Abstract
Background & objectives:
Bone marrow mononuclear cell therapy has emerged as one of the option for the treatment of Stroke. Several preclinical studies have shown that the treatment with mononuclear cell (MNCs) can reduce the infarct size and improve the functional outcome. We evaluated the feasibility, safety and clinical outcome of administering bone marrow mononuclear cell (MNCs) intravenously to patients with subacute ischaemic stroke.
Methods:
In a non-randomized phase-I clinical study, 11 consecutive, eligible and consenting patients, aged 30-70 yr with ischaemic stroke involving anterior circulation within 7 to 30 days of onset of stroke were included. Bone marrow was aspirated from iliac crest and the harvested mononuclear cells were infused into antecubital vein. Outcomes measured for safety included immediate reactions after cell infusion and evidence of tumour formation at one year in whole body PET scan. Patients were followed at week 1, 4-6, 24 and 52 to determine clinical progress using National Institute of Health Stroke Scale (NIHSS), Barthel Index (BI), modified Rankin Scale (mRS), MRI, EEG and PET. Feasibility outcomes included target-dose feasibility. Favourable clinical outcome was defined as mRS score of 2 or less or BI score of 75 to 100 at six months after stem cell therapy.
Results:
Between September 2006 and April 2007, 11 patients were infused with bone-marrow mononuclear cells (mean 80 million with CD-34+ mean 0.92 million). Protocol was target-dose feasible in 9 patients (82%). FDG-PET scan at 24 and 52 wk in nine patients did not reveal evidence of tumour formation. Seven patients had favourable clinical outcome.
Interpretation & conclusions:
Intravenous bone marrow mononuclear cell therapy appears feasible and safe in patients with subacute ischaemic stroke. Further, a randomized controlled trial to examine its efficacy is being conducted.
Keywords: Autologous therapy, bone marrow, ischaemic stroke, mononuclear cell
Stroke has emerged as the third commonest cause of mortality worldwide1. Nearly 50 per cent of stroke survivors are left with disabling sequelae. Acute ischaemic stroke is the most common type and has only tissue plasminogen activator and aspirin as therapeutic options2. There is a clear need for new therapeutic options. Several preclinical studies have shown that the treatment with mononuclear cells (MNCs) can reduce the infarct size and improve the functional outcome in animal models of stroke3–6. MNCs contain several types of stem cells including haematopoietic stem cells (HSCs)7 and mesenchymal stem cells (MSCs) along with the haematopoietic progenitor cells and differentiated cells. These multi-potent stem cells are chemoattracted to the lesioned area and have ability to produce and secrete various cytokines and growth factors8,9. The MNCs have been well established to accelerate angiogenesis/neovasculazation in a number of ischaemic diseases such as limb ischaemia10 and myocardial infarction11. MNCs contribute to the proliferation of endogenous ischaemia-induced neural stem/progenitor cell after cerebral infarction through vascular niche regulation12 and contain endothelial progenitor cells (EPCs)13 which contribute to revascularisation and repair of injured endothelium in stroke14. The MNCs have been shown to accelerate angiogenesis11, to promote endogenous repair through proliferation of host's neural stem cells12, to decrease neuro-degeneration5, to prevent apoptosis and modulate peri-infarct inflammation4.
To our knowledge, there has been one report of intravenous autologous therapy with bone marrow MNCs in patients with stroke15. Two pilot clinical trials of mesenchymal stem cell therapy in subacute ischaemic stroke, through intravenous route16,17 have shown safety, feasibility and preliminary efficacy in subacute ischaemic stroke but MSCs require cell culture for several weeks before treatment which delays the start of intervention and increases the risk of contamination. MNCs are easily harvested from the bone marrow, processed and infused within a few hours of bone marrow aspiration. Thus, MNCs offer some advantages over MSCs in the treatment of subacute phase of ischaemic stroke.
Intravenous route is easier and safer, equally or possibly more effective than intracerebral or intracarotid routes18,19. Savitz et al20 reviewed the current status of cell therapy for stroke and have justified early phase clinical trial using autologous intravenous administration of stem cells in stroke. However, with advancing age, the bone marrow is progressively replaced by fat. Therefore, a question of feasibility is whether it is possible to obtain sufficient number of mononuclear cells from bone marrow. There is also a concern whether the stem cells may cause or promote tumour formation if reinfused after processing. Therefore, the objective of this phase-I study was to determine the feasibility, safety and clinical outcome of bone marrow mononuclear cells therapy in patients with subacute ischaemic stroke.
