Abstract
Background & objectives:
Hyperthyroidism is associated with increased food intake, energy expenditure and altered body composition. This study was aimed to evaluate the role of adipocytokines in weight homeostasis in patients with hyperthyroidism.
Methods:
Patients (n=27, 11men) with hyperthyroidism (20 Graves’ disease, 7 toxic multinodular goiter) with mean age of 31.3±4.2 yr and 28 healthy age and body mass index (BMI) matched controls were studied. They underwent assessment of lean body mass (LBM) and total body fat (TBF) by dual energy X-ray absorptiometer (DXA) and blood sample was taken in the fasting state for measurement of leptin, adiponectin, ghrelin, insulin, glucose and lipids. Patients were re-evaluated after 3 months of treatment as by that time all of them achieved euthyroid state with carbimazole therapy.
Results:
The LBM was higher (P<0.001) in healthy controls as compared to hyperthyroid patients even after adjustment for body weight (BW), whereas total body fat was comparable between the two groups. Serum leptin levels were higher in patients with hyperthyroidism than controls (22.3±3.7 and 4.1±0.34 ng/ml, P<0.001), whereas adiponectin levels were comparable. Plasma acylated ghrelin was higher in patients than in controls (209.8±13.3 vs 106.2±8.2 pg/ml, P<0.05). Achievement of euthyroidism was associated with significant weight gain (P<0.001) and significant increase in lean body mass (P<0.001). The total body fat also increased but insignificantly from 18.4±1.8 to 19.9±1.8 kg. There was significant decrease (P<0.05) in serum leptin and acylated ghrelin but adiponectin levels remained unaltered after treatment. Serum leptin positively correlated with TBF and this correlation persisted even after adjustment for BW, BMI, gender and age (r=0.62, P=0.001). However, serum leptin and acylated ghrelin did not correlate with the presence or absence of hyperphagia.
Interpretation & conclusion:
Patients with hyperthyroidism predominantly had decreased lean body mass which increased after achievement of euthyroidism with carbimazole. The hyperphagia and the alterations in weight homeostasis associated with hyperthyroidism were independent of circulating leptin and ghrelin levels.
Keywords: Adipocytokines, body composition, ghrelin, hyperphagia, hyperthyroidism
Hyperthyroidism is invariably associated with weight loss despite increased appetite1,2. Thyroid hormone excess is associated with increased food intake in humans and rodents, possibly as a compensatory response to increased energy expenditure due to uncoupling of oxidative phosphorylation resulting in increased thermogenesis3,4 and/or its direct effect on hypothalamus.
The mechanisms responsible for the stimulation of food intake by thyroid hormones have not yet been fully elucidated5,6. Food intake is regulated by complex mechanism involving hypothalamic neuropeptides which respond to peripheral satiety signals like leptin and ghrelin. Leptin is considered to be a fundamental signal for satiety to the brain and also increases thermogenesis thereby playing a major role in energy homeostasis7–9. In animal models of T3 induced thyrotoxicosis, it has been found that decreased plasma leptin levels and/or decreased sensitivity of satiety centre to leptin could contribute to hyperphagia7,8.
Ghrelin is a novel peptide secreted from the fundus of the stomach. It enhances hunger and appetite and transduces signals to hypothalamic regulatory nuclei that modulate energy balance in normal physiology10,11. Despite increase in appetite in patients with hyperthyroidism, serum total ghrelin level has been shown to be normal or low12,13. In majority of the earlier studies, serum total ghrelin was measured rather than acylated ghrelin which is the biologically active form10–13.
Adiponectin, an adipocytokine is a peptide preferentially secreted from the smaller adipocytes and enhances insulin sensitivity in target tissues14,15. In patients with hyperthyroidism adiponectin level is expected to be low and may contribute to insulin resistance16. Increase in insulin resistance per se may result in increase in appetite with preferential craving for carbohydrate foods6,11.
Several studies conducted in patients with hyperthyroidism have shown conflicting results regarding the alteration in adipocytokines and ghrelin11–16. These studies were either cross-sectional or had small sample size and more importantly body composition was not assessed with appropriate modalities to correlate with these modulators11–16.
