Abstract
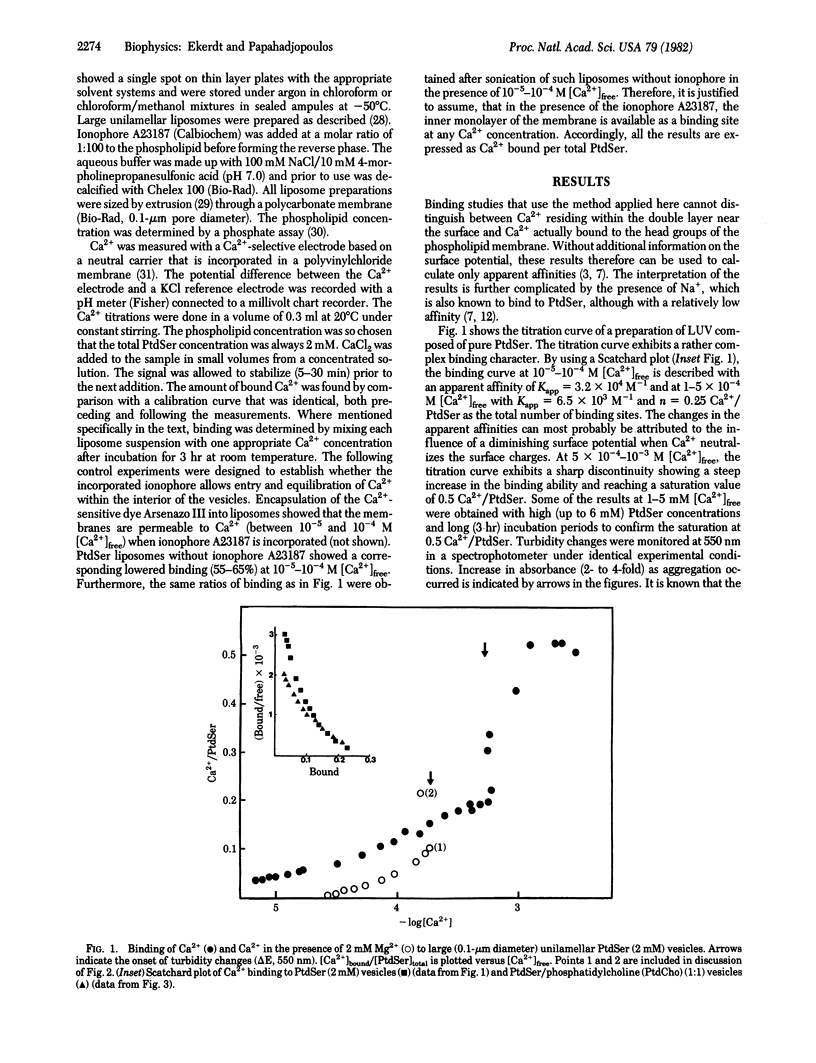
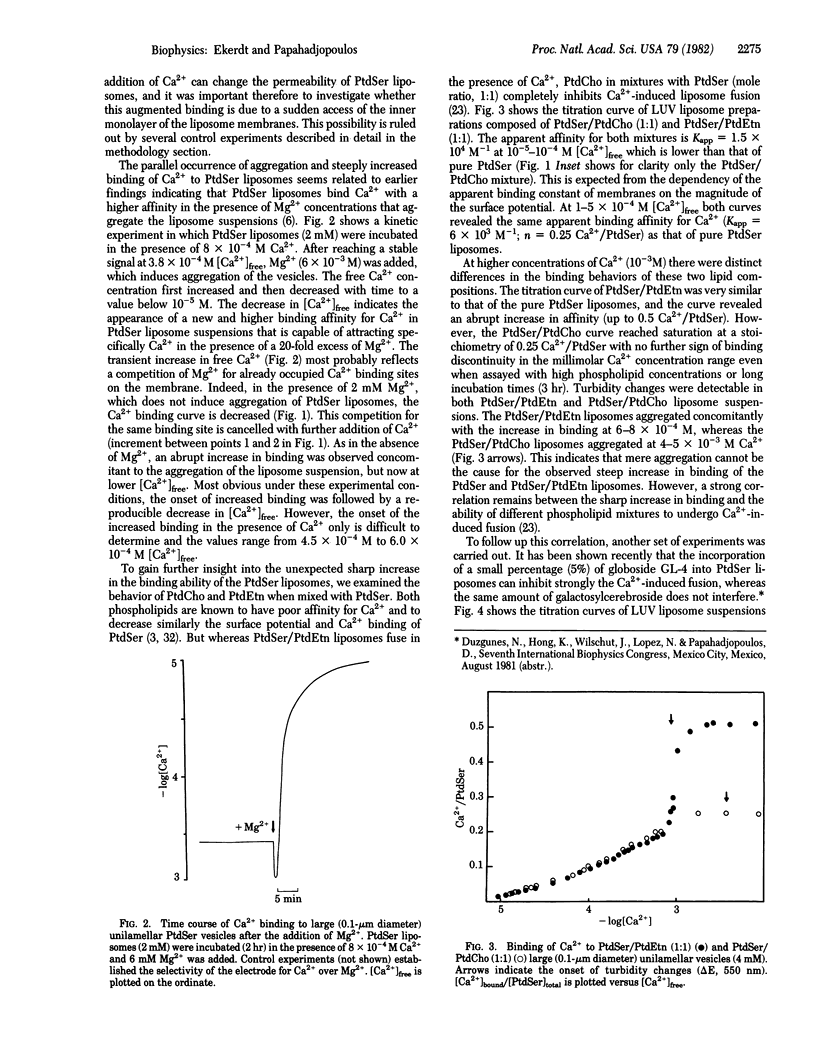
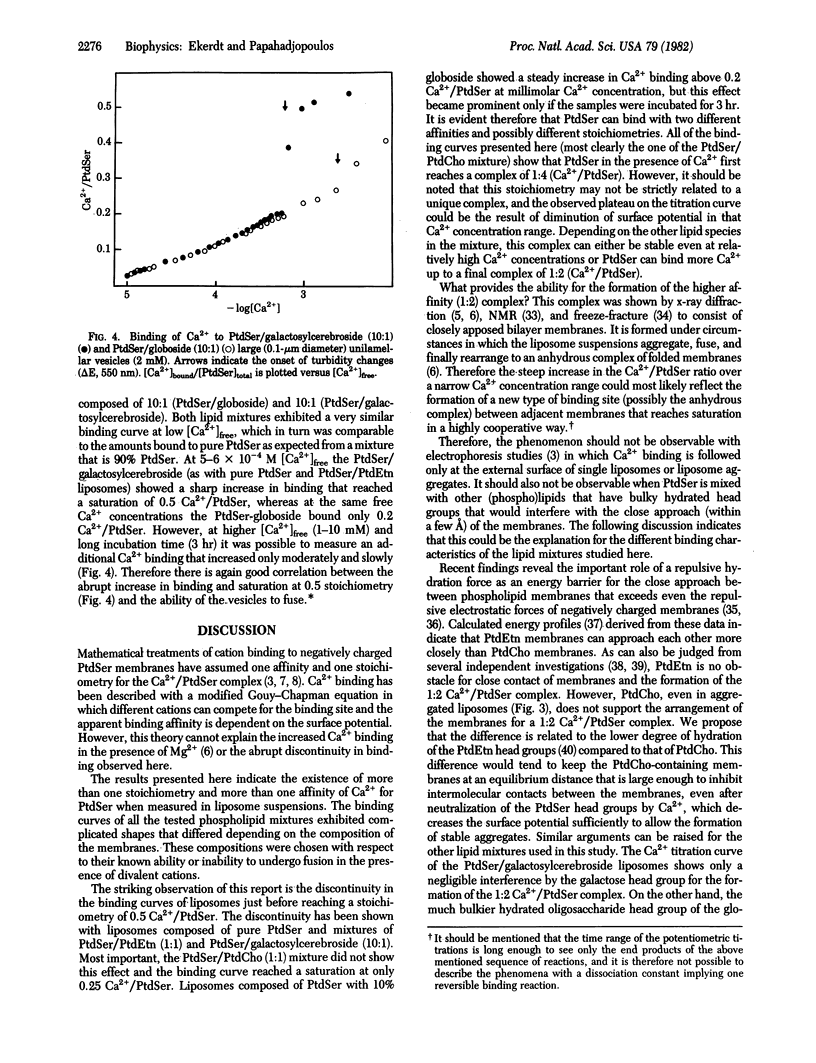
Binding of Ca2+ to liposomes composed of phosphatidylserine (PtdSer) was analyzed by potentiometric titrations. Ca2+ binding to large unilamellar PtdSer vesicles was saturable at a stoichiometry of 1:2 (Ca2+/PtdSer). At approximately 6 X 10(-4) M [Ca2+]free, the binding curve exhibited a discontinuity that can be attributed to the formation of a Ca2+/PtdSer complex with a higher affinity. When both Ca2+ and Mg2+ are present, depending on the relative concentrations, Mg2+ can either complete or can enhance Ca2+ binding. Concomitant to the enhanced binding, the vesicle suspension was found to aggregate, suggesting that close contact of membranes is a prerequisite for the abrupt change in affinity. This concept was tested by binding studies with liposomes of mixed composition. It was found that the incorporation of 50 mol% phosphatidylethanolamine (PtdEtn) into PtdSer liposomes produced a similar binding pattern to that of pure PtdSer with a saturable stoichiometry of 1:2 (Ca2+/PtdSer). However, incorporation of 50 mol% phosphatidylcholine (PtdCho) completely abolished the discontinuous shift in affinity and apparent saturation was reached at a stoichiometry of 1:4 (Ca2+/PtdSer). In addition, Ca2+ binding to PtdSer liposomes with 10 mol% galactosylcerebroside was not altered when compared to pure PtdSer, whereas 10 mol% of the glycolipid GL-4 abolished the increased binding. The results are closely correlated with recent findings on the role of the membrane composition in Ca2+-induced fusion of liposomes and argue in favor of a specific Ca2+/PtdSer complex (with 1:2 stoichiometry) forming only at points of close contact between membranes and serving as the trigger for membrane fusion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barton P. G. The influence of surface charge density of phosphatides on the binding of some cations. J Biol Chem. 1968 Jul 25;243(14):3884–3890. [PubMed] [Google Scholar]
