Sir,
A 44-year-old female presented to us with a history of nocturnal, continuous, and burning dysesthetic low back pain radiating to both lower limbs for the last three months. There were no bladder or bowel complaints. She had a normal neurological examination apart from reduced sensations in the L5 dermatome in both lower limbs. Magnetic Resonance Imaging (MRI) of the lumbar spine showed a well-circumscribed, solid-cystic conus lesion, which was hypointense on T1-weighted images and hyperintense on T2-weighted images, along with peripheral contrast enhancement and an associated rostral syrinx [Figure 1]. The rest of the spine was normal. Her urodynamics revealed low voiding pressures with significant abdominal straining, characteristic of lower motor neuron type of dysfunction. She underwent D11-L2 laminotomy and gross total excision of the tumor. Intraoperatively, the cyst was associated with a grayish solid moderately vascular component [Figure 2], which was adherent to the cord at some places. It was removed piecemeal without the use of an ultrasonic aspirator and almost the entire specimen was sent for pathology. Postoperative recovery was uneventful. Histopathology showed a biphasic pattern of compactly arranged bipolar cells with loose piloid cells, suggestive of pilocytic astrocytoma [Figure 3]. The patient is still under follow-up and has no recurrence to date.
Figure 1.
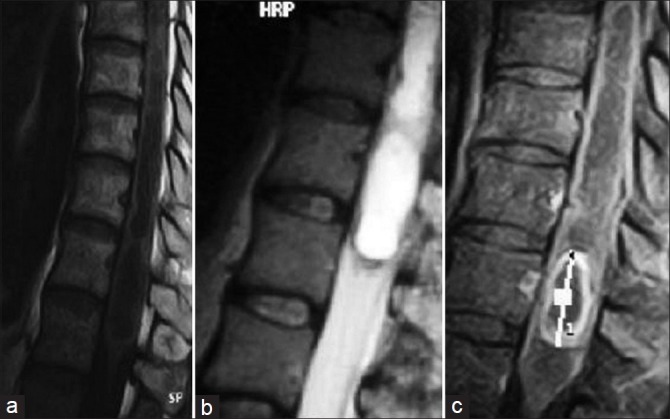
Conus medullaris tumor, which is hypointense on T1‑weighted image and hyperintense on T2‑weighted image, shows peripheral contrast enhancement
Figure 2.
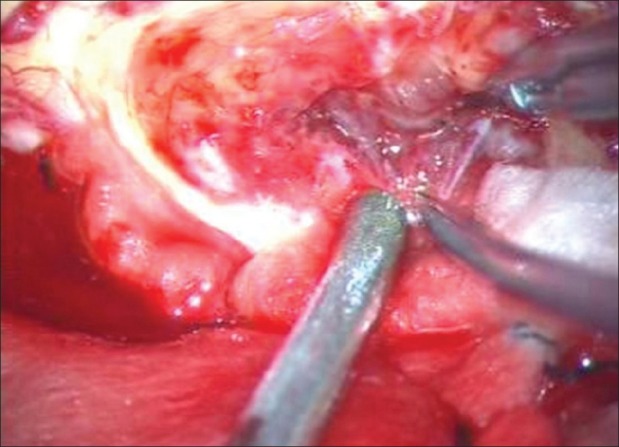
Intraoperative view of a grayish solid component of the tumor, with a good cleavage plane
Figure 3.