The present study was undertaken to evaluate alteration in body composition and its association with adipocytokines and ghrelin in patients with hyperthyroidism.
Material & Methods
Thirty one consecutive patients with hyperthyroidism were recruited from Endocrinology Out Patient Department of Post Graduate Institute of Medical Education and Research, Chandigarh, India, during November 2008 to July 2009. All of them were ambulatory, and treatment naïve. Informed consent was obtained from all patients. Of these, 27 patients (16 female, 2 post menopausal) were included in final analysis. The study protocol was approved by the Institute's Ethics Committee. Considering body weight change (weight gain) as a representative of response parameter, a sample size of 20 was adequate to detect a weight gain of 4 kg after 3 months of treatment with carbimazole, in a two sided paired ‘t’ test with a 5 per cent alpha error and 90 per cent power. Making an allowance for losses during the treatment period, a sample size of at least 25 patients was decided.
Subjects with diabetes, liver and kidney disorders, cardiac failure and underlying infections were excluded. None of them were alcoholic, smoker or receiving medications known to alter glucose, lipid and weight homeostasis. The aetiology of hyperthyroidism was Graves’ disease in 20 patients and the remaining seven had toxic multinodular goiter. Twenty eight euthyroid subjects with similar age and body mass index (BMI) were included as controls. The controls were hospital employees and relatives of patients who were not suffering from any thyroid disorder and were otherwise healthy. Both cases and controls were having sedentary lifestyle. They underwent assessment of body composition and hormone profile including thyroid profile and serum leptin, adiponectin and ghrelin levels. Hyperthyroidism was diagnosed based on typical signs and symptoms, elevated T3 and T4 and suppressed thyroid stimulating hormone (TSH) levels compared to the reference range. “Recall method” was used to assess the food intake for the last three days. Hyperphagia was defined as an increase in calorie intake by >30 per cent as compared to before the onset of the symptoms without any other co-morbidities. This was based on the usual criteria for significant alteration in response to any form of treatment. Blood samples were taken after an overnight fast from an antecubital vein between 0830 and 0900 h. The blood sample was centrifuged and plasma aliquots were kept at -20°C. The body composition including lean body mass (LBM), total body fat (TBF) and bone mineral content (BMC) and bone mineral density (BMD) was assessed by Dual Energy X ray Absorptiometer (DXA, Norland, XR46, USA). The precision error was ±1 per cent if it was repeated within 24 h, and >2.5 per cent if it was repeated after 2-6 months as per manufacturer's details. The DXA was done by a single technician. The patients were treated with a median dose of 30 mg of carbimazole and advised to consume non-iodized salt. None of the patients were on any other medications and they were reassessed at 6 wk (data not shown) and 12th wk as by that time all patients achieved euthyroid state.
Hormone assays: Thyroid hormones (T3 and T4) were estimated by radioimmunoassay and TSH by immunoradiometric assay (IRMA) using commercial kits (Board of Radiation and Isotope Technology, India). The normal range for T3 was 0.6-1.6 ng/ml, T4 4.5-12 μg/dl and TSH 0.5-5.2 μU/ml. Plasma leptin was measured by sandwich ELISA assay (DRG, GmBH, Germany) with a minimum detection limit of 1.0 ng/ml. The intra- and inter-assay coefficients of variation were 3.5 and 6.5 per cent, respectively. Adiponectin levels were measured by enzyme linked immunosorbent assay (ELISA) using the human adiponectin kit (Biovendor laboratory, Medicine, Inc, USA). The intra- and inter-assay coefficients of variation were 6.4 and 7.3 per cent, respectively and minimum detection limit was 0.2 μg/ml. Plasma acylated ghrelin was measured by ELISA (human acylated ghrelin, SPI, Montigmy Le Bretonneux, France) with intra- and inter-assay coefficient of variation being 2.9 and 3.4 per cent, respectively. The lower detection limit of the assay was 1.5 pg/ml and the normal range was 66.2-180 pg/ml.