Material & Methods
Consecutive patients attending the neurology services of All India Institute of Medical Sciences (AIIMS), New Delhi, were considered for the study between September 2006 and April 2007 and followed up for one year. The study was approved by the Ethics Committees of AIIMS and the Department of Biotechnology (DBT), Government of India.
Inclusion criteria: Patients with stroke (defined as rapidly developing clinical symptoms and/or signs of focal loss of cerebral function, with symptoms lasting more than 24 h with no apparent cause other than that of vascular origin (modified from Hatano)21 were considered eligible if they fulfilled all of the following criteria: age between 30-70 yr, between 7th and 30th day after onset, computerized tomographic (CT) and/or MRI scan of the head showing no haematoma, and relevant lesions within the middle cerebral artery (MCA) and/or anterior cerebral artery (ACA) territory, Glasgow Coma Scale (GCS)22 score of above 8 (in patients with aphasia Eye and Motor score of more than 6), Barthel index (BI)score23 of 50 or less, National Institute of Health Stroke Scale (NIHSS) score developed by National Institutes of Health of 7 or more, and inability to walk unaided or raise upper limb by 90° and clinically stable : A patient was defined as stable when he had normal respiration, was afebrile, had BP less than mean arterial pressure of 125 mmHg (but no hypotension defined as systolic BP <90 mmHg), had fasting venous blood sugar level less than 200 mg/dl and normal urea/electrolytes for previous 48 h.
Exclusion criteria: Patients meeting the above criteria were excluded from the study if they had any of the following: lacunar syndrome, intubation, co-morbidity likely to limit survival to less than three years, e.g. malignant diseases, hepatic or renal failure, pre-stroke disability leading to dependence on others for activities of daily living, inaccessibility for follow up, allergy to local anaesthetic, pregnancy or HIV positivity.
Patients meeting all of the inclusion and none of the exclusion criteria were approached for consent to participate in the study. Patients were included in the study only if they or their next of kin provided written informed consent/assent.
Baseline assessment: Baseline assessment included clinical and laboratory tests. Patient characteristics and outcomes are shown in Table I. Carotid Doppler and echocardiography were done when clinically indicated as part of routine management. Flow diagram of study protocol is shown in Fig. 1. All patients had baseline MR examination within 4 wk after onset of neurological deficit with a 1.5T MR scanner (Avonto, Siemens Medical Solutions; Erlangen, Germany) using a circular polarized head coil prior to treatment. MR imaging of brain was done using standard T1, T2 FLAIR and DWI sequences. Spin echo single short echo planner sequence was used to perform DWI (TR 5000 ms, TE 96 ms, 5 mm thickness, 30% gap and 128 × 128 acquisition matrix). All the images were transferred to offline work station. Infarct volume was calculated in FLAIR Images with Analyze 7.0 developed by Biomedical Imaging Resource (BIR) at Mayo Clinic (Analyze Direct, Overland Park, USA) using Region- of -Interest module (ROI). Each patient also underwent whole body 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) with a whole body PET scanner using a Seimens Biograph LSO-PET/CT System, USA. Patients received a single dose of FDG (370 M Bq or 10 mci) intravenously, and emission scanning was performed for a total of 10 min beginning 60 min after injection.
Table I.
