Abstract
Over the past decade, facial rejuvenation procedures to circumvent traditional surgery have become increasingly popular. Office-based, minimally invasive procedures can promote a youthful appearance with minimal downtime and low risk of complications. Injectable botulinum toxin (BoNT), soft-tissue fillers, and chemical peels are among the most popular non-invasive rejuvenation procedures, and each has unique applications for improving facial aesthetics. Despite the simplicity and reliability of office-based procedures, complications can occur even with an astute and experienced injector. The goal of any procedure is to perform it properly and safely; thus, early recognition of complications when they do occur is paramount in dictating prevention of long-term sequelae. The most common complications from BoNT and soft-tissue filler injection are bruising, erythema and pain. With chemical peels, it is not uncommon to have erythema, irritation and burning. Fortunately, these side effects are normally transient and have simple remedies. More serious complications include muscle paralysis from BoNT, granuloma formation from soft-tissue filler placement and scarring from chemical peels. Thankfully, these complications are rare and can be avoided with excellent procedure technique, knowledge of facial anatomy, proper patient selection, and appropriate pre- and post-skin care. This article reviews complications of office-based, minimally invasive procedures, with emphasis on prevention and management. Practitioners providing these treatments should be well versed in this subject matter in order to deliver the highest quality care.
KEYWORDS: Chemical peels, complications, neurotoxins, soft-tissue fillers
INTRODUCTION
Despite predictions that cosmetic procedures would face a decline due to an inclement economy and increased taxes, it appears that cosmetic procedures are at an all-time high with over 13 million procedures performed in 2011 (5% increase from 2010). Interestingly, minimally invasive procedures including injectable neurotoxins, soft-tissue augmentation, chemical peels and laser treatments increased by 123% from 2000, while traditional surgical procedures, including rhytidectomy and belphoroplasty, decreased by 17% over the past decade.[1] Minor office-based procedures can give a more youthful appearance with minimal complications and downtime with the appropriate training.
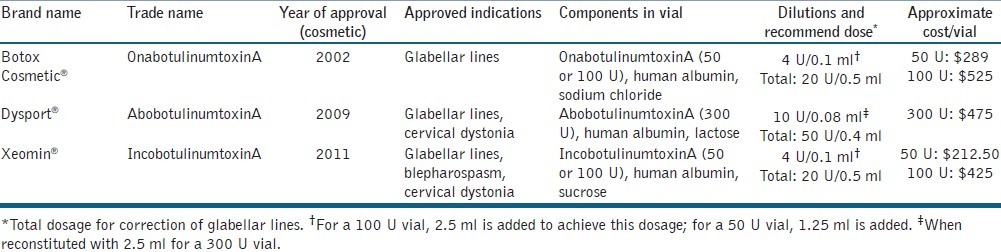
Highly effective procedures used for facial rejuvenation include injectable neurotoxins, soft-tissue fillers and chemical peels. OnabotulinumtoxinA (BoNTA-ONA; Botox/Botox Cosmetic, Allergan Inc., Irvine, CA, USA), abobotulinumtoxinA (BoNTA-ABO; Dysport, Medicis Aesthetics Inc., Scottsdale, AZ, USA) and incobotulinumtoxinA (BoNTA-INCO; Xeomin, Merz Aesthetics, Inc., Franksville, WI, USA) are injectable neurotoxins that have been approved by the United States Food and Drug Administration (FDA) for aesthetic purposes (glabellar lines) since 2002, 2009 and 2011, respectively; however, the list of “off-label” aesthetic applications continues to increase as patients demand full-face (as well as off-face) aesthetic rejuvenation.[2–5] RimabotulinumtoxinB (BoNTB-RIMA; Myobloc, Solstice Neurosciences Inc., South San Francisco, CA, USA) is approved for cervical dystonia, but its use in aesthetic medicine is limited due to worse injection pain and lack of prolonged efficacy.[6] Nevertheless, some practitioners use this product “off-label” for aesthetic purposes.
Soft-tissue fillers are regarded as having an impressive ability to volumise and reshape ageing, sagging skin with a small time commitment and minimally reported side effects.[7,8] There are a variety of filling agents approved by the FDA for the correction of moderate-to-severe facial wrinkles (e.g. nasolabial folds) and are composed of different synthetic materials including hyaluronic acid (HA; Juvaderm Ultra, Allergan, Santa Barbara, CA, USA), poly-L-lactic acid (PLLA; Sculptra, Dermik Laboratories, Bridgewater, NJ, USA), calcium hydroxylapatite (CaHA; Radiesse, Bioform Medical, Inc., Franksville, WI, USA) and polymethylmethacrylate (PMMA; Artefill, Suneva Medical, San Diego, CA, USA). Although not FDA approved for facial aesthetics, silicone is used “off-label” for cosmetic purposes such as the treatment of acne scars or facial augmentation.
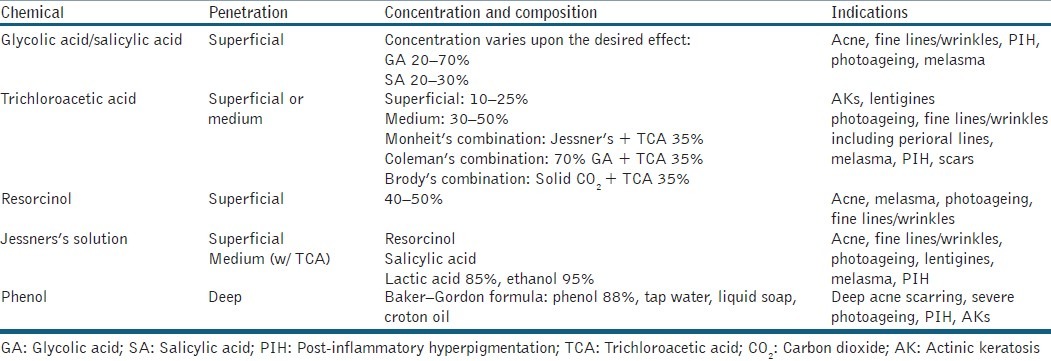
Chemical peels induce skin injury with subsequent re-epithelialisation that ultimately improves the skin appearance by improving texture, pigmentation and tone. Peels penetrate different depths of the epidermis and/or dermis depending on the chemical composition, concentration and/or length of time applied to exposed skin. Superficial peels [e.g. glycolic acid (GA) and salicylic acid (SA)] are used as adjunct therapy for acne treatment to increase the penetration of topical agents, improve post-inflammatory hyperpigmentation and reduce the appearance of superficial scarring.[9,10] Depending on concentration, trichloroacetic acid (TCA) peels can be superficial or medium in depth and are effective for treating photodamage (including actinic damage), melasma and acne scarring. Combination peels such as Jessner's solution, Monheit's combination and Brody's combination can be used for deeper penetration to help improve the appearance and texture of more advanced rhytides.[11]
Office-based, minimally invasive procedures are usually performed safely and effectively. However, even with pristine techniques, there are associated complications that are important to recognize and understand the causes in order to prevent their occurrence or provide appropriate treatment.
BOTULINUM NEUROTOXIN
The various BoNTs produced from isolated Clostridium botulism bacteria work to induce muscle paralysis through inhibition of acetylcholine release at the neuromuscular junction.[12] Each formulation of the available neurotoxins has a different mechanism to induce muscle paralysis, which is utilised for both cosmetic and/or therapeutic benefits [Table 1]. Many studies have documented the efficacy and safety of neurotoxins for aesthetic purposes, specifically for the reduction of glabellar, forehead, periocular and perioral lines.[13,14]
Table 1.