Fasting plasma glucose was estimated by glucose oxidase method (Autopak, Bayer diagnostic Ltd. Boroda, India) and insulin by radioimmunoassay kits (Board of Radiation and Isotope Technology, India). Total cholesterol (TC), triglyceride (TG) and HDL cholesterol levels were measured by Clonital KC kits (Lombardia, Italy) by enzymatic method. Insulin resistance was calculated by homeostasis model of assessment, [HOMA-IR= Fasting plasma insulin (μU/ml) x fasting plasma glucose (mg/dl)/405]8.
Statistical analysis: Statistical analysis was carried out with Microsoft Excel data analysis and SPSS 10.0 for windows, (Chicago Illinois, USA) using simple correlation. Mean values were compared using Student's ‘t’ test. Normality of data was checked by Kolmogorov Smirnov method. Pearsons correlation was used to find out relationship between adipocytokines and ghrelin with various other parameters. The hormone levels measured at baseline and after achievement of euthyroidism were compared by paired t-test.
Results
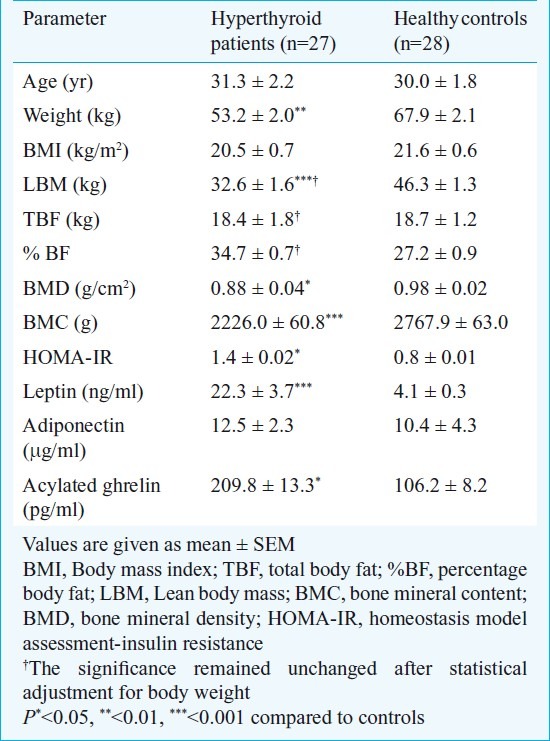
The study group consisted of 31 patients and 27 of them (men 11) completed the study as three patients were lost to follow up and one received radioablation. The mean age of the patients group was 31.3±4.2 yr and median lag time between onset of symptoms and diagnosis was 6 months. Twenty two (81.5%) out of 27 patients had hyperphagia. The mean BMI of the patients’ group was 20.5±0.7 kg/m2 and waist circumference 73.4±2.3 cm. The LBM and TBF as assessed by DXA at baseline were 32.6±1.6 and 18.4±1.8 kg, respectively. The BMD and BMC were 0.88±0.04 g/cm2 and 2226.0±60.8 g, respectively. The controls (men 14) had a comparable BMI of 21.6±0.6 kg/m2 and higher LBM (46.3±1.3 kg, P<0.001). This significantly higher LBM in controls persisted after statistical adjustment for body weight. However, they had similar TBF of 18.7±1.2 kg as compared to patients. The BMD (P<0.05) and BMC (P<0.001) in the control group was higher 0.98±0.02 gm/cm2 (P=0.02) and 2767.9±63.0 gm (P<0.001) as compared to the patients group. The baseline serum T3, T4 and TSH in the patients group were 4.0±0.5 ng/ml, 23.3±2.9 μg/dl and 0.1±0.0 μU/ml respectively. Serum triglyceride level was 150.6±18.0 mg/dl and HOMA IR 1.4±0.02. The mean serum leptin and adiponectin in hyperthyroid patients and healthy controls were 22.3±3.7 vs 4.1±0.3 ng/ml (P<0.001) and 12.5±2.3 μg/ml vs 10.4±4.3 (P=0.75) respectively. The mean plasma acylated ghrelin level at baseline in patients with hyperthyroidism was 209.8±13.3 pg/ml (normal range 66.2-186 pg/ml) which was higher as compared to healthy controls (106.2±8.2 pg/ml, P=0.05) (Table I).
