Abstract
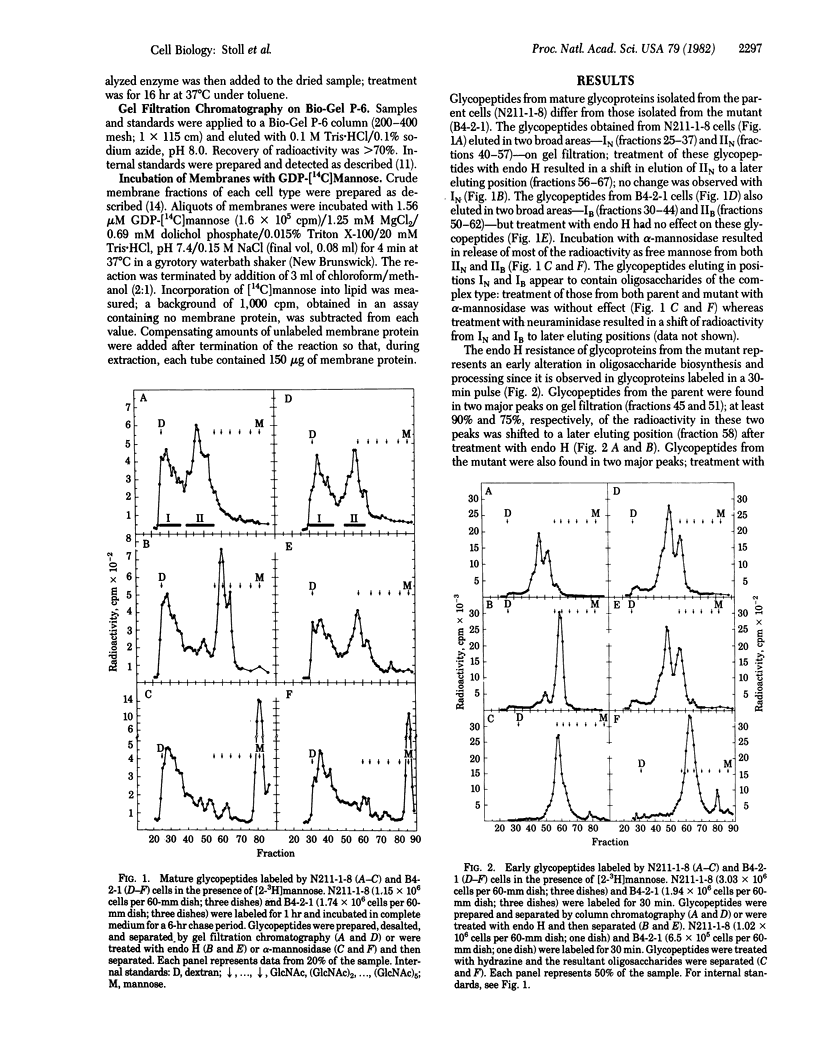
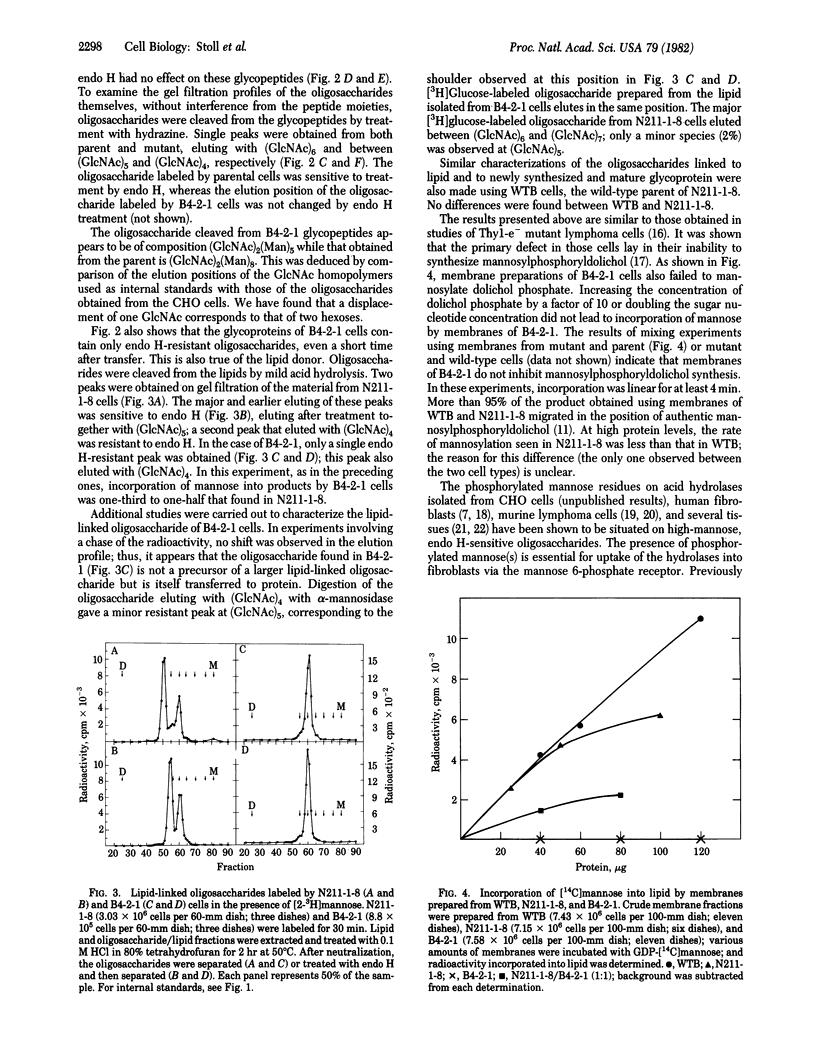
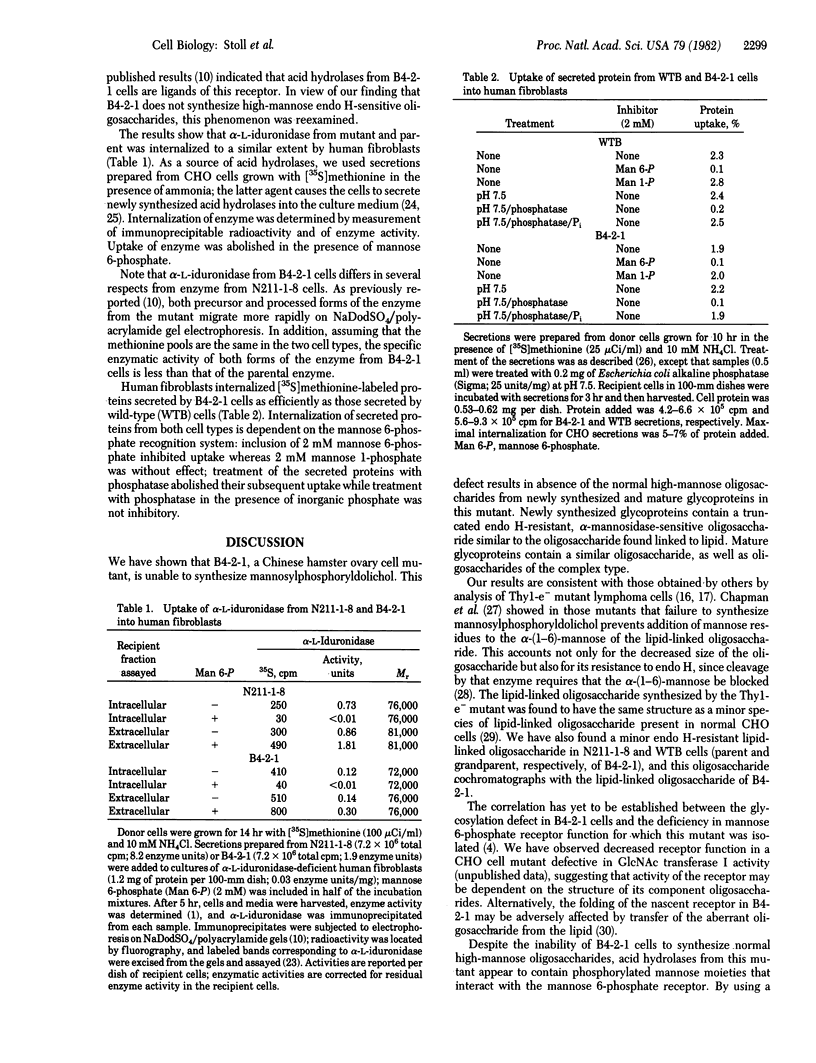
B4-2-1 is a Chinese hamster ovary cell mutant previously isolated and characterized as deficient in mannose 6-phosphate receptor activity. We show here that B4-2-1 is a pleiotropic mutant, defective in biosynthesis of asparagine-linked oligosaccharides, B4-2-1 is unable to synthesize mannosylphosphoryldolichol; the consequences of this defect on glycosylation are (i) biosynthesis of one major lipid-linked oligosaccharide, characterized by its resistance to endoglycosidase H and decreased size; this oligosaccharide is similar to a minor species of lipid-liked oligosaccharide found in parental cells; (ii) transfer of this oligosaccharide to newly synthesized proteins; and (iii) absence of normal "high-mannose" oligosaccharides on mature glycoproteins isolated from B4-2-1; glycoproteins from the mutant contain complex oligosaccharides as well as endoglycosidase H-resistant, alpha-mannosidase-sensitive species. While the glycosylation defect may alter adversely the function of several glycoproteins in the mutant, including that of the mannose 6-phosphate receptor, it appears to have no effect on the formation or function of the mannose 6-phosphate recognition marker on acid hydrolases of B4-2-1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach G., Bargal R., Cantz M. I-cell disease: deficiency of extracellular hydrolase phosphorylation. Biochem Biophys Res Commun. 1979 Dec 14;91(3):976–981. doi: 10.1016/0006-291x(79)91975-2. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Tyo M. A., Krag S. S., Robbins P. W. A new procedure for the preparation of GDP-[U-14C]mannose. Anal Biochem. 1976 Aug;74(2):484–487. doi: 10.1016/0003-2697(76)90229-3. [DOI] [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Chapman A., Trowbridge I. S., Hyman R., Kornfeld S. Structure of the lipid-linked oligosaccharides that accumulate in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):509–515. doi: 10.1016/0092-8674(79)90259-9. [DOI] [PubMed] [Google Scholar]