Approved botulinum toxins for cosmetic usage in the United States
Serious adverse events (SAEs) reported to the FDA of BoNTA for both therapeutic and cosmetic uses over a 13.5-year time period and non-SAEs over a 1-year time period were analysed in an extensive review.[15] There were no reported deaths from cosmetic use of BoNTA, and of the 36 reported SAEs, 30 were cited as possible complications of the FDA-approved label while the other 6 reports were deemed unrelated to the drug treatment. Commonly reported non-SAEs included lack of effect, injection site reaction and ptosis. Therapeutic uses had more SAEs, including 28 deaths, most likely a result of the larger doses required for therapeutic use and the underlying disease state of patients requiring treatment. An earlier meta-analysis of randomised controlled trials for therapeutic uses of BoNTA substantiated the long-term safety profile.[16] Despite the lack of reported SAEs for dermatological use, all neuromodulators have an FDA-mandated black box warning indicating the potential for distant spread resulting in swallowing or breathing difficulties and the risk of death.
More common complications of BoNT injection include local reactions such as bleeding, pain, oedema, erythema and ecchymosis at the injection site, all of which can be significantly reduced through proper technique, knowledge of facial anatomy, and physician education and training.[17] It is important to inform every patient that despite pristine injection technique, a large majority of patients will have bruising due to individual variability of facial veins and wound healing. Injection site pain is also highly variable and dependent on individual sensitivity, but can be minimised through use of small-gauge needles (e.g. 30 or 32), insertion of the needle through the pilosebaceous unit and pinching the skin before injection.[18,19] A good trick is to tap or vibrate the non-injection hand/index finger on the patient's skin, nearby the injection site, to “fool” them into feeling the vibration rather than the injection. Pre-treatment with topical anaesthetic (e.g. 2.5%/2.5% lidocaine/prilocaine or 30% lidocaine) and the use of ice or cold gauze compresses before and after injection can be utilised in more anxious patients and has been reported to significantly decrease injection pain in trials.[20,21]
Injection site ecchymosis reportedly occurs in less than 1% of all injections and can be reduced by avoidance of medications with anticoagulant properties [e.g. aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs)], herbal supplements (e.g. vitamin E, gingko, kava), smoking and alcohol for 7-10 days before the procedure.[22,23] Cold compresses and direct firm pressure immediately after injection can also mitigate the risk of post-injection bruising. Even with the use of proper technique and careful patient selection, the periocular, perioral and lid margins, composed of thin skin with superficial vessels, are very prone to ecchymosis. Up to 25% of patients have demonstrated bruising after receiving treatment of the crow's feet (periocular) with BoNTA-ONA.[24] Similar rates occurred in the placebo group, implying that the ecchymosis resulted from the injection rather than the toxin itself.
Those patients who desire a form of prevention for bruising can be treated pre-operatively with herbal remedies such as Arnica montanta and/or Bromelain, which have been shown to reduce bruising and swelling by both small clinical trials and anecdotally.[25–27] These products are available in over-the-counter regimens such as Bruiseguard MD, which combines a Bromelain complex and A. montana in an easy-to-follow 7-day treatment plan.[28] Topical preparations of vitamin K and C are reported to be beneficial, but data are limited to suggest a clear-cut advantage.
Generalised reactions including nausea, malaise, flu-like symptoms and cutaneous eruptions may result from diffusion of neurotoxin into the systemic circulation, and are treated supportively based on symptoms. Systemic reactions are considered hypersensitivity to BoNT or one of the components (i.e. albumin or lactose) in the suspension. Allergies are rare, but case reports of fixed drug eruption, localised anaphylactic reaction of the leg, and development of sarcoidal nodules from antigenic stimulation following injection have been reported.[29–31] One fatal case of anaphylaxis was reported from a BoNTA-ONA-lidocaine mixture for therapeutic uses; however, there are no reports of anaphylaxis for cosmetic purposes.[32]
Headaches were the most frequently reported adverse event in the initial trials of BoNTA-ONA for the treatment of glabellar lines (BoNTA-ONA, 15.3% vs. placebo, 15.0%; BoNTA-ONA, 11% vs. placebo, 20%), which is most likely a result of the injection technique and not the drug itself.[13,33] A case report of five patients receiving BoNT treatment for cosmetic purposes described severe and debilitating headaches that resolved with appropriate therapy and were postulated to occur from either the microtrauma of needle penetration into the periosteum or toxin-induced muscle spasm.[34] Fortunately, headaches are usually mild and require no specific treatment.
Muscle paralysis/weakness is the ultimate goal of cosmetic uses of BoNT, but can also lead to an unintended effect on neighbouring muscles through local diffusion of up to 3 cm in diameter from the injection point.[35] Local diffusion of the toxin from the procerus and corrugator supercilii muscles to the levator palpebrae superioris through the orbital septum can result in lid ptosis and diplopia.[36] The BoNTA-ONA package insert reports that blepharoptosis occurred in 3% of 405 individuals, while another large multicenter study reported that 5.4% of 264 patients experienced this complication when treated for glabellar lines with 20 units.[3,13]
As described, unwanted paralysis/weakness of neighbouring muscles can result in blepharoptosis; however, midbrow ptosis is, in the author's opinion, more common and underreported in trials and clinical practice. More of a consequence then a complication, high-dose treatment of the eyebrow depressors (orbicularis oculi, depressor supercilii, procerus and corrugator muscles) with concurrent high-dose treatment of the mid-frontalis may subsequently lower the midbrow and cause elevation of the lateral eyebrow (spock effect). This may be prevented by injecting small doses of BoNTA lateral to the temporal fusion lines above the lateral eyebrow at the initial visit. Lateral eyebrow ptosis has been reported in 5% of 183 patients undergoing treatment for periocular lines (crow's feet) in an early dose finding study with BoNTA-ONA.[37] Unfortunately, in the literature, there is little mention regarding the incidence of lateral eyebrow elevation from overzealous eyebrow depressors and/or mid-frontalis treatment.
When injecting into or around the lip, there is high potential for lip ptosis, as many muscles of facial expression are involved in the movement and shape of the lips. When injecting the mentalis, depressor anguli oris or orbicularis oris, unwanted spread or unintentional injection into the depressor labii inferioris may result in difficulty depressing the lower lip, resulting in an an abnormal/asymmetrical smile and/or difficulty when eating. Inadvertent treatment of the zygomaticus major when treating the lower orbicularis oculi or over-treatment of the levator labii superioris alaeque nasi when treating “gummy smile” can also cause an abnormal smile, as these muscles control the movement and shape of the upper lip and lip corners. An extensive analysis of 485 patients treated for various cosmetic purposes with BoNTA reported that of 20 patients injected in the middle and lower face, only 2 patients experienced partial lip ptosis following injection into the upper lip for treatment of nasolabial folds.[38] Additionally, partial lip ptosis occurred in 3 cases in over 1000 patients following injection of 7.5 units of botulinum toxin A to the lateral canthal rhytides (crow's feet); however, these patients had all undergone previous blepharoplasties, suggesting that altered anatomical planes may have resulted in toxin diffusion.[39]
Reduction of unwanted paralysis can be reduced through proper injection and dilution techniques. Application of pressure immediately post-injection, the use of lower dilutions with higher concentrations (lower injection volumes), placement of corrugator injections greater than 1 cm above the orbital bony rim, orbicularis oculi muscle injections at least 1–2 cm from the lateral orbital rim and frontalis injections at least 2.5 cm above the midbrow can all help limit unwanted diffusion [Figure 1]. If lid ptosis does occur, α-adrenergic agonist eye drops can be employed to expedite the resolution of this temporary complication.[39]
Figure 1.