Patient's characteristics and outcome
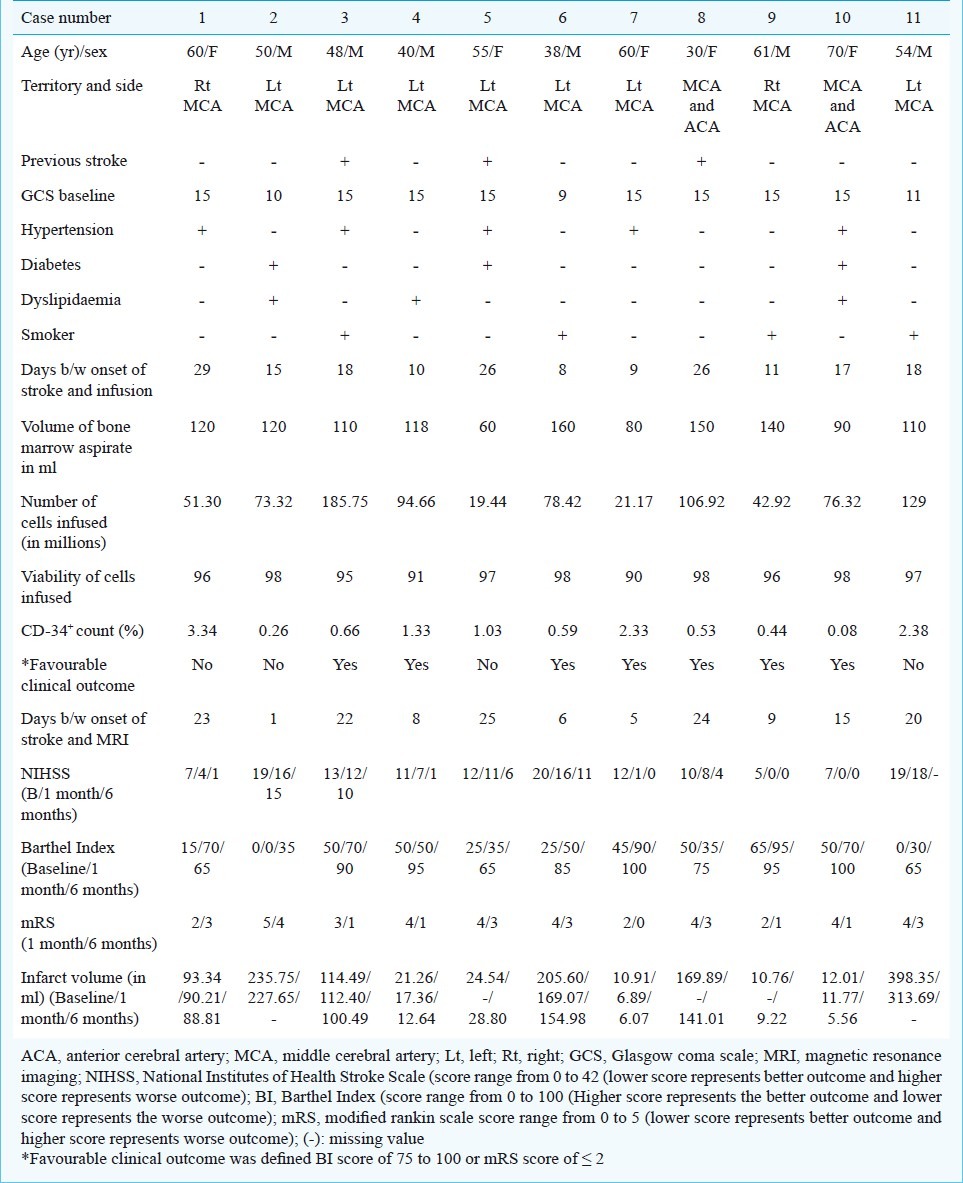
Fig. 1.
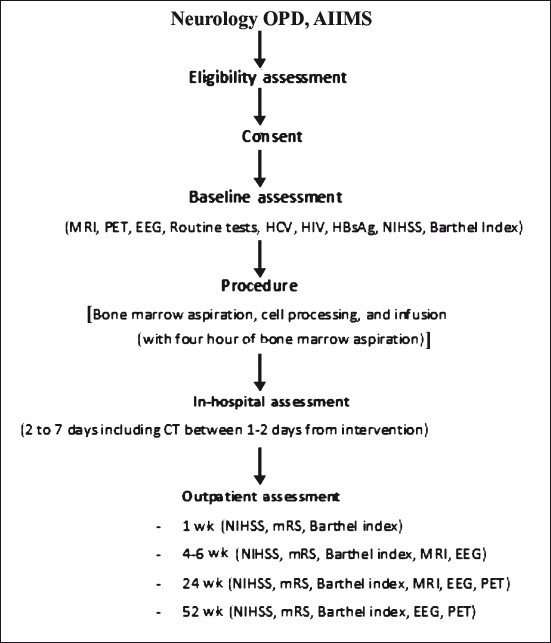
Flow diagram of study protocol. MRI, magnetic resonance imaging; PET, 18F-flurodeoxyglucose position emission tomo-graphy (FDG-PET); EEG, electroencephalography; HCV, hepaitis C virus; HIV, human immunodeficiency virus; HBsAg, hepatitis B virus antigen; NIHSS, National Institutes of Health Stroke Scale; CT, computed tomography; mRS, modified Rankin scale.
