Abstract
Background & objectives:
The cytokines, adipokines, and oxidative stress have been implicated in the pathogenesis of non-alcoholic fatty liver disease (NAFLD); however, such data remain scarce in India. The present study evaluated pro-inflammatory cytokines, adipokines, and markers of oxidative stress in patients with non-alcoholic fatty liver disease (NAFLD), and their association with degree of adiposity, insulin resistance and markers of disease severity.
Methods:
The present prospective cross-sectional pilot study included 79 subjects; 34 NAFLD, 22 chronic hepatitis B (CH-B) and 23 healthy controls (HC). The parameters studied were adiponectin, leptin, tumour necrosis factor α (TNFα), interleukin-1 and 6 (IL-1, IL-6), and systemic markers of oxidative stress.
Results:
The mean body mass index (kg/m2) in NAFLD patients, CHB, and HC were 26.4±3.7, 21.3±2.3, and 22.3±2.7, respectively. The median serum levels of all pro-inflammatory cytokines were significantly higher (P<0.001) in NAFLD compared to control groups. Compared to HC, levels of adiponectin and leptin were significantly (P<0.05, P<0.01) reduced in both NAFLD and CHB. IL-6 showed marked and selective increase only in NAFLD patients. The levels of IL-6 were significantly (P<0.02) higher in NAFLD patients with advanced histology grade and correlated with IR (r=0.42, P=0.02). In a sub-group, markers of oxidative stress were significantly higher, and that of antioxidant potential were significantly lower among NAFLD patients compared to control subjects.
Interpretation & conclusions:
Patients with NAFLD revealed significantly elevated levels of pro-inflammatory cytokines, increased oxidative stress, and a significant association of IL-6 with IR and advanced histopathology.
Keywords: Adipokines, chronic hepatitis B, cytokines, insulin resistance, NAFLD, oxidative stress
Nonalcoholic fatty liver disease (NAFLD) is a major cause of chronic liver disease worldwide1–3. The spectrum of NAFLD ranges from simple steatosis, non-alcoholic steatohepatitis (NASH), to cirrhosis. The pathogenesis of NAFLD appears to involve a multi-hit process1,4. The first hit is the steatosis which is believed to be triggered by insulin resistance (IR), and the second hit, which involves cytokines alteration and oxidative stress, results in disease progression. The pro-inflammatory cytokines and adipokines have been implicated in the pathogenesis of NAFLD; however, the data have been derived mainly from animal studies, and studies on the Western population where profiles of NAFLD patients appear to be different5–7. The differences in Indian NAFLD patients include a lower frequency of metabolic syndrome8,9, lesser degree of adiposity2, lower frequencies of abnormal iron indices10, and histological milder disease at presentation8. A study has revealed that the Indians in comparison to matched Caucasians, Hispanics, Black and Eastern Asians had 2 to 3-fold increase in IR, and 2-fold increase in hepatic triglyceride content11.
The alteration of cytokines in NAFLD has been linked to degree of adiposity. Lower preponderance of adiposity in Indian NAFLD is well documented2,8,9 but the profiles of various adipokines, and cytokines have not been evaluated. Further, there are evidences to suggest a role of oxidative stress in pathogenesis of NAFLD12,13. However, many studies have shown conflicting results, and a weak association. Also, description of the antioxidant capacity among NAFLD patients is scarce. The present prospective study was designed to evaluate the profiles of pro-inflammatory cytokines, adipokines and oxidative stress in NAFLD patients presenting at a tertiary care centre in India compared to healthy and disease controls, and to study their association with degree of adiposity, IR, and markers of disease severity.
Material & Methods
The study was conducted at Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi, India, from June 2005 to December 2008. Consecutive patients who attended the liver clinic, satisfied the inclusion criteria, and showed willingness to participate in the study were included. The healthy controls were selected from hospital staffs, or those who accompanied patients with minor illnesses to our outpatient clinic. The study protocol was approved by the ethics committee of the institute and a written consent was obtained from all study subjects. This exploratory cross-sectional study was performed as a pilot project in small groups of patients and controls to generate preliminary data for the Indian NAFLD patients.