Table I.
Baseline characteristics of the patients and controls
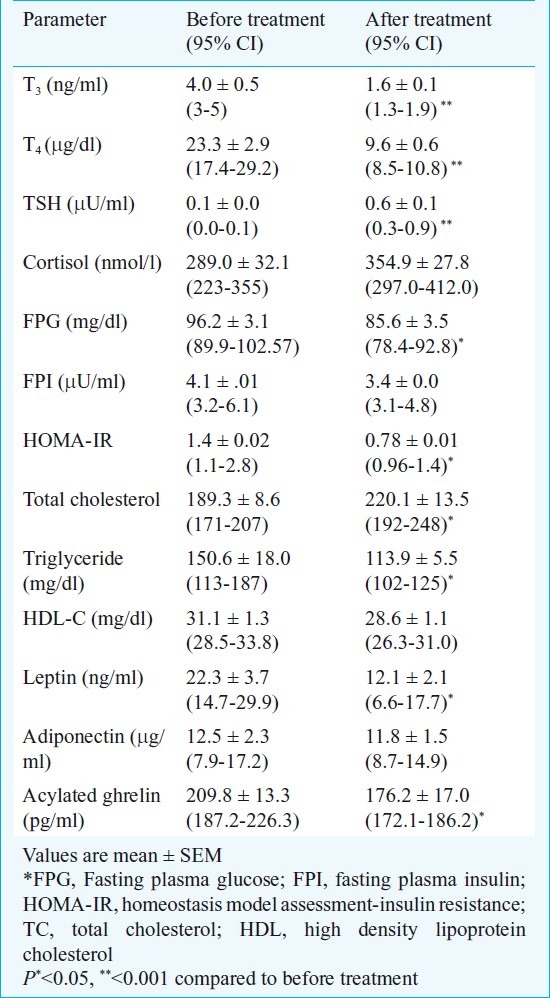
After 12 wk of treatment with carbimazole (median dose of 30 mg/day), all patients achieved an euthyroid state with a significant decrease in T3 (1.6±0.1 ng/ml), T4 (9.6±0.6 μg/dl) and increase in TSH levels (0.6±0.1 μU/ml, range 0.6-3.3). All patients had a significant (P<0.001) weight gain after carbimazole therapy and had an increase (P<0.001) in lean body mass. Though the total body fat also increased from 18.4±1.8 to 19.9±1.8 kg, but it could not achieve statistical significance. The BMD did not increase but BMC increased significantly after treatment (P<0.05). There was a significant improvement in HOMA-IR (P<0.05) after treatment and a decrease (P<0.05) in leptin levels, however, the adiponectin level did not change. The plasma acylated ghrelin level decreased significantly (P<0.05) after achievement of euthyroidism (Tables II and III). The improvement in insulin resistance commensurated with decrease in serum triglyceride (r=0.62, P=0.01) and decrease in leptin levels (r=0.43, P=0.02).
Table II.
Body composition parameters in hyperthyroid patients before and after treatment with carbimazole
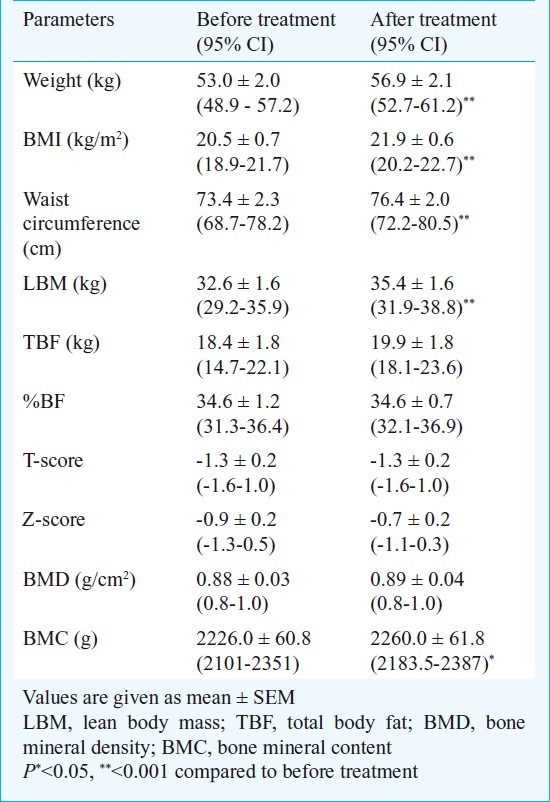
Table III.