Intervention: Bone marrow was aspirated from iliac crest under local anaesthesia by the trained neurology residents. The procedure continued till either the patient requested stopping the procedure (due to unacceptable level of discomfort/pain) or volume of 160 ml aspirate was obtained. MNCs were separated by Ficoll density centrifugation method. A small aliquot of harvested mononuclear cells was used for (i) cell count, (ii) CD-34+ cell count by flow cytometry, (iii) cell viability test by trypan blue dye exclusion test24, and (iv) microbiological sterility testing. All the isolated mononuclear cells once received after processing (within 4 h) were immediately infused into antecubital vein over five minutes through canula in the forearm.
Outcomes: Feasibility was defined as target dose feasibility i.e. infusion of 40 million viable mononuclear cells intravenously within four hours of aspiration.
We assessed the safety of intravenous autologous MNCs infusion by recording the development of immediate or delayed complications recorded in a form with pre-specified list of safety parameters. The list included allergic reactions (tachycardia, fever, skin eruption, leukocytosis), local complications (haematoma, local infection at the site of bone marrow aspiration and thrombophlebitis at the site of injection), clinical features suggestive of vascular obstruction (tachypnoea, oliguria, peripheral vascular insufficiency, worsening of stroke) and systemic complications (septicaemia, increased SGOT, SGPT or rise in blood urea, creatinine levels) and development of epileptiform discharges in brain by EEG. To evaluate tumour formation as a delayed complication, physical examination was performed including inspection of the skin and oral mucosa, and a follow up whole-body FDG-PET scan at the end of 52 wk. Favourable clinical outcome was defined as any of the following at 24 wk: NIHSS <2, A score of 0 to 2 on modified Rankin Scale (mRS)24 and A BI score of 75 to 10026.
The NIHSS score was recorded by a research worker certified by NIH through online training and examination27. The BI, and mRS during follow up were measured by a resident blinded to baseline and previous scores on these scales. This was achieved with the use of blank data collection form at each visit.
Follow up: Patients were followed up for adverse events with clinical and laboratory evaluations. A follow up CT was done 24-48 h after the stem cell infusion to detect any haemorrhagic transformation of infarct. Patients were assessed daily during hospital stay, at wk 1 (NIHSS, BI, mRS), wk 4-6 (NIHSS, BI, mRS, EEG, MRI), wk 24 (NIHSS, BI, mRS, MRI, EEG, PET) and wk 52 (NIHSS, BI, mRS, EEG, PET). BI and mRS for one patient who did not turn up for follow up, were recorded by telephone.
Statistical analysis: Descriptive statistics were used to summarize baseline characteristics, feasibility and safety data. The data were analyzed using SPSS for Windows (Version 17.0).
Results
Eleven patients were included in this study. The number (in parentheses) completing follow up visits were: one week (all), 4-6 wk (all), 24 wk (all, one by telephone) and 52 wk (all, one by telephone). One patient was found ineligible after MRI (reported as intracerebral haemorrhage). Fig. 2 shows baseline MRI scans of three study participants.
Fig. 2.
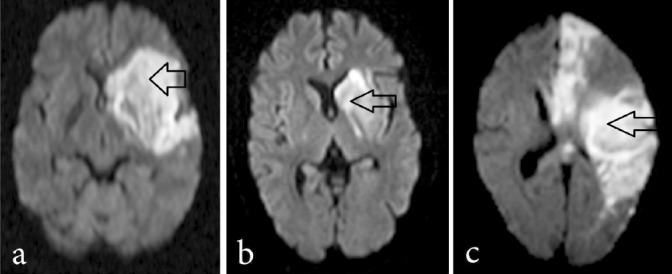
Magnetic resonance imaging (diffusion weighted) showing site and size of infarct in three study patients. Arrows show location of the infarct.
Feasibility outcomes: Protocol was target-dose feasible in 9/11 (82%) (Table I). Mean volume of aspirate was 114.4 ml (SD 29.8, range 60 to 160 ml). The mean number of mononuclear cells obtained was 85.6 million (SD 50.5, range 21 to 188.6 million) of which CD-34 positive cells (Fig. 3) constituted an average of 1.18 per cent (SD 1.1, range .08 to 3.2) and mean per cent of viable cells was 95.8 per cent (SD 2.8, range 90 to 98). Mean number of mononuclear cells infused was 80 million (SD 48.9, range 19.4 to 185.7). All infusions were completed within four hours of bone marrow aspiration. Infusion was given in four patients during 2nd wk, three in 3rd wk, and three in 4th wk, and one at day 29 after onset of stroke.