Diabetes mellitus (DM) was defined as per definition given by the World Health Organization14. Patients with body mass index (BMI) of more than 23 kg/m2 were defined as overweight and those with a BMI of >25 were labelled as obese according to Asian standards15. Metabolic syndrome was diagnosed as per modified Adult Treatment Panel III criteria15.
Patients: They were NAFLD patients (n=34) with raised serum alanine transaminase (ALT) to at least 1.5 times upper limit of normal (upper limit was 41 IU/l for men and 30 IU/l for women). The diagnosis of NAFLD was made on the basis of ultrasonography, presence of insulin resistance or features of metabolic syndrome, and histologic confirmation whenever possible.
Controls: Because the alteration in the profiles of cytokines and oxidative stress can occur in several inflammatory conditions of liver such as chronic viral hepatitis, both healthy as well as disease controls were included for the purpose of comparison. Healthy controls (n=23) were healthy subjects with normal liver function tests, and without ultrasonographic evidence of fatty liver. Disease controls (n=22) were patients with chronic hepatitis B (CHB) with raised serum ALT to at least 1.5 times upper limit, and without histologic and/or ultrasonographic evidence of fatty liver. The rationale of using disease control was to assess whether the profiles of cytokines, adipokines, and markers of oxidative stress in NAFLD patients differ significantly from patients of other inflammatory conditions of liver without hepatic steatosis. Patients with CHB were chosen over patients with chronic hepatitis C because steatosis, which could have been a confounder, is quite common in latter condition.th
Exclusion criteria: Patients and controls consuming alcohol >20 g ⁄day, having other known liver diseases (hepatitis viruses A to E, autoimmune disease, Wilson's disease, alpha 1 anti-trypsin deficiency and haemochromatosis) and those on medications known to induce fatty liver or insulin sensitization such as estrogens, amiodarone, methotrexate, tamoxifen, glitazones and metformin were excluded.
Patients evaluation and procedure: A thorough clinical history and examination including anthropometric measurements were done in all patients. After an overnight fast, blood samples (20 ml) were collected for blood count, biochemical profiles and other investigations. Serum from each patient was tested for markers of viral hepatitis A, B, C and E. Serum ferritin, copper and autoantibody tests were done using conventional techniques. After obtaining informed consent, liver biopsy was done using 18-gauge Menghini's aspiration needle. The classification given by Brunt et al16 was used to grade and stage NASH. For known CHB patients, histological grading and staging was done according to Knodell's histological activity index17 and the International Classification for staging17.
Measurements of pro-inflammatory cytokines, adipokines, insulin, and insulin resistance: Commercial ELISA assays kits were used to measure serum level of TNFα, IL-1, IL-6, leptin (Cayman Chemical Company, Michigan, USA, Website: http//www.cayman), adiponectin (BioVendor Laboratory, Medicine Inc, Modrice, Czech Republic), and insulin (Ray BioTech, Norcross Ga 30092, USA) according to manufacturer's manual. IR was measured as homeostasis model assessment insulin resistance (HOMA-IR). Value of HOMA-IR more than 2.5 was taken as the presence of insulin resistance18.
Measurement of oxidative stress and antioxidant capacity: Plasma malondialdehyde (MDA), the end product of lipid peroxidation was determined by thiobarbituric acid (TBA) reactivity19. Superoxide dismutase (SOD) was estimated by the 50 per cent inhibition of auto-oxidation of pyrogallol at pH 8.020. Plasma vitamin C was measured by dinitrophenyl hydrazine method in which vitamin C reacts with dinitrophenyl hydrazine to give orange chromophore when heated at 60°C for 1 h and solubilized by concentrated acid and absorbance is read at 520 nm21. Total antioxidant capacities, measured as ferric reducing ability of plasma (FRAP), by the methodology given by Benzie and Strain22. The chemicals for lipid peroxidation and antioxidant potential were procured from Sigma Aldrich Co., USA.
Data analysis: Normally distributed continuous variables were expressed as mean±SD, and the continuous variables with skewed distribution were expressed as median (range). For the comparison of continuous variables between three groups namely NAFLD, CHB and healthy control, one-way ANOVA with Bonferroni correction as a post-hoc test was used. Similarly, the comparison between groups for skewed data was done by Kruskal-Wallis test, followed by Mann Whitney test with adjusted P values. Several confounders (DM, dyslipidaemia, and adiposity) are known to affect the levels of cytokines. The adjustment for these confounders were done by restriction of the analysis. We made homgenous sub-groups by including only those patients and control subjects who did not have any of these confounders. Associations between variables were calculated using Spearman's correlation coefficients. Data were analyzed by using SPSS software version15.0 (SPSS, Chicago, IL, USA) and P<0.05 was taken as significant.