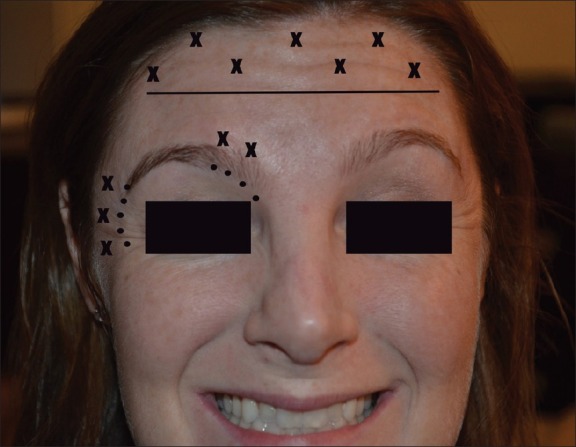
Safe injection locations for neurotoxins. Injections (“x”) 2.5 cm above the bony orbit (horizontal line), 1 cm lateral to and above the orbital rim (small dots) should avoid unwanted diffusion and paralysis
Injection into the palmoplantar region for hyperhidrosis can result in temporary hand weakness and reduction in fine motor skills which can last several weeks; thus, injection sites should be spaced appropriately and injections should be placed superficially [Figure 2].[40,41] Recently, a 17-year-old girl reported the inability to engage in cellular communication via texting following BoNT-A injection.[42] Full disclosure of possible complications is necessary when using neurotoxin for cosmetic purposes.
Figure 2.
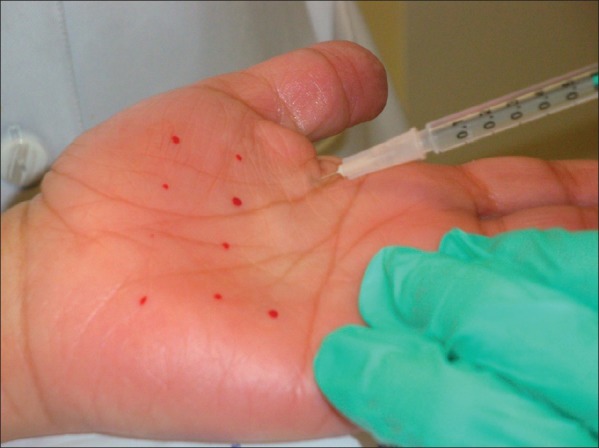
Injection pattern for palmar hyperhidrosis injection. Roughly 20 injection points spaced 1 cm apart should equate to 50 U of BoNTA-ONA (4 ml bacteriostatic water into a 100 U vial; 0.1 ml = 2.5 U) to each palm
SOFT-TISSUE FILLERS
Demand for soft-tissue fillers continues to increase, specifically because they are a safe, easy and non-invasive alternative to surgical modalities for facial augmentation and/or volume improvement. Filler efficacy has been demonstrated through multiple clinical trials and has the ability to provide dramatic instantaneous results (HA and CaHA) and prolonged improvements (PLLA and PMMA) with impressive safety profiles when proper injection technique is adhered to. The armamentarium of FDA-approved soft-tissue fillers is extensive, and each varies in composition and approved uses [Table 2]. Reported complications following soft-tissue augmentation are unique and a likely consequence of poor injection technique, the filler material itself, or improper procedure sterilisation. Each filler product has unique complications due to the composition of the product, injection techniques and dilutions [Table 3].
Table 2.
Commonly used injectable soft-tissue fillers in the United States
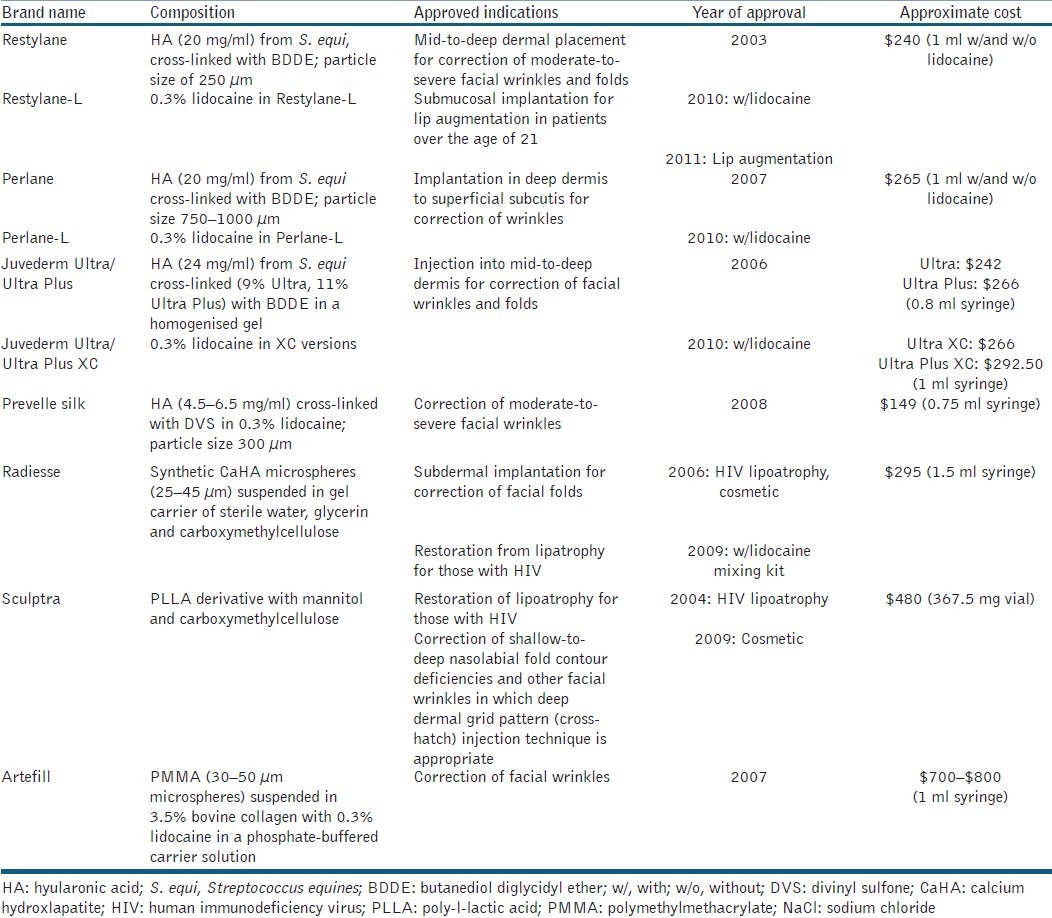
Table 3.
Complications, management and tips for each filler type

Local injection site reactions such as swelling, bruising, pain and pruritus are the most commonly reported adverse events [Figure 3]. These reactions can be prevented through proper technique including slow and precise injections, use of a small-gauge needles, immediate application of cold compresses or pressure to the area following injection, and stopping the use of alcohol, aspirin or an alike blood-thinning agent 7–10 days before injections.[19] In a clinical trial comparing a collagen (Zyplast, McGhan Medical Inc., Santa Barbara, CA, USA) and an HA-filler (Restylane Q-Medical, Uppsala, Sweden) in the treatment of nasolabial folds, 90.6% and 93.5% patients, respectively, experienced local injection site reactions following initial injection of 1 ml of product with a 30-gauge, 0.5-inch needle.[43] These local reactions lasted less than 1 week and were classified as moderate-to-severe by patient-reported data.
Figure 3.
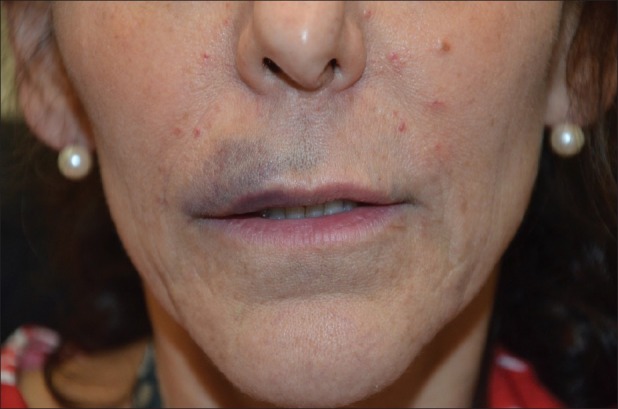
Lip haematoma after injection with HA acid filler (Juvederm). The patient admitted to alcohol ingestion the night before therapy. This complication healed without any sequelae
The incidence of infection following filler placement is low; however, reactivation of herpes simplex virus may occur, thus warranting either prophylactic anti-viral treatment for those with known outbreaks or procedure postponement for those with active lesions. Bacterial infection with Mycobacterium abscessus was reported following soft-tissue augmentation with an unapproved product composed of Hyacell, an illegally imported HA derivative.[44] Other soft-tissue infections of the face, including facial cellulitis and periorbital and buccal space abscesses, associated with soft-tissue filler placement were reported in a recent case series of 22 patients of whom 18 were hospitalised after injection with various filler agents including collagen, silicone, HA and autologous fat.[45] A subset (n = 9) of these patients underwent their procedure at a non-medical facility, including from beauticians at salons, while the majority of this cohort (n = 13) received their treatment at a private plastic surgery or dermatology office. Practitioners should be wary of these potential complications in order to initiate and organise prompt medical treatment if necessary or refer patients for appropriate surgical treatment.