Fig. 3.
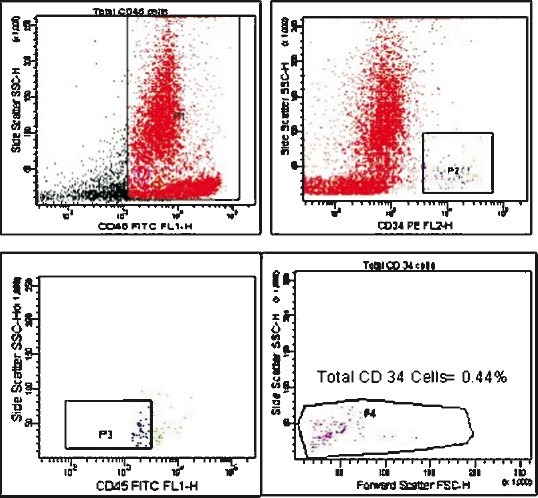
Flow cytometric analysis of CD-34+ epitope on bone marrow derived mononuclear cells of one study participant.
Safety outcomes: No serious adverse event was observed during the study. The bone marrow aspiration, cell processing and cell infusion were uneventful. MRI done after 4-6 wk of the first detected a small new infarct (less than a week old) on the same side as the index one but was clinically silent. None of the patients showed deterioration in NIHSS, BI or mRS during the follow up. There was no haemorrhagic transformation of infarct in any patient. No patient developed any feature suggestive of vascular obstruction, septicemia, liver or renal dysfunction or thrombophlebitis. None of the patients’ scalp EEGs showed any epileptiform discharges in the brain. Nine patients had PET scan at one year but none showed any evidence of tumour formation.
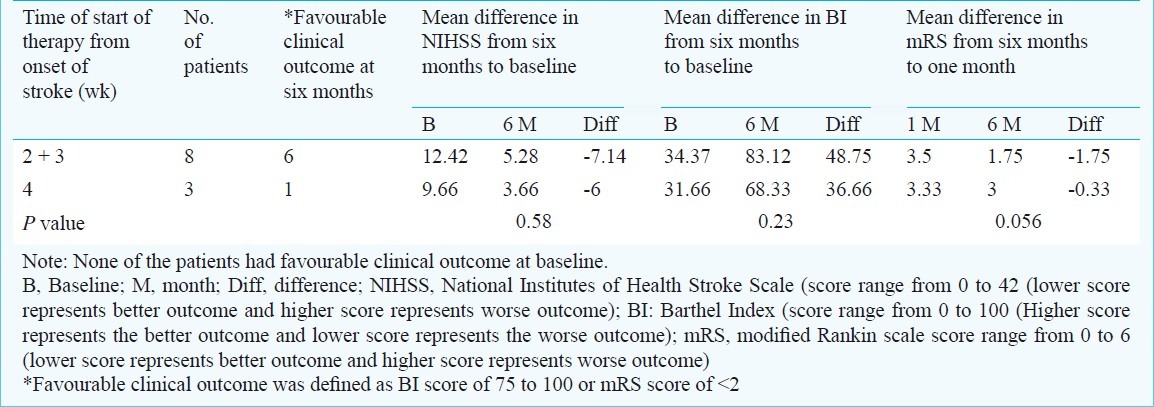
Clinical outcomes: All scales showed improvement from baseline in mean score that were statistically significant. Favourable outcome was found in 7 of 11 (64%): as per scales- BI 7 of 11 (64%), NIHSS 5 of 10 (50%) and mRS 6 of 11 (54.5%). Number of patients who achieved favourable clinical outcome with dose less than 75 million and greater than 75 million and start of stem cell therapy from the onset of stroke in weeks is represented in Table II.
Table II.