Results
The baseline clinical and demographic profiles of patients and controls are depicted in Table I. The mean age of NAFLD patients was similar to that of healthy control, but was higher (P<0.001) than that of CHB patients. The BMI, waist hip ratio (WHR), plasma insulin levels, and HOMA-IR were significantly higher in NAFLD patients compared to both control groups (P<0.001). The liver function tests were similar between NAFLD and CHB patients. Also, except for steatosis, the histological grades and stages of fibrosis were similar between NAFLD and CHB. Twenty one (61.7%) patients of NAFLD were obese as per Asian criteria, and 22 (62%) had central obesity. Among NAFLD patients, hypertriglyceridaemia and DM were present in 17 (50%) and four (13%) patients, respectively. The criterion for metabolic syndrome was fulfilled in 12 (35%) patients of NAFLD.
Table I.
Baseline clinical, anthropometric and biochemical characteristics of study populations
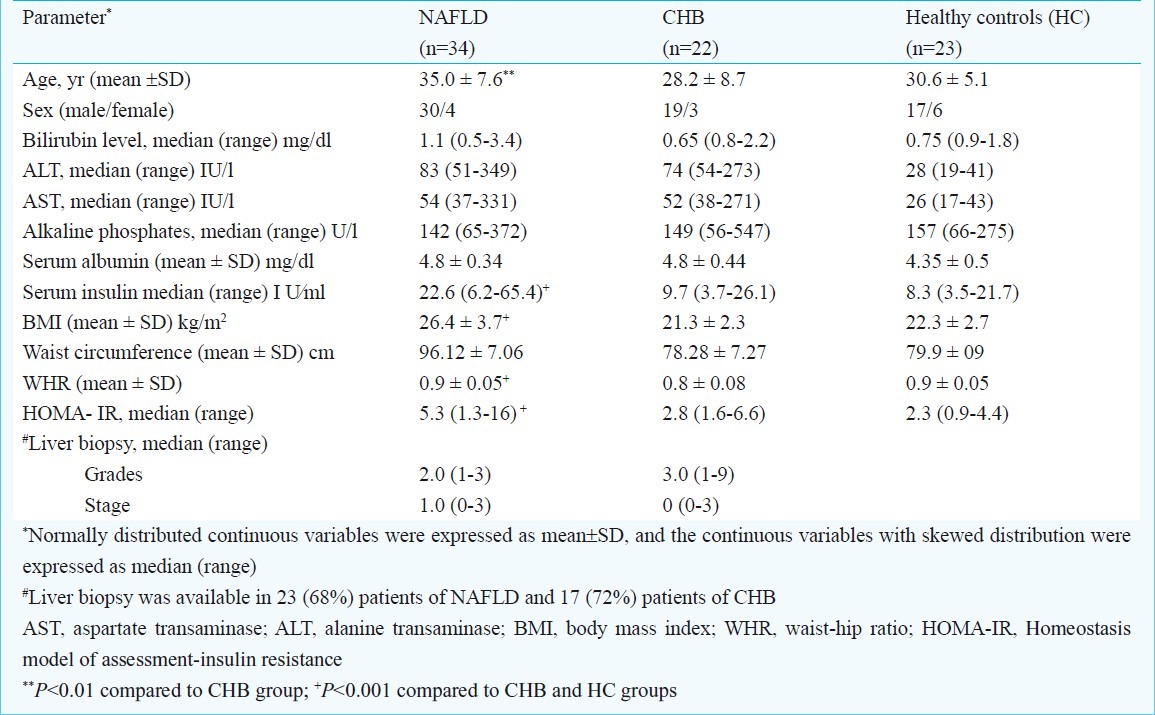
Comparison of pro-inflammatory cytokines and adipokines between groups (Table II): The serum levels of all the pro-inflammatory cytokines (TNFα, IL-1, and IL-6) were significantly (P <0.001) higher among NAFLD patients compared to control groups. The cytokine which showed maximum increase in the levels among NAFLD was IL-6. Interestingly, the levels of IL-6 were similar between CHB and HC suggesting its selective increase only in NAFLD patients. In contrast, the levels of adiponectin and leptin were similarly reduced both in NAFLD and CHB patients compared to healthy control (Table II). Because the blood levels of cytokines may be affected by certain demographic and metabolic confounders such as DM, dyslipidemia, and adiposity, a subgroup analysis was performed after adjusting these confounders by restriction analysis. Still, results remained the same as before (Table III).