Biochemical parameters in patients with hyperthyroidism before and after treatment with carbimazole
There was a significant positive correlation observed between serum leptin with hip circumference (r=0.41, P=0.03), TBF (r=0.62, P<0.001), BMD (r=0.45, P=0.02) and serum triglyceride levels (r=0.55, P<0.001). However, no significant correlation could be observed with the presence or absence of hyperphagia, BMI, LBM, serum T3, T4 and HOMA-IR. After adjustment for BMI, gender and age, the correlation between serum leptin with TBF (r=0.50, P<0.02) and BMD (r=0.60, P=<0.001) persisted. The serum adiponectin level had only positive correlation with T3 (r=0.59, P=0.001) and T4 (r=0.44, P=0.02), while no correlation was observed with total body fat, HOMA-IR and triglyceride. After adjustment for BMI, age and gender the correlation between adiponectin and T3 persisted (r=0.85, P<0.001). Ghrelin positively correlated with serum total cholesterol (r=0.45, P=0.021) and negatively correlated with serum T3- level (r=0.40, P=0.04). However, no correlation was found with the presence or absence of hyperphagia, serum insulin, blood glucose, triglyceride, HOMA-IR, TBF, LBM, BMC and BMD. After treatment with carbimazole, no correlation was observed between serum leptin, adiponectin, ghrelin with TBF, BMD and presence or absence of hyperphagia.
Discussion
This study shows that patients with hyperthyroidism had weight loss predominantly due to decrease in lean body mass despite increased food intake. It was accompanied with increased serum leptin and ghrelin levels. Treatment with carbimazole resulted in substantial increase in lean body mass with modest increase in fat mass and decrease in both leptin and ghrelin levels. Alterations in body composition and appetite in subjects with hyperthyroidism appear to be independent of circulating leptin and ghrelin levels.
The effects of thyroid hormones on body composition have been described extensively in the literature1,2. In patients with hyperthyroidism, prior to antithyroid drug therapy, a substantial decrease in lean body mass, a modest decrease in fat mass and BMD have been described2. Our study confers the same observations. After treatment with antithyroid therapy, there was weight gain, which was mainly contributed by increase in lean body mass and modest gain in fat mass, probably due to decrease in thermogenesis and protein catabolism, which is consistent with the previous studies2–4.
At the time of diagnosis of hyperthyroidism, there is a substantial weight loss despite increase in hunger and food intake in majority of the patients. Weight loss in patients with hyperthyroidism is attributable to exaggerated thermogenesis mediated by uncoupling of oxidative phosphorylation by thyroid hormones5. Weight loss despite increased hunger can be explained by the fact that either the calorie intake was not substantial to tide over the exaggerated basal metabolic rate or some other factors like adipocytokines might be modulating weight loss in patients with hyperthyroidism. Previous studies have shown variable plasma leptin levels (high or low normal to normal) in patients with hyperthyroidism which can be explained by variable duration and severity of disease, varying amount of body fat and variability in leptin assay18–22. We tried to overcome these limitations in the present study and showed that the plasma leptin levels were higher in these patients as compared to healthy controls with similar BMI and body fat. This may be attributed to direct effect of thyroid hormones on leptin mRNA expression as shown by in vitro studies and altered leptin metabolism and increased insulin resistance in hyperthyroid state16,22. In normal physiology, leptin is associated with weight loss by its effect on hypothalamus through neuropeptide Y (NPY) expression thereby resulting in decreased food intake and also enhancing thermogenesis3,23. The observation of weight loss despite increased appetite in these patients with higher leptin levels can be explained by differential leptin sensitivity, that is its decreased sensitivity at hypothalamus (feeding centre) while preserved action at periphery resulting in increased appetite and exaggerated thermogenesis, respectively. Alternatively, increased hunger and food intake may be related to undefined signaling pathway from periphery, induced by exaggerated thermogenesis to the hypothalamus. The lack of correlation between leptin and hyperphagia and thyroid hormones suggests that effect of leptin is overcome by increased thermogenesis induced by thyroid hormones. Decrease in serum leptin despite increase in body fat after treatment with carbimazole may be due to these mechanisms and significant improvement in insulin resistance as serum leptin level and insulin resistance go hand in hand16,21,22.