- Bentz J., Nir S. Cation binding to membranes: competition between mono-, di- and trivalent cations. Bull Math Biol. 1980;42(2):191–220. doi: 10.1007/BF02464638. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruijff B. Polymorphic phase behaviour of lipid mixtures as detected by 31P NMR. Evidence that cholesterol may destabilize bilayer structure in membrane systems containing phosphatidylethanolamine. Biochim Biophys Acta. 1978 Feb 21;507(2):207–218. doi: 10.1016/0005-2736(78)90417-0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Düzgünes N., Nir S., Wilschut J., Bentz J., Newton C., Portis A., Papahadjopoulos D. Calcium- and magnesium-induced fusion of mixed phosphatidylserine/phosphatidylcholine vesicles: effect of ion binding. J Membr Biol. 1981 Apr 15;59(2):115–125. doi: 10.1007/BF01875709. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Fraley R., Wilschut J., Düzgüneş N., Smith C., Papahadjopoulos D. Studies on the mechanism of membrane fusion: role of phosphate in promoting calcium ion induced fusion of phospholipid vesicles. Biochemistry. 1980 Dec 23;19(26):6021–6029. doi: 10.1021/bi00567a012. [DOI] [PubMed] [Google Scholar]
- Hauser H., Darke A., Phillips M. C. Ion-binding to phospholipids. Interaction of calcium with phosphatidylserine. Eur J Biochem. 1976 Feb 16;62(2):335–344. doi: 10.1111/j.1432-1033.1976.tb10165.x. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Phillips M. C., Barratt M. D. Differences in the interaction of inorganic and organic (hydrophobic) cations with phosphatidylserine membranes. Biochim Biophys Acta. 1975 Dec 16;413(3):341–353. doi: 10.1016/0005-2736(75)90120-0. [DOI] [PubMed] [Google Scholar]