Table II.
Comparisons of median levels of adipokines and pro-inflammatory cytokines between study groups
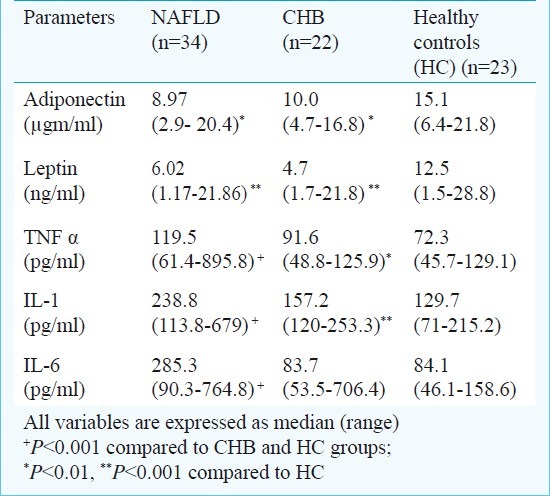
Table III.
Comparison of various adipokines and proinflammatory cytokines between groups adjusted for metabolic and demographic confounders by restriction analysis
Association of histological severity with pro-inflammatory cytokines and adipokines: Liver biopsy could be done in 23 (68%) patients of NAFLD, and 17 (72%) patients of CHB. The histological grades among NAFLD patients were as follows: grade I - 35 per cent, grade II - 53 per cent, and grade III - 12 per cent patients. Similarly, 39, 26, 26, and 9 per cent of NAFLD patients had stage 0, 1, 2, and 3 of fibrosis, respectively. The levels of pro-inflammatory cytokines were similar between grade I and II disease. However, among patients with grade III histology, levels of IL-6 [560 (523-575) pg/ml] were significantly higher than that in grade I [206 (181-413) pg/ml, P<0.05], and in grade II [226 (91-507) pg/ml, P<0.05] disease, respectively (Fig. 1A). The levels of all three pro-inflammatory cytokines were noticeably higher in patients with stage 3 fibrosis; however, the overallth differences in levels between different stages of fibrosis were not statistically significant (Fig. 1B), and only IL-6 revealed a significantly higher levels in patients with stage 3 fibrosis compared to stage 0 (P<0.05) and stage 1 (P<0.05). This may be because of small number of patients with advanced fibrosis. With increasing histological severity of NAFLD, the levels of adiponectin and leptin did not reveal any significant difference. Among patients with CHB, the levels of all cytokines and adipokines were similar between different grades and stages of fibrosis (data not shown).
Fig. 1.
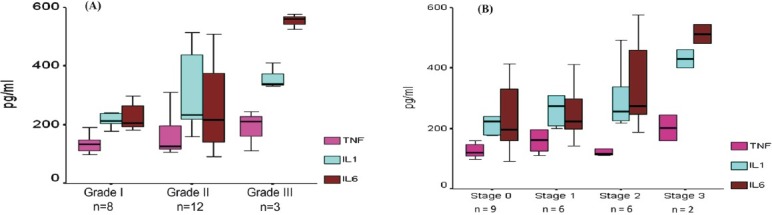
Box plots of pro-inflammatory cytokines levels in NAFLD patients as per histological grades (A) , and stages of fibrosis (B). The level of IL-6 was significantly (P<0.05) higher among patients with grade III histology than grade I and grade II disease. Only IL-6 revealed a significantly (P<0.05) higher levels in patients with stage 3 fibrosis compared to stage 0.
Association with degree of adiposities: Among NAFLD patients, none of adipokines or cytokines had significant correlation with the degree of adiposities. No significant differences were observed in the levels of adipokines or cytokines between NAFLD patients with high BMI versus normal BMI, or between patients with or without central obesity (Fig. 2 A and B). The degrees of adiposities (BMI and WHR) did not differ significantly between patients with normal and increased insulin resistance defined as HOMA-IR cut-off at 2.5.