The development of a biofilm, defined as a collection of encapsulated microorganisms adherent to inert surfaces, may present as deep erythematous nodules weeks to years after product placement.[46] Practitioners should keep a high level of suspicion for this complication. It is likely that the development ensues due to inadequate skin cleaning or excessive product placement that creates the perfect environment for bacterial overgrowth. Proper skin preparation with antiseptic (Cetaphil or CeraVe wash then chlorhexidine) is necessary for prevention. Although filler injections are considered non-invasive, they should be treated as surgical procedures and patients should be prepared in a fashion that keeps the field as clean as possible. In addition, avoidance of injecting large volumes, multiple filler types during one session and injection into inflammatory lesions (e.g. acne or eczema) may prevent infections.[47]
If an infection occurs, expressible exudate should be cultured before initiating empiric antibiotic therapy and purulent collections should be drained. Clarithromycin offers acid-fast bacterial coverage and minocycline covers methicillin-resistant Staphylococcus aureus (MRSA) and atypical mycobacterial infections. One or the combination of both may be necessary as empiric treatment for infection or “angry red bumps” following filler placement.[48] Depending on the disease severity, antibiotic treatment should last several weeks. Some infections (such as those involving the orbit or other danger zones of the face) may require hospital admission for intravenous antibiotics before prolonged oral therapy. If possible, the nidus of infection should be removed through either aspiration or incision and drainage.[45]
Vascular occlusion is a devastating, rare consequence that can lead to tissue necrosis and permanent scarring and is fortunately estimated to occur in only 0.001% of injections.[49] Occlusion can occur through compression of the skin's blood supply by excessive external filler material exerting pressure on the involved vessel, venous congestion or by direct intra-articular injection. The glabellar region is an extremely high-risk area due to the small, substantial vessels (supraorbital and supratrochlear) that have little collateral circulation in this area. The alar grove is the location of the angular artery (continuance of the facial artery) and is another high-risk area. Injections at a 90° angle or fanning perpendicular to the most medial aspect of the nasolabial fold towards the alar grove can puncture the vessel or lead to excessive external pressure resulting in vessel compression and excessive necrosis of the nasal ala, tip, medial nasolabial fold and upper lip. Thus, an extensive knowledge of facial anatomy and superior injection technique can help limit the potential for skin necrosis.[50]
Techniques to minimise the likelihood of this complication include aspirating before injection (although this may be difficult with more viscous fillers such as undiluted CaHA or silicone), using a small-gauge needle, minimising the volume of product used in one injection session, avoiding anaesthesia with epinephrine near a vascular bundle to prevent vasospasm and tenting the skin to avoid the vascular supply. Vascular occlusion must be treated emergently to give any chance of full recovery without serious long-term sequelae. Any concern for these complications, such as extreme pain, skin blanching and/or skin mottling, needs empiric treatment with rapid vasodilation as the primary goal. This can be accomplished by the application of warm compresses and/or nitroglycerin paste, taking an oral aspirin or clopidogrel and/or administration of hyaluronidase to relieve vascular compression if an HA material was used.[51] Murine experiments suggest that early intervention with hyaluronidase is beneficial, but administration after 24 h is unlikely to help.[52] Ulceration following skin necrosis can be treated supportively through wound care with wet-to-dry dressings or Xeroform™ (petrolatum gauze with 3% bismuth tribromophenate), emollients (e.g. biafine), topical antibiotics (e.g. silvadene, mupirocin) and debridement, if necessary, to facilitate healing.
Most soft-tissue fillers are indicated for mid-to-deep dermal placement. If placed too superficially, complications such as visible product, nodule formation, hypertrophic scarring, and Tyndall effect (bluish discolouration from HA fillers) can occur.[53] Attention to visual cues during injection, such as assessing the colour and shape of the needle and response of the subcutaneous tissue during placement, can help prevent misplaced products.[54] Periosteal injection can avoid the majority of complications, but requires pristine placement and sufficient experience, as needles tapping the periosteum will dull quickly and potentiate pain during injection and increase the chance of a local skin reaction (e.g. bruising or swelling).
Local massage, aspiration and the use of hyaluronidase are the methods used to treat imperfections caused by superficial product placement. A recent trend is the use of small-gauge, blunt cannulas that allow for a large distribution of product in the correct plane without the need for multiple needle sticks. Cannulas can limit the potential for improper placement and lower the risk of bruising (given that they are not sharp); however, this technique should be limited to experienced injectors with confidence in typical injection patterns, proper placement locations, as well as facial anatomy and its variations.[55–57]
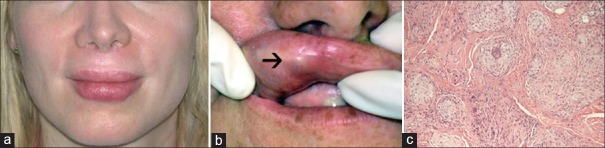
Persistent erythematous nodules (angry red bumps) represent granulomatous reactions and have been associated with every injectable filler type [Figure 4a–c].[58] Treatment of persistent inflammatory nodules presents a challenge. An algorithm was developed for management which suggests evaluation for persistent infection with close clinical follow-up and empiric antibiotic treatment with clarithromycin followed by culture of any exudates. If there is no improvement, intralesional steroids [±5-fluorouracil (FU)] can be employed at 3 or 4 week intervals; systemic steroids are unlikely to result in improvement. Incision and drainage and/or needle aspiration in combination with oral antibiotic therapy is necessary for any fluctuant lesion. Biopsy and tissue culture are warranted to assess histology and to assess for fungal or bacterial infections if there is no improvement or worsening of the lesion after the above-mentioned treatments are attempted without success.[49]
Figure 4.
(a) Erythematous indurated papules on the outer and (b) inner lip weeks after HA filler injection (Restylane). (c) Histology demonstrated aggregates of non-caseating granulomas (Courtesy of George Anastassov, MD, DDS.New York, Ny)
Late-onset persistent nodules are a primary complication of PLLA, with development ranging from 5% to over 40% [Figure 5]. Fortunately, nodule development can be avoided through higher product dilutions (9-12 ml total volume per vial), product placement in the subcutaneous and periosteal planes, minimising volume during injection sessions and aggressive massage by the patient for several days after injection (minimum 5 times a day, 5 min at a time, over 5 days).[59–61] A retrospective review of over 100 patients injected with PLLA for aesthetic purposes found that nodules were most likely to occur in the hands (12.5%) and cheek (7.2%), so care should be taken when treating these areas.[62] Injection into (or even close to) the lips should also be avoided with PLLA and CaHA, as nodule formation is most likely to occur.
Figure 5.