Our study also showed that serum ghrelin levels were higher at baseline and normalized after achievement of euthyroidism as shown earlier11,12. A few studies in patients with hyperthyroidism have shown variable ghrelin levels. One of the previous study has shown a minute to minute variation in serum ghrelin level related to insulin sensitivity during hyperinsulinaemic, euglycaemic clamp study rather than thyroid hormone level11. The higher ghrelin level in our study was possibly a compensatory response to negative energy balance as described in patients with anorexia nervosa and obese patients on weight loss programme24–26. The previous studies did not show increased ghrelin levels possibly because of polyclonal assays and measuring total rather than acylated ghrelin12. In the present study, plasma ghrelin levels did not correlate with hyperphagia as also conferred in previous studies12,21,25–27.
Serum adiponectin levels are inversely related to the degree of adiposity and insulin resistance in healthy subjects and in type 2 diabetes mellitus14,15,21. In the present study serum adiponectin levels were comparable between patients and controls possibly because of similar total body fat mass. Moreover, serum adiponectin levels remained unaltered even after achievement of euthyroidism because of insignificant increase in total body fat. This is further substantiated by the observation that thyroid hormones do not affect adiponectin mRNA expression as shown by in vitro studies16,22. Similar observation has been made in previous studies21,23.
The strengths of the study includes the presence of control group, estimation of acylated ghrelin and follow up after anti-thyroid therapy, while limitations included lack of objective criteria to define hyperphagia, lack of assessment of thermogenesis and short duration of follow up.
In conclusion, patients with hyperthyroidism have decrease in lean body mass, which was regained after achievement of euthyroidism with carbimazole therapy. The hyperphagia accompanied with weight loss in hyperthyroidism was independent of leptin and ghrelin.
References
- 1.Ingbar SH. The thyroid gland. In: Wilson JD, Foster DW, editors. Text book of endocrinology. Philadelphia: Saunders; 1985. pp. 975–1170. [Google Scholar]
- 2.Lonn L, Stenlof K, Ottosson M, Lindroos AK, Nystrom E, Sjostrom L. Body weight and body composition changes after treatment with hyperthyroidism. J Clin Endocrinol Metab. 1998;83:4269–73. doi: 10.1210/jcem.83.12.5338. [DOI] [PubMed] [Google Scholar]
- 3.Ishii S, Kenegai J, Tanura H, Takako S, Sugihava H, Okawa S. Hypothalamic neuropeptide y/y, receptor pathway activated by a reduction in circulating leptin, but not by an increase in circulating ghrelin contributes to hyperphagia associated with triiodothyronine induced thyrotoxicosis. Neuroendocrinology. 2003;78:321–30. doi: 10.1159/000074885. [DOI] [PubMed] [Google Scholar]
- 4.Weetman AP. Graves’ disease. N Engl J Med. 2000;343:1236–48. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 5.Kileverik LP, Coomans CP, Endert E, Sauerwein HP, Havekes LM, Voshol PJ, et al. Thyroid hormone effects on whole body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 2009;150:5639–48. doi: 10.1210/en.2009-0297. [DOI] [PubMed] [Google Scholar]
- 6.Pijl H, de Meijer PH, Langius J, Coenegracht CI, Van den bank AH, Chandie Shaw PK, et al. Food choice in hyperthyroidism: Potential influence of the autonomic nervous system and brain serotonin precursor availability. J Clin Endocrinol Metab. 2001;86:5848–53. doi: 10.1210/jcem.86.12.8112. [DOI] [PubMed] [Google Scholar]