Fig. 2.
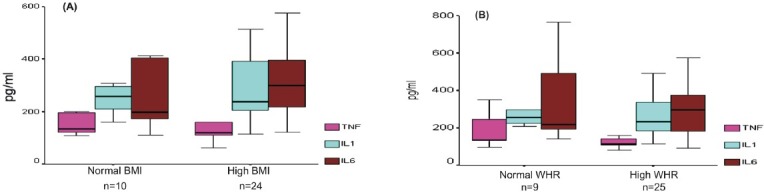
Pro-inflammatory cytokines in relation to (A) BMI, and (B) Waist-hip ratio (WHR). No significant differences were observed between the subgroups.
Adipokines and pro-inflammatory cytokines in association with insulin resistance: Among NAFLD patients, 76.4 per cent (26/34) had IR defined by HOMA-IR >2.5. The HOMA-IR was significantly (P<0.001) higher in NAFLD patients in comparison to CHB patients, and HC (Table I). Among all studied adipokines and cytokines, only IL-6 had a significant positive correlation with HOMA-IR (r=0.42, P=0.02). Also, the levels of IL-1 and IL-6 had significant correlation with the serum insulin levels [IL-1/insulin (r 0.35 P= 0.04), and IL-6/insulin (r 0.37, P= 0.03)].
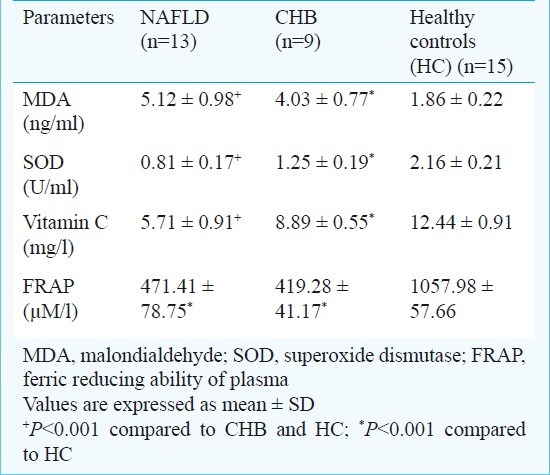
Measures of systemic oxidative stress and antioxidant capacity: Due to technical reasons, these measurements could be done only in 37 study subjects (13 NAFLD, 09 CHB, and 15 HC). The serum levels of MDA were significantly higher among NAFLD patients compared to either of control groups (Table IV). In contrast, the levels of vitamin C, and SOD were significantly lower among NAFLD patients as compared to controls. FRAP, a measure of total antioxidant capacity of the body, was significantly lower among NAFLD and CHB patients as compared to HC. However, it was similar between NAFLD and CHB suggesting this reduction may not be specific to NAFLD.
Table IV.
Comparison of systemic measures of oxidative and anti-oxidative capacity between NAFLD patients and controls
Association between different disease variables: A positive correlation was noted between levels of different pro-inflammatory cytokines, such as between IL-1 and IL-6 (r 0.47, P=0.005), and between IL-1 and TNFα (r 0.63 P =0.001). There were no significant correlations between levels of any of cytokines and serum ALT. Serum adiponectin was found to vary inversely with pro-inflammatory cytokines, but the correlation was not significant. No significant correlation was found between any measures of oxidative stress and histological severity, insulin levels, ALT, adipokines, and cytokines. Also measures of oxidative stress or antioxidant potential were independent of BMI or WHR.
Discussion
The present study evaluated comprehensive profiles of cytokines, adipokines, and oxidative stress in Indian NAFLD patients, and studied their associations with markers of disease severity, adiposity, and IR. The levels of pro-inflammatory cytokines (TNFα, IL-1, and IL-6) were found to be significantly higher in NAFLD patients compared to healthy controls as well as patients of chronic hepatitis B, an inflammatory condition of liver without steatosis. A higher amplification of cytokines in NAFLD over CHB suggests that this may not be just a non-specific inflammatory response.