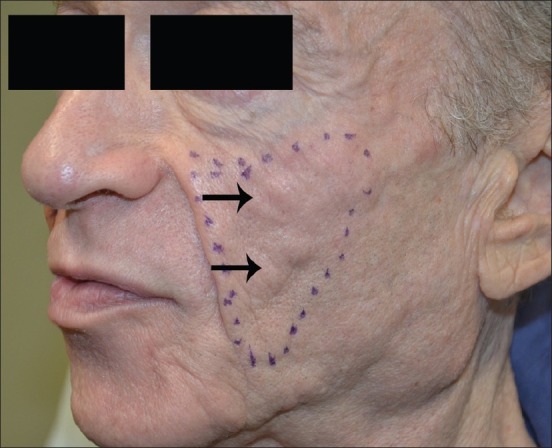
PLLA nodules on the left cheek of a patient treated for HIV lipoatrophy with improper dilution and poor mixing of product before injections
Silicone injections are still a popular permanent filler option. Liquid silicone for facial and body contouring became popular in the 1950s when it was deemed to be inert, permanent and non-pyogenic. Since then, many horror stories have been reported in the medical literature and through the media regarding cosmetic use of this substance.[63] It should be noted that many of these devastating long-lasting complications stem from injection of large volumes of industrial-grade impure products from either unlicensed or unskilled practitioners.[64,65] Currently, liquid silicone products available in the United States include Silikon 1000 (purified polydimethylsiloxane; Alcon Laboratoires Inc., Fort Worth, TX, USA) and AdatoSil 5000 (Bausch and Lomb, Rodchester, NY, USA) and are FDA approved for retinal detachment. Any use of silicone for cosmetic purposes is considered “off-label,” but experienced practitioners are dedicated to its use for soft-tissue augmentation, treatment of acne scars, lipoatrophy and correction of post-rhinoplasty nasal irregularities.
Serious complications of silicone injections range from 1 in 10,000 to 1 in 1000, and include infection, scarring and granuloma formation resulting in permanent deformities.[66] Siliconomas (granulomatous foreign body reactions to silicone deposition) can develop from several months to years after implantation [Figure 6a and b]. Clinical presentation includes subcutaneous nodules that can progress to cellulitis, ulceration and lymph node involvement. Histologically, there is an inflammatory foreign body reaction at the injection site or even a distant site due to migration (metastasis) of the material. Migration is usually a result of improper and unnecessary injection of large volumes (i.e. not adhering to the microdroplet technique).[65] The pathogenesis of granuloma formation has not been fully elucidated, but is suspected to result from T-cell activation after injection trauma, secondary infection or product additives in the filler material.[67]
Figure 6.
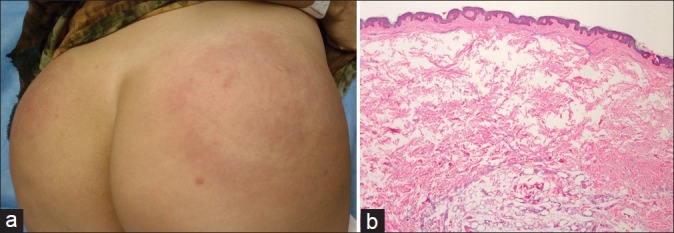
(a) Erythematous indurated plaques on bilateral buttocks. (b) Histology demonstrated disruption of the adipose tissue architecture and dermal collections of a vacuolated material between collage bundles admixed with histiocytes and rare giant cells. The material was non-polarisable and the larger vacuoles in the deep dermis suggested the possibility of an adulterated mixture of non-medical grade silicone
Granulomas are notoriously difficult to treat, thus proper technique with a known sterile product is critically important. Silicone injections should only be performed by practitioners trained in this method, as the permanence of this product makes it less forgiving than other filling agents. Specifically, the product should be injected in small amounts (0.01–0.03 ml/depot) in the subdermal plane, as superficial injections will result in visible papules and large volumes can result in product migration and large asymmetries. Deviations from this technique or injections with unapproved products can result in the aforementioned siliconoma formation or systemic toxicities such as pneumonitis, hepatic granulomas, severe infection or even death.[68,69]
Various modalities including systemic and intralesional corticosteroids, minocycline, anti-tumor necrosis factor antibodies or surgical removal can be employed to treat silicone granuloma formation. Treatment with minocycline 100 mg daily improved extensive facial granulomas after 4 weeks, and when continued for a total of 10 months, improvement was sustained 1 year after antibiotics’ initiation.[70] Oral corticosteroids may provide temporary improvement, but relapse often occurs during tapering and many patients are unable to tolerate the side effects of long-term therapy. Immunomodulating drugs, such as tumor necrosis factor inhibitors (e.g. etanercept) or imiquimod cream, are other potential treatment modalities that have been reported in the literature.[67,71,72] Imiquimod 5% topical cream applied twice daily for 2 weeks significantly improved extensive granulomas from an unapproved silicone injection on both the upper or lower lips.[72] Surgical excision should be a last resort after medical treatment has failed, as scarring is a common disfiguring consequence.
Product hypersensitivity and allergy is nowadays considered a very rare complication as compared to the incidence seen in the past with collagen (in patients not skin tested). The original bovine collagen products fell out of favour due to the potential for allergic reactions and the need to perform multiple skin tests before administration.[73] PMMA, the only remaining filler containing bovine collagen, requires skin testing with 0.1 ml intradermal injection of collagen to the volar forearm 4 weeks before treatment, and those patients with a positive test (erythema or induration) or two equivocal tests (systemic reactions such as rash, arthralgia or myalgia) should forgo treatment.[74,75] HA fillers are approved without allergy testing, as the products are highly purified and contain only a minimal amount of protein, although rare cases of angioedema and other hypersensitivity reactions have been reported in the literature.[76]
CHEMICAL PEELS
Chemical peels are increasingly popular for facial rejuvenation, as a solo treatment or in combination with topical regimens and/or with other minimally invasive techniques such as those described above [Table 4]. Complications from chemical peels include prolonged erythema and pruritus, delayed wound healing, infection, textural changes to the skin, induction of acne or milia, pigmentation changes such as post-inflammatory hyperpigmentation, and scarring. Deep chemical peels (e.g. phenol) necessitate specialised care such as cardiopulmonary monitoring, as phenol can enter the circulation resulting in cardiotoxicity.[77] Practitioners should fully evaluate the patient's Fitzpatrick skin type and perform a complete history with emphasis on prior sun exposure including any history of blistering sunburns or skin cancers, isotretinoin use, keloid formation, post-inflammatory hyperpigmentation, poor wound healing, severe herpetic infections of the face or lips and habitual picking from compulsive personalities.
Table 4.
Chemical peels for facial rejuvenation
Erythema and pruritus is a normal reaction to re-epithelialisation that usually subsides in 30–90 days depending on peel depth (typically sooner). This complication is easily managed by setting expectations before peeling, application of cool wet gauze or cold compresses as needed, administration of anti-itch creams and oral antihistamines, and keeping the skin moist with non-irritating emollients similar to petrolatum or topical barrier repair/emulsion creams such as Biafine (Ortho Dermatologics, Los Angeles, CA, USA) or Eletone (Ferndale Laboratories Inc., Ferndale, MI, USA). For deeper peels (e.g. phenol, TCA >30% peels, or combination peels), it may be necessary to provide oral anti-anxiety medications (e.g. benzodiazepines) or oral sleeping agents (e.g. zolpidem, trazodone or doxepin) for those with compulsive picking or anxiety during the healing phase.
Persistent erythema is common in deeper dermal peels including combination Jessner's plus TCA peels, especially if multiple coatings are applied. Setting expectations before peel application is extremely beneficial, as patients will appreciate being informed of what to expect in the post-peel period [Figure 7]. Erythema can easily be treated with topical corticosteroids, continued emollients, sun protection with hats and sunscreens, as well as cosmetic camouflage. If severe erythema, pruritus or pain persists beyond 3–7 days post-peel, evaluation for contact dermatitis or infection (i.e. fungal, viral, bacterial) is mandatory. If induration is appreciated in association with erythema, this may indicate impending scar formation and oral, injectable or topical corticosteroids may be necessary to prevent scar formation.
Figure 7.