Outcome with timing of stroke onset and bone marrow derived mononuclear stem cells infusion
Discussion
Our study suggests that use of autologous MNCs through intravenous route is safe and feasible in patients with subacute ischaemic stroke between 7 to 30 days after its onset. In the setting of subacute stroke, a feasibility question arises: whether adequate bone marrow aspiration is possible without sedating stroke patients who are usually old in age. Sedation is avoided in patients with subacute stroke because decreased level of consciousness is an important clinical parameter to indicate deterioration in their condition that requires immediate attention. Our study shows that it is possible to successfully obtain adequate bone marrow (mean 114.36 ml), MNCs (mean 80 million) and CD-34+ cells (mean 0.9 million) without using sedation and from iliac crest of one side.
A safety question that often arises in the use of autologous stem cells is whether cells homing in organs other than bone marrow, pose a risk of development of tumour. Our patients followed for one year with clinical examination and FDG-PET did not reveal any indication of such complication. Although the small number of patients with one-year follow up may not be considered definitive, the study allays fear of any major safety concern from the intervention over one year period and provides assurance to conduct further research.
Three points deserve some comments: (i) intravenous route of infusion; (ii) time window of day 7 to day 30 after onset of stroke; (iii) advantages of MNCs. Intravenous route has been the second most commonly used route in preclinical studies after intracerebral route. Two studies comparing the intravenous, intracerebral and intracarotid routes in middle cerebral artery occlusion model (MCAO) models showed that intravenous route is either superior18 or similar19 to the other routes. Meta-analysis of preclinical studies in neurological disorders favours intravenous route28. Moreover, ease of administration of the cells and permissibility to use greater volume of infusate through intravenous than intracarotid route argues in favour of using intravenous route. One matter of concern with intravenous route may be the dilution of the infusate leading to small number of cells homing into the brain. Indeed, preclinical studies indicate that only 1 per cent of injected cells home into the brain18. However, even with intracarotid route, large majority of stem cells would return to venous system after first transit and undergo dilution. Considering that the major mechanism of action of MNCs seems to be through release of growth factors, angiogenesis factor and anti-apoptotic factors, it may not be important whether these home in the affected organ or other organs. The growth factors are probably released with change in the microenvironment of the stem cells and may enter the affected organ through blood stream and exert their effects. This is supported by a study in rat MCAo model29 which suggested that homing of injected cells into brain is not a prerequisite for acute neuroprotection. Intravenous route, therefore, appears safe, effective, devoid of risks associated with catheter angiography and consistent with preclinical evidence.
We selected the time window between day 7 to 30 after onset of stroke so that at least five days are available to stabilize the patients with acute stroke and the stable state continues for at least two days before bone marrow aspiration. The upper limit of 30 days was taken because the blood brain barrier is shown to be permeable upto 30 days in most cases of moderately severe stroke30 and there is experimental evidence that apoptosis continues for four to six weeks after onset of ischaemic stroke31. Preclinical work suggests that intravenous route maintains efficacy even when treatment is delayed for one month32. As anti-apoptosis is one of the main mechanisms of action of stem cells through intravenous route29, there is potential for benefit throughout the time window used in the study. The wide window will allow us to examine this potential during various time periods in phase-2 study and if established, it increases the generalizability of the therapy.
Autologous MNCs offer certain advantages over other stem cell types: (i) being autologous substantially less risk, if any, of tumour formation compared to embryonic stem cells; (ii) no risk of rejection; and (iii) ready availability in substantial numbers compared to MSCs. Ready availability allows the early administration of MNCs after stroke onset.
Our sample size was consistent with the ongoing and completed phase-I studies registered in NIH website (www.clinicaltrials.gov) having sample size of 12 or less33. The field of stem cell therapy is new, and phase-I studies are being undertaken on a few patients as matter of abundant caution. The results from such studies form the basis for larger phase-II studies, some of which are ongoing. Cell tracking and mechanism of action of stem cell therapy are other important issues. However, expert groups have recently noted that “there are no accepted techniques to label and monitor cells for clinical testing” and “understanding the mechanism of action is not essential before initiating clinical trials”34.
The present study was small and without comparison group. Safety beyond one year was not examined in this study. The large time window makes it difficult to ascertain average changes in functional outcomes over time. We do not know whether bone marrow volume used in our study was enough to treat stroke patients but at the current state of knowledge exact volume is unknown and hence this is a limitation common to all bone marrow autologous stem cell studies involving stem cell therapy. The preclinical study indicating efficacy at this time window period of stem cell therapy used MSCs and it is not known whether extrapolating this to MNCs is valid. Some groups used multiple antibodies to characterize different types of other cells in MNCs. One of the limitations of this study was use of only one antibody to characterize only CD-34+ cells.