- Distler J., Hieber V., Sahagian G., Schmickel R., Jourdian G. W. Identification of mannose 6-phosphate in glycoproteins that inhibit the assimilation of beta-galactosidase by fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4235–4239. doi: 10.1073/pnas.76.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S. Beta-glucuronidase binding to human fibroblast membrane receptors. J Biol Chem. 1980 Jun 10;255(11):5069–5074. [PubMed] [Google Scholar]
- Gibson R., Kornfeld S., Schlesinger S. The effect of oligosaccharide chains of different sizes on the maturation and physical properties of the G protein of vesicular stomatitis virus. J Biol Chem. 1981 Jan 10;256(1):456–462. [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Waheed A., von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981 Feb 12;98(3):761–767. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- Kobata A. endo-beta-N-Acetylglucosaminidases CI and CII from Clostridium perfringens. Methods Enzymol. 1978;50:567–574. doi: 10.1016/0076-6879(78)50064-5. [DOI] [PubMed] [Google Scholar]
- Krag S. S. A concanavalin A-resistant Chinese hamster ovary cell line is deficient in the synthesis of [3H]glucosyl oligosaccharide-lipid. J Biol Chem. 1979 Sep 25;254(18):9167–9177. [PubMed] [Google Scholar]
- Krag S. S., Robbins P. W. Sindbis envelope proteins as endogenous acceptors in reactions of guanosine diphosphate-[14C]Mannose with preparations of infected chicken embryo fibroblasts. J Biol Chem. 1977 Apr 25;252(8):2621–2629. [PubMed] [Google Scholar]
- Li E., Kornfeld S. Biosynthesis of lipid-linked oligosaccharides. Isolation and structure of a second lipid-linked oligosaccharide in Chinese hamster ovary cells. J Biol Chem. 1979 Apr 25;254(8):2754–2758. [PubMed] [Google Scholar]