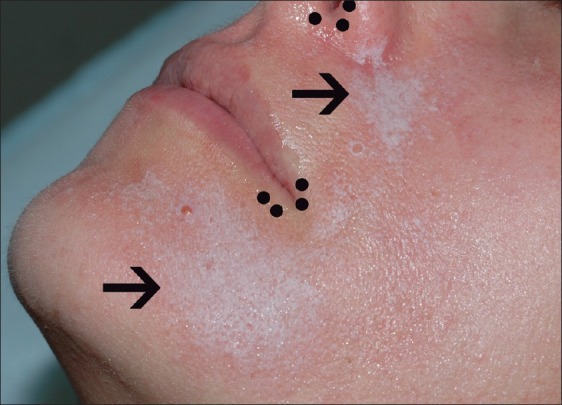
White frost after a combination of Jessner's and TCA 20% chemical peel of the face (arrows). Sparing of the lip corners and nasal opening (small dots) is evident due to the use of a petrolatum barrier protection during peeling
Scars are the most dreaded complications of chemical peels and are fortunately rare, reportedly occurring in less than 1% of medium-depth peels (e.g. Jessner's solution or GA plus TCA 35%). Higher concentrations and multiple applications of any peeling agent should be utilised with caution, as this technique is more likely to result in complications.[78] Application to areas other than the face (e.g. neck) or around the eyes (e.g. periorbital) can be fraught with more severe complications when not preformed conservatively. Facial scars are most commonly found in the lower part of the face due to active moments during wound healing. Avoidance of isotretinoin for at least 6 months before peeling can reduce the risk of scarring. Treatment strategies for resulting scars include the use of occlusive covers (e.g. silicone gel), intralesional triamcinolone (±5-FU), laser treatment, radiation, surgical excision or topical imiquimod cream.
Dyspigmentation such as hypopigmentation or hyperpigmentation can occur following a peel of any depth and is more likely to occur in patients of Fitzpatrick skin types IV–VI. Hyperpigmentation is often temporary and results from post-inflammatory changes, but the use of bleaching creams (e.g. hydroquinone, kojic acid, azelaic acid and/or ascorbic acid) and sun protection can help. Hypopigmentation is a manifestation of melanocyte destruction from deeper chemical peels and presents as an unfortunate challenge to treat.[79] The 308-nm xenon chloride excimer laser is a ultraviolet laser which can selectively target hypopigmented patches and is a treatment for lesions commonly seen in vitiligo and pityriasis alba (PA). After biweekly treatment with the excimer laser for 3 months in 12 pediatric patients with PA, there was an extensive decrease in facial hypopigmentation and some with complete resolution.[80] There were no SAEs, and side effects including erythema or a “warm sensation” post-treatment were short-term, implying that this modality may be a possible option for facial hypopigmentation resulting from chemical peels. Testing the product before use, priming the skin with hydroquinone and/or tretinoin cream at least 2–4 weeks before superficial and medium-depth peels, and proper peel selection rather than using a similar peel for every patient can help avoid unnecessary pigmentary changes.
Bacterial and viral infections have rarely been reported in association with chemical peels. Reactivation of herpes simplex virus can be prevented with anti-viral prophylaxis such as valacyclovir 500 mg twice daily starting 2–3 days before the procedure and finishing 7 days post-procedure. Bacterial infections such as staphylococcal folliculitis are usually a result of improper wound care or predisposing factors such as acne, carrier states or history of eczema, and should be cultured if possible and treated with empiric antibiotic therapy (e.g. cephalosporin or doxycycline).
Acne may occur during the re-epithelialisation process and can be treated with topical and oral antibiotics, topical azelaic acid, low-dose intralesional corticosteroids or low-dose isotretinoin, if necessary. Milia has been reported to occur in up to 20% of patients after chemical peels 8–16 weeks after the procedure and can be removed with either electrodessication or surgical extraction with #11 blade.[81]
CONCLUSIONS
Office-based, minimally invasive techniques are in high demand since they provide a quick and reliable service for skin care rejuvenation with low risk of complications and high patient satisfaction. However, as with any surgical procedure, complications can occur and should be discussed before procedure initiation. Proper patient selection is of utmost importance, as is significant knowledge of facial anatomy, skin pathology, and procedure techniques and options. Immediate recognition and treatment of complications can help minimise the likelihood of permanent consequences. With significant patient demand and the rising rates of non-trained practitioners performing these procedures, the recognition and management of complications will become increasingly important in clinical practice.
ACRONYMS
BoNT, botulinum toxin; FDA, Food and Drug Administration; HA, hyaluronic acid; PLLA, Poly-l-lactic acid; CaHA, calcium hydroxylapatite; PMMA, polymethylmethacrylate; GA, glycolic acid; SA, salicylic acid; TCA, trichloroacetic acid; SAE, serious adverse event; NSAIDs, nonsteroidal anti-inflammatory drugs; 5-FU, 5-fluorouracil; nm, nanometer; PA, pityriasis alba.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.American Society of Plastic Surgeons. Cosmetic Plastic Surgery Statistics. 2011. [Last accessed on 2012, March 17]. Available from: http://www.plasticsurgery.org/Documents/news-resources/statistics/2011-statistics/2011-cosmetic-procedurestrends-statistics.pdf .
- 2.Lu DW, Lippitz J. Complications of botulinum neurotoxin. Dis Mon. 2009;55:198–211. doi: 10.1016/j.disamonth.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Irvine CA: Allergan; 2011. Botox Cosmetic [package insert] [Google Scholar]
- 4.Scottsdale, AZ: Medicis Aesthetics Inc; 2009. Dysport[package insert] [Google Scholar]
- 5.Franksville, WI: Merz Aesthetics, Inc; 2011. Xeomin[package insert] [Google Scholar]
- 6.Alster TS, Lupton JR. Botulinum toxin type B for dynamic glabellar rhytides refractory to botulinum toxin type A. Dermatol Surg. 2003;29:516–8. doi: 10.1046/j.1524-4725.2003.29123.x. [DOI] [PubMed] [Google Scholar]
- 7.André P. Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASHA - Q-Medical, Sweden) in European countries: A retrospective study from1997 to 2001. J Eur Acad Dermatol Venereol. 2004;18:422–5. doi: 10.1111/j.1468-3083.2004.00934.x. [DOI] [PubMed] [Google Scholar]
- 8.Bray D, Hopkins C, Roberts DN. A review of dermal fillers in facial plastic surgery. Curr Opin Otolaryngol Head Neck Surg. 2010;18:295–302. doi: 10.1097/MOO.0b013e32833b5162. [DOI] [PubMed] [Google Scholar]
- 9.Berson DS, Cohen JL, Rendon MI, Roberts WE, Starker I, Wang B. Clinical role and application of superficial chemical peels in today's practice. J Drugs Dermatol. 2009;8:803–11. [PubMed] [Google Scholar]
- 10.Kessler E, Flanagan K, Chia C, Rogers C, Glaser DA. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34:45–50. doi: 10.1111/j.1524-4725.2007.34007.x. [DOI] [PubMed] [Google Scholar]
- 11.Monheit GD. The Jessner's-trichloroacetic acid peel. An enhanced medium-depth chemical peel. Dermatol Clin. 1995;13:277–83. [PubMed] [Google Scholar]
- 12.Aoki KR. Pharmacology and immunology of botulinum neurotoxins. Int Ophthalmol Clin. 2005;45:25–37. doi: 10.1097/01.iio.0000167167.10402.74. [DOI] [PubMed] [Google Scholar]
