2) and 100 per cent (95% CI 91.2 - 100) for anti-HBc. Samples with difference in results for HBsAg and anti-HBc had close to cut-off values both in CMIA and EIA analysis. For the anti-HCV analysis the pattern was different, the two samples being clearly positive by CMIA and clearly negative by EIA (Table V).
Table IV.
Comparison of EIA and CMIA test outcomes
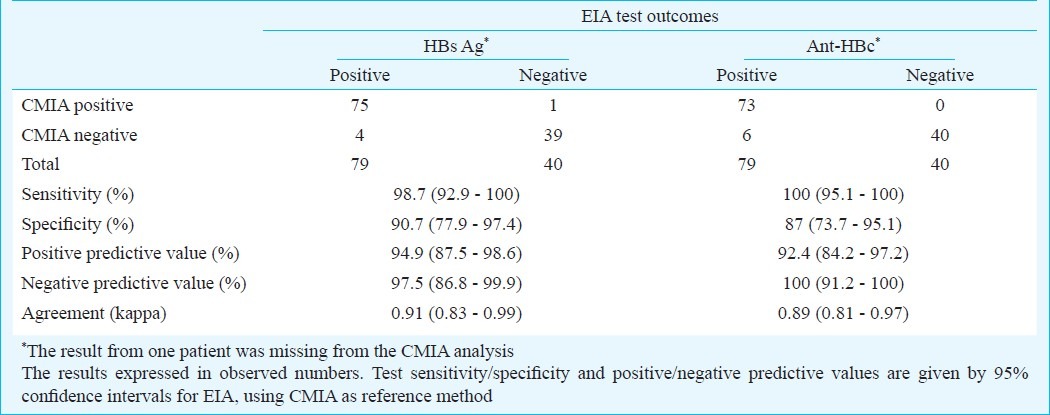
Table V.
Mismatched EIA and CMIA test results
Discussion
The present study documents high prevalence of HBV infection in the study area and thus confirms findings in previous smaller epidemiological studies conducted in rural as well as urban Vietnamese populations reporting prevalence rates of HBV infection in the range of 8 to 25 per cent12–15. Due to the size of the present study population, the prevalence estimates are strong with narrow confidence limits. Although there was a gender bias in the main sample, the study demonstrated that gender was a risk variable, men having a higher risk of HBV infection than women. The study also documents a high prevalence of anti-HBc (51.7%) in the study area. The finding is consistent with survey estimates from Thanh Hoa province in Vietnam (49.7%)14. The large number of samples being HBsAg negative and anti-HBc positive (42.2%) indicated that HBV infections have been endemic in the study area for some time. However, there may be large local prevalence variations as reported in studies of HBV infection in Thailand19. Comparing two communities with different infrastructural levels, we found higher prevalence of anti-HBc in the less developed community. However, the difference was moderate (10%) and hardly of clinical significance. Of the 1,200 samples included in the present study, only two were classified as anti-HCV positive with EIA technique. Of these two, one was also positive for both HBsAg and anti-HBc; the other was HBsAg negative and anti-HBc positive. Two more anti-HCV positive samples were found with CMIA technique in Norway. The finding corresponds well with prevalence estimates at 0.4 per cent HCV infection reported in two different districts of Thanh Hoa province14.
A recent study in rural Cambodia reports high prevalence rate of HCV antibodies (14.7%)20. The low prevalence in Vietnam as compared to Cambodia is interesting and can hardly be explained by differences in health care policy. Roughly 80 per cent of anti-HCV positive individuals are considered to be infectious21. The two clearly missed anti-HCV positive blood samples by EIA picked up by CMIA analysis, may be due to test properties. Both for HBV and HCV, there are genotype differences between Western countries and East Asia. Genotype C6 of HCV is dominant in South East Asia, China and South Africa whereas it is less common in other part of the world22. The test in use was developed by a Western company, and it may well be that this test did not identify the antibodies produced by the genotypes in the actual study population. Regardless of the reason, the study documented that the accuracy of hepatitis screening in potential blood donors in Vietnam was suboptimal. The false-negative HBsAg rate was 2.5 per cent, which increases the risk of transmitting HBV infections through blood products. Also the false-positive rate for anti-HBc of 7.6 per cent is unacceptably high and would exclude large numbers of potential blood donors.