Several evidences support a role for cytokines, including TNFα, IL-1, IL-6, IL-8, and IL-18, in the pathogenesis of the IR and NAFLD5,6,23,24. In our study, IL-6 emerged as the most important cytokine in NAFLD patients. Interestingly, levels of IL-6 increased selectively in NAFLD patients only. The levels of IL-6 were significantly higher among NAFLD patients with advanced histopathology, and also had significant correlation with HOMA-IR and serum insulin. Therefore, IL-6 may have a role in the pathogenesis of IR and NAFLD. IL-6 has, in fact, gained attention lately after a small study that showed a markedly increased serum levels and liver expression of IL-6 in NASH patients which correlated with inflammation and fibrosis24. We did not find TNFα to be associated with any important disease variables, though its levels were significantly higher in NAFLD patients than the controls. This may be due to different study populations or the lack of adjustment for some factors that might have influenced its serum levels.
In West, there is a strong clinical association between obesity and NAFLD. The source of cytokines is thought to be macrophages residing in the adipose tissue25. However, NAFLD in India occurs at lower BMI and even in lean subjects2,8,9. We found that the cytokines profiles were similar between NAFLD patients with higher versus normal degree of adiposities measured by BMI and WHR. This raises a question as to whether the adipose tissues of these patients have more pro-inflammatory potential or some other factor(s) are responsible for cytokines amplification. The location and size of adipocytes have an important link with secretion of cytokines. Visceral adipocytes tend to be smaller than subcutaneous adipocytes but have greater potential to secrete cytokines26,27. Thus, our NAFLD patients may be having more visceral adiposity. However, we did not find a correlation between levels of cytokines and central obesity. This may be because of the fact that measured central obesity, which also includes metabolically inert subcutaneous abdominal fat, does not exactly correspond to visceral adiposity. Another mechanism by which cytokines amplification can occur in NAFLD patients is endotoxaemia resulting from small intestinal bacterial overgrowth (SIBO)28. IL-6 production by macrophages has been shown to be enhanced by addition of endotoxins (lipopolysaccharide)29. Also, patients with SIBO have revealed an increased mucosal production of IL-630. Thus, SIBO may account for cytokines amplification in Indian NAFLD; however, data supporting this are not yet available.th
A reduced level of adiponectin has been implicated in the pathogenesis of NAFLD. Our study showed a significant reduction of serum adiponectin levels among NAFLD. The role of leptin in human NAFLD is still controversial because of conflicting results in different studies. While leptin was found to be significantly higher in NAFLD patients compared to control in one report31, another study found no significant role of leptin in NAFLD32. In our study, leptin levels in NAFLD patients were significantly lower than that in HC. However, we could not find any significant association between either leptin or adiponectin and any of disease variables and their levels were similarly decreased in patients with CHB.
In consistence with previous reports12,13, we found a significantly increased rate of lipid peroxidation among NAFLD patients compared to control groups. The markers of antioxidant capacity were significantly lower among NAFLD patients compared to HC. Other studies have also demonstrated significant reductions in levels of antioxidants such as SOD and FRAP in NAFLD patients33. However, the reduction in total antioxidant capacity, as measured by FRAP, was similar in NAFLD and CHB. Also, the correlation between oxidative stress and histological severity or biochemical activity was not significant. Therefore, their association with disease pathogenesis may be weak and non-specific. Also, we had measured only the systemic markers of oxidative stress rather markers of oxidative stress in liver biopsy tissues which could have been more direct evidence of its role in pathogenesis. No significant correlation was observed between measures of oxidative stress and levels of cytokines or adipokines. Thus the cytokines alteration and oxidative stress may have an independent role in the pathogenesis of NAFLD.
This preliminary study in Indian NAFLD patients showed some novel findings. We observed a significant amplification of pro-inflammatory cytokines in Indian NAFLD patients even at a lower degree of adiposity, and it was IL-6 levels (and not TNFα) which showed the maximum increase and association with IR, and histopathological severity. It will be interesting to see whether modification of IL-6 can change the course of IR and NAFLD in Indian patients. Further, occurrence of NAFLD in India at a lower degree of adiposity also raises question as to whether some other trigger like SIBO plays a role in the pathogenesis. An obvious limitation is the cross-sectional design from which it is difficult to make a causal inference. In view of small sample size and the fact that association studies are susceptible to various biases, confirmatory studies using larger sample are needed.