- 13.Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, et al. BOTOX Glabellar Lines I Study Group. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46:840–9. doi: 10.1067/mjd.2002.121356. [DOI] [PubMed] [Google Scholar]
- 14.Semchyshyn N, Sengelmann RD. Botulinum toxin A treatment of perioral rhytides. Dermatol Surg. 2003;29:490–5. doi: 10.1046/j.1524-4725.2003.29118.x. [DOI] [PubMed] [Google Scholar]
- 15.Coté TR, Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: Adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407–15. doi: 10.1016/j.jaad.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Naumann M, Jankovic J. Safety of botulinum toxin type A: A systematic review and meta-analysis. Curr Med Res Opin. 2004;20:981–90. doi: 10.1185/030079904125003962. [DOI] [PubMed] [Google Scholar]
- 17.Klein AW. Complications and adverse reactions with the use of botulinum toxin. Semin Cutan Med Surg. 2001;20:109–20. doi: 10.1053/sder.2001.25964. [DOI] [PubMed] [Google Scholar]
- 18.Pena MA, Alam M, Yoo SS. Complications with the use of botulinum toxin type A for cosmetic applications and hyperhidrosis. Semin Cutan Med Surg. 2007;26:29–33. doi: 10.1016/j.sder.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Emer J, Waldorf H. Injectable neurotoxins and fillers: There is no free lunch. Clin Dermatol. 2011;29:678–90. doi: 10.1016/j.clindermatol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Söylev MF, Koçak N, Kuvaki B, Ozkan SB, Kir E. Anesthesia with EMLA cream for botulinum A toxin injection into eyelids. Ophthalmologica. 2002;216:355–8. doi: 10.1159/000066186. [DOI] [PubMed] [Google Scholar]
- 21.Linder JS, Edmonson BC, Laquis SJ, Drewry RD, Jr, Fleming JC. Skin cooling before periocular botulinum toxin A injection. Ophthal Plast Reconstr Surg. 2002;18:441–2. doi: 10.1097/00002341-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A: A focus on cosmetic procedures. Am J Clin Dermatol. 2005;6:141–50. doi: 10.2165/00128071-200506030-00001. [DOI] [PubMed] [Google Scholar]
- 23.Broughton G, 2nd, Crosby MA, Coleman J, Rohrich RJ. Use of herbal supplements and vitamins in plastic surgery: A practical review. Plast Reconstr Surg. 2007;119:48e–66e. doi: 10.1097/01.prs.0000252661.72071.8d. [DOI] [PubMed] [Google Scholar]
- 24.Lowe NJ, Lask G, Yamauchi P, Moore D. Bilateral, double-blind, randomized comparison of 3 doses of botulinum toxin type A and placebo in patients with crow's feet. J Am Acad Dermatol. 2002;47:834–40. doi: 10.1067/mjd.2002.124070. [DOI] [PubMed] [Google Scholar]
- 25.Kouzi SA, Nuzum DS. Arnica for bruising and swelling. Am J Health Syst Pharm. 2007;64:2434–43. doi: 10.2146/ajhp070155. [DOI] [PubMed] [Google Scholar]
- 26.Leu S, Havey J, White LE, Martin N, Yoo SS, Rademaker AW, et al. Accelerated resolution of laser-induced bruising with topical 20% arnica: A rater-blinded randomized controlled trial. Br J Dermatol. 2010;163:557–63. doi: 10.1111/j.1365-2133.2010.09813.x. [DOI] [PubMed] [Google Scholar]
- 27.MacKay D, Miller AL. Nutritional support for wound healing. Altern Med Rev. 2003;8:359–77. [PubMed] [Google Scholar]
- 28.Skinelite.com [internet]. ScarGuard Bruiseguard MD. Skinelite. 2007. [Last accessed on 2012 April 3]. Available at http://www.skinelite.com/scar-004.html .
- 29.Cox NH, Duffey P, Royle J. Fixed drug eruption caused by lactose in an injected botulinum toxin preparation. J Am Acad Dermatol. 1999;40:263–4. doi: 10.1016/s0190-9622(99)70202-1. [DOI] [PubMed] [Google Scholar]
- 30.LeWitt PA, Trosch RM. Idiosyncratic adverse reactions to intramuscular botulinum toxin type A injection. Mov Disord. 1997;12:1064–7. doi: 10.1002/mds.870120637. [DOI] [PubMed] [Google Scholar]
- 31.Ahbib S, Lachapelle JM, Marot L. [Sarcoidal granulomas following injections of botulic toxin A (Botox) for corrections of wrinkles] Ann Dermatol Venereol. 2006;133:43–5. doi: 10.1016/s0151-9638(06)70842-0. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Goldberger BA, Hopkins C. Fatal case of BOTOX-related anaphylaxis? J Forensic Sci. 2005;50:169–72. [PubMed] [Google Scholar]
- 33.Carruthers JD, Lowe NJ, Menter MA, Gibson J, Eadie N Botox Glabellar Lines II Study Group. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg. 2003;112:1089–98. doi: 10.1097/01.PRS.0000076504.79727.62. [DOI] [PubMed] [Google Scholar]
- 34.Alam M, Arndt KA, Dover JS. Severe, intractable headache after injection with botulinum a exotoxin: Report of 5 cases. J Am Acad Dermatol. 2002;46:62–5. doi: 10.1067/mjd.2001.118342. [DOI] [PubMed] [Google Scholar]
- 35.Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43:249–59. doi: 10.1067/mjd.2000.105567. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira MC, Salles AG, Gimenez R, Soares MF. Complications with the use of botulinum toxin type a in facial rejuvenation: Report of 8 cases. Aesthetic Plast Surg. 2004;28:441–4. doi: 10.1007/s00266-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 37.Garcia A, Fulton JE., Jr Cosmetic denervation of the muscles of facial expression with botulinum toxin. A dose-response study. Dermatol Surg. 1996;2:39–43. doi: 10.1111/j.1524-4725.1996.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 38.Sarrabayrouse MA. Indications and limitations for the use of botulinum toxin for the treatment of facial wrinkles. Aesthetic Plast Surg. 2002;26:233–8. doi: 10.1007/s00266-002-2030-x. [DOI] [PubMed] [Google Scholar]
- 39.Matarasso SL, Matarasso A. Treatment guidelines for botulinum toxin type A for the periocular region and a report on partial upper lip ptosis following injections to the lateral canthal rhytids. Plast Reconstr Surg. 2001;108:208–14. doi: 10.1097/00006534-200107000-00033. discussion 215-7. [DOI] [PubMed] [Google Scholar]
- 40.Klein AW. Complications with the use of botulinum toxin. Dermatol Clin. 2004;22:197–205. doi: 10.1016/s0733-8635(03)00122-0. vii. [DOI] [PubMed] [Google Scholar]
- 41.Simonetta Moreau M, Cauhepe C, Magues JP, Senard JM. A double-blind, randomized, comparative study of Dysport vs. Botox in primary palmar hyperhidrosis. Br J Dermatol. 2003;149:1041–5. doi: 10.1111/j.1365-2133.2003.05620.x. [DOI] [PubMed] [Google Scholar]
- 42.Lehman JS. Writer's block: “Texting” impairment as a complication of botulinum toxin type A therapy for palmar hyperhidrosis. Arch Dermatol. 2011;147:752. doi: 10.1001/archdermatol.2011.143. [DOI] [PubMed] [Google Scholar]
- 43.Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–95. doi: 10.1046/j.1524-4725.2003.29150.x. [DOI] [PubMed] [Google Scholar]
- 44.Toy BR, Frank PJ. Outbreak of Mycobacterium abscessus infection after soft tissue augmentation. Dermatol Surg. 2003;29:971–3. doi: 10.1046/j.1524-4725.2003.29262.x. [DOI] [PubMed] [Google Scholar]
- 45.Schütz P, Ibrahim HH, Hussain SS, Ali TS, El-Bassuoni K, Thomas J. Infected facial tissue fillers: Case series and review of the literature. J Oral Maxillofac Surg. 2012 doi: 10.1016/j.joms.2011.11.014. [in press] [DOI] [PubMed] [Google Scholar]