An important problem is the risk of virus transmission from OBI donors. In the actual study population, the rate of HBsAg negative-anti-HBc positive participants was high (42%) which raised the question of how many of these were also OBI cases and potential transmitters of HBV infection? Accurate estimates cannot be given because the actual study participants due to resource restrictions could not be tested for HBV-DNA. The rate of HBV DNA detected in HBsAg-negative samples is reported to vary with the prevalence of HBV infection. Less than 5 per cent of HBsAg negative-anti-HBc positive blood donor samples are reported to have detectable HBV DNA in European countries with low HBV infection, whereas the corresponding rate is as high as 24 per cent in areas with high HBV prevalence2,8,23. A study conducted in China showed that the prevalence of occult HBV infection among HBsAg negative healthy individuals was 10.6 per cent24. In SE Asia, including Vietnam, routine screening of donors for HIV, syphilis, HBV, HCV, and malaria is well in place – but not routine screening for anti-HBc. On one hand, screening of blood donors for anti-HBc might reduce the risk of HBV transmission; but on the other hand, half the Vietnamese population would then be excluded from donation which has obvious consequences for blood product availability in the country. Based on the present state of knowledge it is not possible to come up with actual risk estimates for HBV transmission by HBsAg negative-anti-HBc positive blood products in areas of high HBV endemicity. NAT studies of HBV-DNA in HBsAg negative individuals are required to get a precise risk estimates for HBV transmission by HBsAg negative, anti-HBc positive blood products.
In conclusion, HBV is endemic in rural Vietnam and constitutes a major public health problem. The study shows that half of the rural population has detectable anti-HBc antibodies and that the prevalence of potential transmitters of HBV infection is high. The prevalence of HCV seems to be low, however, genotype differences of the virus may affect the accuracy of test results. The study also indicated that the EIA performance in blood donor screening in Vietnam was sub-optimal. The screening tests should be validated by nucleic acid amplification test (NAT) technology.
Acknowledgment
The authors thank the authorities, health workers and the civil organizations in Trieu Trach and Cam Thuy for their commitment, and acknowledge co-operation and logistic support from Quang Tri Provincial People's Committee, Dr Tran Kim Phung at Quang Tri Health Service, and Project RENEW, as well as professional co-operation of the research teams at Trauma Care Foundation, Cambodia and the University Hospital, North Norway. The Norwegian Plasma Fraction Foundation, and Tromsoe Mine Victim Resource Centre, University Hospital North Norway sponsored the study.
References
- 1.Wang JT, Lee CZ, Chen PJ, Wang TH, Chen DS. Transfusion transmitted HBV infection in an endemic area: the necessity of more sensitive screening for HBV carriers. Transfusion. 2002;42:1592–9. doi: 10.1046/j.1537-2995.2002.00274.x. [DOI] [PubMed] [Google Scholar]
- 2.Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008;48:1001–26. doi: 10.1111/j.1537-2995.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685–90. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 4.Weber B. Recent developments in the diagnosis and monitoring of HBV infection and the role of the genetic variability of the S gene. Expert Rev Mol Diagn. 2005;5:75–91. doi: 10.1586/14737159.5.1.75. [DOI] [PubMed] [Google Scholar]