- Natowicz M. R., Chi M. M., Lowry O. H., Sly W. S. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4322–4326. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R. The mannose 6-phosphate receptor of Chinese hamster ovary cells. Compartmentalization of acid hydrolases in mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10623–10627. [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R., Youle R. J., Murray G. J., Neville D. M., Jr The mannose 6-phosphate receptor of Chinese Hamster ovary cells. Isolation of mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10618–10622. [PubMed] [Google Scholar]
- Robey P. G., Neufeld E. F. Defective phosphorylation and processing of beta-hexosaminidase by intact cultured fibroblasts from patients with mucolipidosis III. Arch Biochem Biophys. 1982 Jan;213(1):251–257. doi: 10.1016/0003-9861(82)90459-3. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Weissmann B., Neufeld E. F. Direct demonstration of binding of a lysosomal enzyme, alpha-L-iduronidase, to receptors on cultured fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2331–2334. doi: 10.1073/pnas.76.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Distler J., Jourdian G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4289–4293. doi: 10.1073/pnas.78.7.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Biosynthetic intermediates of beta-glucuronidase contain high mannose oligosaccharides with blocked phosphate residues. J Biol Chem. 1980 Jul 25;255(14):6633–6639. [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R. Abnormal lipid-linked oligosaccharides in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):503–508. doi: 10.1016/0092-8674(79)90258-7. [DOI] [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Structural studies of phosphorylated high mannose-type oligosaccharides. J Biol Chem. 1980 Nov 25;255(22):10847–10858. [PubMed] [Google Scholar]
- Yoshima H., Nakanishi M., Okada Y., Kobata A. Carbohydrate structures of HVJ (Sendai virus) glycoproteins. J Biol Chem. 1981 Jun 10;256(11):5355–5361. [PubMed] [Google Scholar]
- von Figura K., Klein U. Isolation and characterization of phosphorylated oligosaccharides from alpha-N-acetylglucosaminidase that are recognized by cell-surface receptors. Eur J Biochem. 1979 Mar;94(2):347–354. doi: 10.1111/j.1432-1033.1979.tb12900.x. [DOI] [PubMed] [Google Scholar]