To conclude, the findings of our study suggest that pro-inflammatory cytokines are markedly increased in Indian NAFLD patients. The levels of IL-6 were more selectively increased in NAFLD, and revealed significant association with IR and histopathological severity. The systemic oxidative stress was increased in NAFLD; however, its association with pathogenesis was not significant.
Acknowledgment
The study was financially supported by the Indian Council of Medical Research (ICMR), New Delhi.
References
- 1.McCullough AJ. Pathophysiology of Nonalcoholic Steatohepatitis. J Clin Gastroenteol. 2006;40(Suppl1):S17–29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 2.Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 3.Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL for the Asia-Pacific Working Party on NAFLD. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–93. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- 4.Day CP, James OF. Steatohepatitis: a tale of two “hits” ? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 5.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 7.Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–21. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 8.Madan K, Batra Y, Gupta SD, Chander B, Rajan KD, Tewari MS, et al. Non-alcoholic fatty liver disease may not be a severe disease at presentation among Asian Indians. World J Gastroenterol. 2006;12:3400–5. doi: 10.3748/wjg.v12.i21.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duseje A, Das A, Das R, Dhiman RK, Chawla A, Bhansali A, et al. The Clinicopathological Profile of Indian Patients with Nonalcoholic Fatty Liver Disease (NAFLD) is Different from that in the west. Dig Dis Sci. 2007;52:2368–74. doi: 10.1007/s10620-006-9136-y. [DOI] [PubMed] [Google Scholar]
- 10.Duseja A, Das R, Nanda M, Das A, Garewal G, Chawla Y. Nonalcoholic steatohepatitis in Asian Indians is neither associ-ated with iron overload nor with HFE gene mutations. World J Gastroenterol. 2005;11:393–5. doi: 10.3748/wjg.v11.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Man CD, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Nat Acad Sci. 2006;103:18273–7. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–8. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259–68. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 14.Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Geneva: WHO; 1999. [accessed on July 8, 2001]. World Health Organization, Department of Noncommunicable Disease Surveillance. Available at: http://www.staff.ncl.ac.uk/philip.home/who_dmg.pdf . [Google Scholar]
- 15.Heng D, Ma S, Lee JJM, Tai BC, Mak KH, Hughes K, et al. Modification of the NCEP ATP III definitions of the metabolic syndrome for use in Asians identifies individuals at risk of ischemic heart disease. Atherosclerosis. 2006;186:367–73. doi: 10.1016/j.atherosclerosis.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Belch JJF, Bridges AB, Scott N. Oxygen free radicals and congestive heart failure. Br Heart J. 1991;65:215–8. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for SOD. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 21.Okamura M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin Chim Acta. 1980;103:259–68. doi: 10.1016/0009-8981(80)90144-8. [DOI] [PubMed] [Google Scholar]
- 22.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 23.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–10. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–9. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 25.Bouloumié A, Curat CA, Sengenès C, Lolmède K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8:347–54. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 26.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 27.Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Goldstein BJ. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–7. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 28.Wigg AJ, Robert-Thomson IC, Dymock RB, Mc Carthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxemia and tumor necrosis factor alfa in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–11. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita A, Soga Y, Iwamoto Y, Yoshizawa S, Iwata H, Kokeguchi S, et al. Macrophage-adipocyte interaction: marked interleukin-6 production by lipopolysaccharide. Obesity (Silver Spring) 2007;15:2549–52. doi: 10.1038/oby.2007.305. [DOI] [PubMed] [Google Scholar]
- 30.Rioda SM, Mclver CJ, Wakefield D, Duncombe VM, Bolin TD, Thomas MC. Mucosal cytokine production in small-intestinal bacterial overgrowth. Scand J Gastroenterol. 1996;31:977–84. doi: 10.3109/00365529609003117. [DOI] [PubMed] [Google Scholar]
- 31.Tsochatzis E, Papatheodoridis GV, Hadziyannis E, Georgiou A, Kafiri G, Tiniakos DG, et al. Serum adipokine levels in chronic liver diseases: association of resistin levels with fibrosis severity. Scand J Gastroenterol. 2008;43:1128–36. doi: 10.1080/00365520802085387. [DOI] [PubMed] [Google Scholar]
- 32.Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41:943–9. doi: 10.1016/j.jhep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci. 2004;106:261–8. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]