In conclusion, our findings indicate that MNCs therapy in patients with subacute ischaemic stroke appears feasible and safe. Its efficacy needs examination in a randomized controlled trial that is already initiated (registration number CTRI -PROVCTRI/2008/091/000046)35 and clinicaltrial.gov (NCT01501773).
Acknowledgment
The study was funded by Department of Biotechnology, Ministry of Science & Technology, Government of India, New Delhi.
References
- 1.Sarti C, Rastenyte D, Cepaitis Z, Tuomilehto J. International trends in mortality from stroke, 1968 to 1994. Stroke. 2000;31:1588–601. doi: 10.1161/01.str.31.7.1588. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 3.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–9. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83:433–7. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Giraldi-Guimarães A, Rezende-Lima M, Bruno FP, Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009;1256:108–20. doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 6.Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Park JS, Choi YJ, Kim MO, Huh YH, Kim SW, et al. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells. 2009;27:1686–96. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 9.Shyu WC, Lee YJ, Liu DD, Lin SZ, Li H. Homing genes, cell therapy and stroke. Front Biosci. 2006;11:899–907. doi: 10.2741/1846. [DOI] [PubMed] [Google Scholar]
- 10.Duong Van Huyen JP, Smadja DM, Bruneval P, Gaussem P, Dal-Cortivo L, Julia P, et al. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol. 2008;21:837–46. doi: 10.1038/modpathol.2008.48. [DOI] [PubMed] [Google Scholar]
- 11.Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–9. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 12.Nakano-Doi A, Nakagomi T, Fujikawa M, Nakagomi N, Kubo S, Lu S, et al. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells. 2010;28:1292–302. doi: 10.1002/stem.454. [DOI] [PubMed] [Google Scholar]
- 13.Wang QR, Wang BH, Huang YH, Dai G, Li WM, Yan Q. Purification and growth of endothelial progenitor cells from murine bone marrow mononuclear cells. J Cell Biochem. 2008;103:21–9. doi: 10.1002/jcb.21377. [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–97. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY for the STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 17.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 18.Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosc Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 19.Jin K, Sun Y, Xie L, Mao XO, Childs J, Peel A, et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis. 2005;18:366–74. doi: 10.1016/j.nbd.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Savitz SI, Dinsmore JH, Wechsler LR, Rosenbaum DM, Caplan LR. Cell therapy for stroke. NeuroRx. 2004;1:406–14. doi: 10.1602/neurorx.1.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 22.Teasdale G, Jennett B. Assessment of coma and impaired consciousness.A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 23.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–9. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 24.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. Appendix 3: Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 25.Mateen FJ, Nasser M, Spencer BR, Freeman WD, Shuaib A, Demaerschalk BM, et al. Outcomes of intravenous tissue plasminogen activator for acute ischemic stroke in patients aged 90 years or older. Mayo Clin Proc. 2009;84:334–8. doi: 10.1016/S0025-6196(11)60542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, et al. Ebselen Study Group. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Stroke. 1998;29:12–7. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 27.NIHSS online education program. [accessed on March 10, 2008]. Available from: http://nihss-english.trainingcampus.net/uas/modules/trees/windex.aspx .
- 28.Janowski M, Walczak P, Date I. Intravenous route of cell delivery for treatment of neurological disorders: a meta-analysis of preclinical results. Stem Cells Dev. 2010;19:5–16. doi: 10.1089/scd.2009.0271. [DOI] [PubMed] [Google Scholar]
- 29.Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–9. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, et al. Changes in background blood brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke. 2008;39:1327–32. doi: 10.1161/STROKEAHA.107.500124. [DOI] [PubMed] [Google Scholar]
- 31.Gladstone DJ, Black SE, Hakim AM. Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 32.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:1311–9. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 33.Burns TC, Steinberg GK. Stem cells and stroke: opportunities, challenges and strategies. Expert Opin Biol Ther. 2011;11:447–61. doi: 10.1517/14712598.2011.552883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L. Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke. 2011;42:825–9. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- 35.National Institute of Medical Statistics (NIMS) The Clinical Trial Registry (CTRI) [accessed on May 30, 2012]. Available from: http://www.ctri.in:8080/Clinicaltrials/trials_jsp/index.jsp. (CTRIPROVCTRI/2008/091/000046 .