- Kurland R., Newton C., Nir S., Papahadjopoulos D. Specificity of Na+ binding to phosphatidylserine vesicles from a 23Na NMR relaxation rate study. Biochim Biophys Acta. 1979 Feb 20;551(1):137–147. doi: 10.1016/0005-2736(79)90360-2. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P., Parsegian V. A. Measurement of forces between lecithin bilayers. Nature. 1976 Feb 19;259(5544):601–603. doi: 10.1038/259601a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin A., Grathwohl C., McLaughlin S. The adsorption of divalent cations to phosphatidylcholine bilayer membranes. Biochim Biophys Acta. 1978 Nov 16;513(3):338–357. doi: 10.1016/0005-2736(78)90203-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C., Pangborn W., Nir S., Papahadjopoulos D. Specificity of Ca2+ and Mg2+ binding to phosphatidylserine vesicles and resultant phase changes of bilayer membrane structure. Biochim Biophys Acta. 1978 Jan 19;506(2):281–287. doi: 10.1016/0005-2736(78)90398-x. [DOI] [PubMed] [Google Scholar]
- Nir S., Bentz J., Wilschut J. Mass action kinetics of phosphatidylserine vesicle fusion as monitored by coalescence of internal vesicle volumes. Biochemistry. 1980 Dec 23;19(26):6030–6036. doi: 10.1021/bi00567a013. [DOI] [PubMed] [Google Scholar]
- Ohki S., Sauve R. Surface potential of phosphatidylserine monolayers. I. Divalent ion binding effect. Biochim Biophys Acta. 1978 Aug 17;511(3):377–387. doi: 10.1016/0005-2736(78)90274-2. [DOI] [PubMed] [Google Scholar]
- Olson F., Hunt C. A., Szoka F. C., Vail W. J., Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta. 1979 Oct 19;557(1):9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977 Mar 17;465(3):579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Pangborn W. A., Poste G. Studies on membrane fusion. II. Induction of fusion in pure phospholipid membranes by calcium ions and other divalent metals. Biochim Biophys Acta. 1976 Oct 5;448(2):265–283. doi: 10.1016/0005-2736(76)90241-8. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., Papahadjopoulos D. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 1979 Mar 6;18(5):780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- Puskin J. S., Coene M. T. Na+ and H+ dependent Mn2+ binding to phosphatidylserine vesicles as a test of the Gouy-Chapman-Stern theory. J Membr Biol. 1980 Jan 31;52(1):69–74. doi: 10.1007/BF01869007. [DOI] [PubMed] [Google Scholar]
- Puskin J. S. Divalent cation binding to phospholipids: an EPR study. J Membr Biol. 1977 Jun 24;35(1):39–55. doi: 10.1007/BF01869939. [DOI] [PubMed] [Google Scholar]
- Radin N. S. Preparative isolation of cerebrosides (galactosyl and glucosyl ceramide). J Lipid Res. 1976 May;17(3):290–293. [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Rehfeld S. J., Düzgünes N., Newton C., Papahadjopoulos D., Eatough D. J. The exothermic reaction of calcium with unilamellar phosphatidylserine vesicles: titration microcalorimetry. FEBS Lett. 1981 Jan 26;123(2):249–251. doi: 10.1016/0014-5793(81)80299-2. [DOI] [PubMed] [Google Scholar]
- Sundler R., Düzgüneş N., Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. II. The role of phosphatidylethanolamine in mixtures with phosphatidate and phosphatidylinositol. Biochim Biophys Acta. 1981 Dec 21;649(3):751–758. doi: 10.1016/0005-2736(81)90180-2. [DOI] [PubMed] [Google Scholar]
- Sundler R., Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. I. Phosphatidate/phosphatidylinositol specificity. Biochim Biophys Acta. 1981 Dec 21;649(3):743–750. doi: 10.1016/0005-2736(81)90179-6. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980 Dec 23;19(26):6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Papahadjopoulos D. Calcium/magnesium specificity in membrane fusion: kinetics of aggregation and fusion of phosphatidylserine vesicles and the role of bilayer curvature. Biochemistry. 1981 May 26;20(11):3126–3133. doi: 10.1021/bi00514a022. [DOI] [PubMed] [Google Scholar]