- 7.Wilding JP. Neuropeptides and appetite control. Diabetic Med. 2002;19:619–27. doi: 10.1046/j.1464-5491.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 8.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: Leptin, acylation stimulating protein and adiponectin. Curr Opin Lipidol. 2002;8:1288–95. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Ukkola O, Santaniem M. Adiponectin : a link between excess adiposity and associated co-morbidities? J Mol Med. 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone releasing peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 11.Gimenez-Palop O, Gimenez Perez G, Mauricio D, Berlanga E, Potau N, Vilardell C, et al. Circulating ghrelin in thyroid dysfunction is related to insulin resistance and not to hunger, food intake or anthropometric changes. Eur J Endocrinol. 2005;153:73–9. doi: 10.1530/eje.1.01934. [DOI] [PubMed] [Google Scholar]
- 12.Riis ALD, Hansen TK, Moller N, Weeke J, Jorgensen JOL. Hyperthyroidism is associated with suppressed circulating ghrelin levels. J Clin Endocrinol Metab. 2007;88:853–7. doi: 10.1210/jc.2002-021302. [DOI] [PubMed] [Google Scholar]
- 13.Corbetta S, Englaro P, Giambona S, Persani L, Blum WF, Beck-Peccoz P. Lack of effects of circulating thyroid hormone levels on serum leptin concentrations. Eur J Endocrinol. 1997;137:659–63. doi: 10.1530/eje.0.1370659. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 15.Dimitriadis G, Baker B, Marsh H, Mandarino L, Rizza R, Bergman R, et al. Effect of thyroid hormone excess on action, secretion and metabolism of insulin in humans. Am J Physiol. 1985;248:593–601. doi: 10.1152/ajpendo.1985.248.5.E593. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias P, Alvarez FP, Codoceo R, Diez JJ. Serum concentrations of adipocytokines in patients with hyperthyroidism and hypothyroidism before and after control of thyroid function. Clin Endocrinol. 2003;59:621–9. doi: 10.1046/j.1365-2265.2003.01897.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Momatani N, Hayashi M, Monkawa T, Ito K, Saruta T. Serum leptin concentrations in patients with thyroid disorders. Clin Endocrinol. 1998;48:229–302. doi: 10.1046/j.1365-2265.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh CJ, Wang PW, Wang ST, Liu RT, Tung SC, Chien WY, et al. Serum leptin concentrations of patients with sequential thyroid function changes. Clin Endocrinol. 2002;57:29–34. doi: 10.1046/j.1365-2265.2002.01543.x. [DOI] [PubMed] [Google Scholar]
- 19.Sreenan S, Caro JF, Refetoff S. Thyroid dysfunction is not associated with alterations in serum leptin levels. Thyroid. 1997;7:407–9. doi: 10.1089/thy.1997.7.407. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–7. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 21.Iglesias P, Diez JJ. Influence of thyroid dysfunction on serum concentrations of adipocytokines. Cytokine. 2007;40:61–70. doi: 10.1016/j.cyto.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2009;290:1084–9. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 23.Al-Shoumer KA, Vasanthy BA, AL-Zaid MM. Effects of treatment of hyperthyroidism on glucose homeostasis, insulin secretion and markers of bone turnover. Endocr Pract. 2006;12:121–30. doi: 10.4158/EP.12.2.121. [DOI] [PubMed] [Google Scholar]
- 24.Purnell JQ, Cummings D, Weigle DS. Changes in 24h area under the curve ghrelin values following diet induced weight loss are associated with loss of fat free mass, but not with changes in fat mass, insulin levels or insulin sensitivity. Int J Obes. 2007;31:385–9. doi: 10.1038/sj.ijo.0803401. [DOI] [PubMed] [Google Scholar]
- 25.Hansen TK, Dall R, Hasoda H, Koijma M, Kangawa K, Christansen JS, et al. Weight loss increase circulating levels of ghrelin in human obesity. Clin Endocrinol. 2002;56:203–6. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 26.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Ripel RL, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–73. [PubMed] [Google Scholar]
- 27.Sadegholvad A, Afkhamizadeh M, Omrani GR. Serum ghrelin changes in thyroid dysfunction. Arch Iranian Med. 2007;10:168–70. [PubMed] [Google Scholar]