- 46.Christensen LH. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol Surg. 2009;35(Suppl 2):1612–9. doi: 10.1111/j.1524-4725.2009.01338.x. [DOI] [PubMed] [Google Scholar]
- 47.Bachmann F, Erdmann R, Hartmann V, Becker-Wegerich P, Wiest L, Rzany B. Adverse reactions caused by consecutive injections of different fillers in the same facial region: Risk assessment based on the results from the Injectable Filler Safety study. J Eur Acad Dermatol Venereol. 2011;25:902–12. doi: 10.1111/j.1468-3083.2010.03878.x. [DOI] [PubMed] [Google Scholar]
- 48.Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008;34(Suppl 1):S92–9. doi: 10.1111/j.1524-4725.2008.34249.x. [DOI] [PubMed] [Google Scholar]
- 49.Narins RS, Jewell M, Rubin M, Cohen J, Strobos J. Clinical conference: Management of rare events following dermal fillers-focal necrosis and angry red bumps. Dermatol Surg. 2006;32:426–34. doi: 10.1111/j.1524-4725.2006.32086.x. [DOI] [PubMed] [Google Scholar]
- 50.Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: Protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006;32:276–81. doi: 10.1111/j.1524-4725.2006.32052.x. [DOI] [PubMed] [Google Scholar]
- 51.Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: Case report and review of the literature. J Cosmet Dermatol. 2011;10:224–31. doi: 10.1111/j.1473-2165.2011.00562.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim DW, Yoon ES, Ji YH, Park SH, Lee BI, Dhong ES. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64:1590–5. doi: 10.1016/j.bjps.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 53.Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26:34–9. doi: 10.1016/j.sder.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31:110–21. doi: 10.1177/1090820X10391083. [DOI] [PubMed] [Google Scholar]
- 55.Zeichner JA, Cohen JL. Use of blunt tipped cannulas for soft tissue fillers. J Drugs Dermatol. 2012;11:70–2. [PubMed] [Google Scholar]
- 56.Niamtu J., 3rd Filler injection with micro-cannula instead of needles. Dermatol Surg. 2009;35:2005–8. doi: 10.1111/j.1524-4725.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 57.Cohen JL, Brown MR. Anatomic considerations for soft tissue augmentation of the face. J Drugs Dermatol. 2009;8:13–6. [PubMed] [Google Scholar]
- 58.Dadzie OE, Mahalingam M, Parada M, El Helou T, Philips T, Bhawan J. Adverse cutaneous reactions to soft tissue fillers-A review of the histological features. J Cutan Pathol. 2008;35:536–48. doi: 10.1111/j.1600-0560.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 59.Bridgewater, NJ: Dermik Laboratories; 2009. Sculptra[package insert] [Google Scholar]
- 60.Valantin MA, Aubron-Olivier C, Ghosn J, Laglenne E, Pauchard M, Schoen H, et al. Polylactic acid implants (New-Fill) to correct facial lipoatrophy in HIV-infected patients: Results of the open-labelstudy VEGA. AIDS. 2003;17:2471–7. doi: 10.1097/00002030-200311210-00009. [DOI] [PubMed] [Google Scholar]
- 61.Lam SM, Azizzadeh B, Graivier M. Injectable poly-L-lactic acid (Sculptra): Technical considerations in soft-tissue contouring. Plast Reconstr Surg. 2006;118(3 Suppl):55S–63S. doi: 10.1097/01.prs.0000234612.20611.5a. [DOI] [PubMed] [Google Scholar]
- 62.Palm MD, Woodhall KE, Butterwick KJ, Goldman MP. Cosmetic use of poly-l-lactic acid: A retrospective study of 130 patients. Dermatol Surg. 2010;36:161–70. doi: 10.1111/j.1524-4725.2009.01419.x. [DOI] [PubMed] [Google Scholar]
- 63.Narins RS, Beer K. Liquid injectable silicone: A review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(3 Suppl):77S–84S. doi: 10.1097/01.prs.0000234919.25096.67. [DOI] [PubMed] [Google Scholar]
- 64.Anastassov GE, Schulhof S, Lumerman H. Complications after facial contour augmentation with injectable silicone. diagnosis and treatment. Report of a severe case. Int J Oral Maxillofac Surg. 2008;37:955–60. doi: 10.1016/j.ijom.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 65.Schwartzfarb EM, Hametti JM, Romanelli P, Ricotti C. Foreign body granuloma formation secondary to silicone injection. Dermatol Online J. 2008;14:20. [PubMed] [Google Scholar]
- 66.Duffy DM. Liquid silicone for soft tissue augmentation. Dermatol Surg. 2005;31()(11 Pt 2):1530–41. doi: 10.2310/6350.2005.31238. [DOI] [PubMed] [Google Scholar]
- 67.Pasternack FR, Fox LP, Engler DE. Silicone granulomas treated with etanercept. Arch Dermatol. 2005;141:13–5. doi: 10.1001/archderm.141.1.13. [DOI] [PubMed] [Google Scholar]
- 68.Chastre J, Basset F, Viau F, Dournovo P, Bouchama A, Akesbi A, et al. Acute pneumonitis after subcutaneous injections of silicone in transsexual men. N Engl J Med. 1983;308:764–7. doi: 10.1056/NEJM198303313081307. [DOI] [PubMed] [Google Scholar]
- 69.Ellenbogen R, Rubin L. Injectable fluid silicone therapy.Human morbidity and mortality. JAMA. 1975;234:308–9. [PubMed] [Google Scholar]
- 70.Arin MJ, Bäte J, Krieg T, Hunzelmann N. Silicone granuloma of the face treated with minocycline. J Am Acad Dermatol. 2005;52(2 Suppl 1):53–6. doi: 10.1016/j.jaad.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Desai AM, Browning J, Rosen T. Etanercept therapy for silicone granuloma. J Drugs Dermatol. 2006;5:894–6. [PubMed] [Google Scholar]
- 72.Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with aldara (imiquimod 5%) Dermatol Surg. 2003;29:429–3. doi: 10.1046/j.1524-4725.2003.29102.x. [DOI] [PubMed] [Google Scholar]
- 73.Alam M, Gladstone H, Kramer EM, Murphy JP, Jr, Nouri K, Neuhaus IM, et al. American Society for Dermatologic Surgery. ASDS guidelines of care: Injectable fillers. Dermatol Surg. 2008;34(Suppl 1):S115–48. doi: 10.1111/j.1524-4725.2008.34253.x. [DOI] [PubMed] [Google Scholar]
- 74.Artefill Instructions for Use. [Last accessed on 2012 April 3]. Available at http://www.doctor.artefill.com/about-artefill/artefill-instructions-for-use .
- 75.Emer J, Waldorf H, Cohen J. Complications and their management. In: Sadick N, Carniol P, Roy D, Wiest L, editors. Illustrated Manual of Injectable Fillers. New York: Informa Healthcare; 2011. p. 141. [Google Scholar]
- 76.Leonhardt JM, Lawrence N, Narins RS. Angioedema acute hypersensitivity reaction to injectable hyaluronic acid. Dermatol Surg. 2005;31:577–9. doi: 10.1111/j.1524-4725.2005.31166. [DOI] [PubMed] [Google Scholar]
- 77.Landau M. Cardiac complications in deep chemical peels. Dermatol Surg. 2007;33:190–3. doi: 10.1111/j.1524-4725.2006.33037.x. [DOI] [PubMed] [Google Scholar]
- 78.Brody HJ. Complications of chemical resurfacing. (vii-viii).Dermatol Clin. 2001;19:427–38. doi: 10.1016/s0733-8635(05)70283-7. [DOI] [PubMed] [Google Scholar]
- 79.Camacho FM. Medium-depth and deep chemical peels. J Cosmet Dermatol. 2005;4:117–28. doi: 10.1111/j.1473-2165.2005.40213.x. [DOI] [PubMed] [Google Scholar]
- 80.Al-Mutairi N, Hadad AA. Efficacy of 308-nm Xenon Chloride Excimer Laser in Pityriasis Alba. Dermatol Surg. 2011 doi: 10.1111/j.1524-4725.2011.02223.x. [in press] [DOI] [PubMed] [Google Scholar]
- 81.Landau M. Chemical peels. Clin Dermatol. 2008;26:200–8. doi: 10.1016/j.clindermatol.2007.09.012. [DOI] [PubMed] [Google Scholar]