- 5.Liu CJ, Lo SC, Kao JH, Tseng PT, Lai MY, Ni YH, et al. Transmission of occult hepatitis B virus by transfusion to adult and pediatric recipients in Taiwan. J Hepatol. 2006;44:39–46. doi: 10.1016/j.jhep.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya P, Chandra PK, Datta S, Banerjee A, Chakraborty S, Rajendran K, et al. Significant increase in HBV, HCV, HIV and syphilis infections among blood donors in West Bengal, Eastern India 2004-2005: Exploratory screening reveals high frequency of occult HBV infection. World J Gastroenterol. 2007;13:3730–3. doi: 10.3748/wjg.v13.i27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol. 2004;11:18–25. doi: 10.1016/j.tracli.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Behzad-Behbahani A, Mafi-Nejad A, Tabei SZ, Lankarani KB, Torab A, Moaddeb A. Anti-HBc & HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian J Med Res. 2006;123:37–42. [PubMed] [Google Scholar]
- 9.Hennig H, Puchta I, Luhm J, Schlenke P, Goerg S, Kirchner H. Frequency and load of hepatitis B virus DNA in first-time blood donors with antibodies to hepatitis B core antigen. Blood. 2002;100:2637–41. doi: 10.1182/blood-2002-03-0798. [DOI] [PubMed] [Google Scholar]
- 10.Biswas R, Tabor E, Hsia CC, Wright DJ, Laycock ME, Fiebig EW, et al. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion. 2003;43:788–98. doi: 10.1046/j.1537-2995.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen VTT, McLaws ML, Dore GJ. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22:2093–100. doi: 10.1111/j.1440-1746.2007.05010.x. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen VT, Law MG, Dore GJ. An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025. Liver Int. 2008;28:525–31. doi: 10.1111/j.1478-3231.2007.01646.x. [DOI] [PubMed] [Google Scholar]
- 13.Chien VC, Loan LTK, Tung LT, Nguyen HT, Van NT, Anh NT, et al., editors. Summary record of science works. Vietnam: Nha Tarang University; 1995-2001. Investigation into hepatits B virus infection among population in Nha Trang City; pp. 31–40. [Google Scholar]
- 14.Long HT, Van NT, Dat DT, Tran HQ, Cuong NV, Hao NH, et al. Situation of hepatitis B and C infections in Thanh Hoa. J Prev Med. 1999;2:5–10. [Google Scholar]
- 15.Duc DD. The epidemiology of hepatitis virus in Vietnam. J Pract Med. 1997;339:1–3. [Google Scholar]
- 16.Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. Manual of clinical microbiology. Washington DC: ASM Press; 2007. pp. 1424–36. [Google Scholar]
- 17.Abbott diagnostics. Products/instruments. [accessed on January 11, 2009]. Available from: http://www.abbottdiagnostics.com/Products/Instruments_by_Platform/systests.cfm?system=architect&suffix=i2000 .
- 18.Altman DC. Practical statistics for medical research. London: Chapman & Hall/CRC; 1999. [Google Scholar]
- 19.Ishida T, Takao S, Wannapa S, Tiwawech D. Prevalence of hepatitis B and C virus infection in rural ethnic populations of Northern Thailand. J Clin Virol. 2002;24:31–5. doi: 10.1016/s1386-6532(01)00222-0. [DOI] [PubMed] [Google Scholar]
- 20.Ol HS, Bjoerkvoll B, Sothy S, Heng YV, Hoel H, Husebekk A, et al. Prevalence of hepatitis B and hepatitis C virus infections in potential blood donors in rural Cambodia. SE Asian J Trop Med Public Health. 2009;40:963–71. [PubMed] [Google Scholar]
- 21.Lemon SM, Walker C, Alter MJ, Yi M. Hepatitis C virus. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1253–305. [Google Scholar]
- 22.Valsamakis A. Molecular testing in the diagnosis and management of chronic hepatitis B. Clin Microbiol Rev. 2007;20:426–39. doi: 10.1128/CMR.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Montalvo BM, Farfan-Ale JA, Acosta-Viana KY, Puerto-Manzano FI. Hepatitis B virus DNA in blood donor with anti-HBc as a possible indicator of active hepatitis B virus infection in Yucatan, Mexico. Transfus Med. 2005;15:371–8. doi: 10.1111/j.1365-3148.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 24.Fang Y, Shang QL, Liu JY, Li D, Xu WZ, Teng X, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. J Infect. 2009;58:383–8. doi: 10.1016/j.jinf.2009.02.013. [DOI] [PubMed] [Google Scholar]