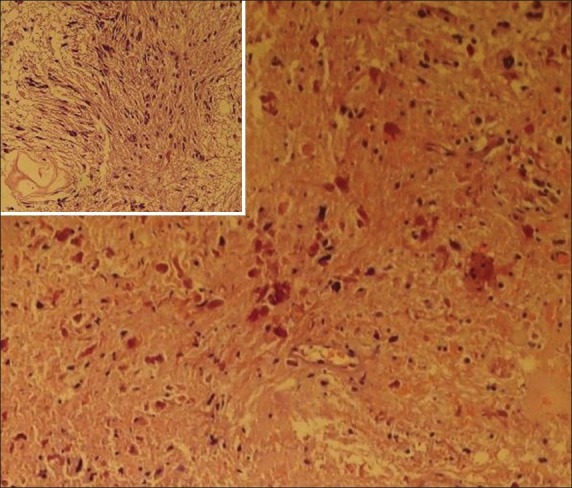
Histopathology showing rosenthal fibers and compact cells with loosely arranged piloid cells (inset)
In adults, pilocytic astrocytomas (grade I) are rare tumors in the lower spinal cord, although other low-grade astrocytomas (grade II) have been reported in this region in the past. The difference between grade I and grade II tumors is paramount, as the recurrence rates drop significantly in the former. Only a single case report of a conus pilocytic astrocytoma in a 20-year-old was found in the literature after an extensive search.[1] Our patient, however, was 44 years old, where the possibility of a pilocytic astrocytoma was never thought of preoperatively. In adults, only 5% of spinal tumors are intramedullary in location and are chiefly myxopapillary ependymomas, affecting the lumbosacral cord. In one of the largest series of 164 cases of intramedullary tumors in children and adults, low-grade astrocytomas consisted of 46% of all the tumors and mainly affected the cervicodorsal region.[2] Surprisingly, there was not a single pilocytic astrocytoma in this series, which is one of the largest ones to date. Glioblastoma multiforme in conus medullaris has been reported and such an isolated involvement of the conus region by an astrocytoma is seen in approximately 3% of the cases.[3] Uchiyama et al. reported as many as 58% of the patients with conus tumors, having urinary symptoms and detrusor areflexia detected by urodynamics.[4] This was also evident in our patient and it helped to quantify the preoperative deficit and in prognosticating future sphincteric control. It is essential to predict the pathology before surgery in eloquent areas like the conus, so as to minimize neural tissue handling in cases of benign tumors, and to achieve maximal resection with least postoperative morbidity. Han et al. did not find any significant difference between the resection rates of intramedullary tumors elsewhere and those of the conus medullaris, as the overall rate of tumor excision was around 90% for ependymomas and hemangioblastomas and 50–76% for low-grade astrocytomas.[5] However, according to them, this was more dependent on the pathology and the surgeon's experience, technique, and attitude. We had gross total excision in our case and the patient did not develop any new postoperative deficit. The vital point in such cases is to stay within the tumor and stop immediately as soon as the normal firm cord parenchyma is encountered peripherally. These tumors are generally suckable and excessive traction on the normal parenchyma is not expected. Although, use of neurophysiological monitoring (Motor and Somato-Sensory Evoked Potentials) is quintessential in the conus region, low-grade gliomas like pilocytic astrocytomas generally have good cleavage planes and can be excised completely, without injuries to the normal cord parenchyma. Ideally, all such patients should undergo postoperative MRI at three monthly intervals initially, followed by yearly scans, but this was not possible in our case, due to financial constraints. No adjuvant therapy is deemed necessary for pilocytic astrocytomas and we intend to repeat imaging now, after one year of surgery, as she remains clinically stable.
To summarize, pilocytic astrocytoma is rare in conus medullaris. Surgical excision with minimal tissue handling can lead to an excellent outcome. Awareness with regard to a possible pilocytic astrocytoma in the conus should lead to more aggressive resections whenever possible, even in the eloquent regions.
REFERENCES
- 1.Baréa D, Richez P, Gueguen E, Clavel G, Grisoli F, Briant JF. Pilocytic astrocytoma of the conus medullaris. J Radiol. 1999;80:736–8. [PubMed] [Google Scholar]
- 2.Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: Surgical morbidity and long-term follow-up evaluation in164 children and young adults. J Neurosurg. 2000;93(2 Suppl):183–93. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 3.Stecco A, Quirico C, Giampietro A, Sessa G, Boldorini R, Carriero A. Glioblastoma multiforme of the conus medullaris in a child: Description of a case and literature review. AJNR Am J Neuroradiol. 2005;26:2157–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Uchiyama T, Sakakibara R, Hattori T, Yamanishi T. Lower urinary tract dysfunctions in patients with spinal cord tumors. Neurourol Urodyn. 2004;23:68–75. doi: 10.1002/nau.10070. [DOI] [PubMed] [Google Scholar]
- 5.Han IH, Kuh SU, Chin DK, Kim KS, Jin BH, Cho YE. Surgical treatment of primary spinal tumors in the conus medullaris. J Korean Neurosurg Soc. 2008;44:72–7. doi: 10.3340/jkns.2008.44.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]


