Abstract
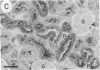
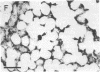
The distribution of a heavy metal binding protein, metallothionein, was studied immunocytochemically by using antimetallothionein antibody and the immunoperoxidase staining technique on histological sections of liver, kidney, intestine, lung, and testis from cadmium-treated rats. These tissues either accumulate heavy metals (e.g., liver, kidney, and testis) or are exposed to metal by ingestion or inhalation (intestine and lung). Staining for metallothionein was observed intracellularly in epithelial parenchymal cells of the liver and kidney; all hepatocytes and most renal tubular cells stained for the protein. Accumulation of metallothionein was not seen in connective tissue cells surrounding either blood vessels or renal tubules. Extracellular localization of metallothionein was also observed in the liver sinusoids and within the lumina of the renal tubules, suggesting a metal transport or excretory function for this protein. Surface columnar epithelial cells of the intestinal villi indicated the presence of metallothionein but connective tissue cells of the lamina propria were negative for the protein. The granular secretory Paneth cells of the small intestine also stained strongly for metallothionein as did respiratory epithelial cells of the lung. In the testis, metallothionein was detected in the Sertoli cells and interstitial cells but not within the spermatogonia. Sertoli cells are closely associated with the developing spermatogonia and appear to serve a nutritive role in spermatogenesis. Because of the secretory, absorptive, or nutritive function of the metallothionein-localizing cells in the organs studied, we suggest that metallothionein may be involved in metal storage or transport in addition to its commonly proposed detoxification role.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLIN M., ULLBERG S. THE FATE OF CD109 IN THE MOUSE. AN AUTORADIOGRAPHIC STUDY AFTER A SINGLE INTRAVENOUS INJECTION OF CD109CL2. Arch Environ Health. 1963 Dec;7:686–693. doi: 10.1080/00039896.1963.10663601. [DOI] [PubMed] [Google Scholar]
- Bremner I., Davies N. T. The induction of metallothionein in rat liver by zinc injection and restriction of food intake. Biochem J. 1975 Sep;149(3):733–738. doi: 10.1042/bj1490733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan S. E., Hidalgo H. A. Nuclear 115cadmium: uptake and disappearance correlated with cadmium-binding protein synthesis. Biochem Biophys Res Commun. 1976 Feb 9;68(3):858–866. doi: 10.1016/0006-291x(76)91224-9. [DOI] [PubMed] [Google Scholar]
- Chen R. W., Whanger P. D. Some immunochemical properties of rat liver and kidney metallothioneins. Biochem Med. 1980 Aug;24(50):71–81. doi: 10.1016/0006-2944(80)90089-7. [DOI] [PubMed] [Google Scholar]
- Failla M. L., Cousins R. J. Zinc accumulation and metabolism in primary cultures of adult rat liver cells. Regulation by glucocorticoids. Biochim Biophys Acta. 1978 Oct 18;543(3):293–304. doi: 10.1016/0304-4165(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Hidalgo H. A., Bryan S. E. Cadmium-115 bound to nuclear and cytoplasmic proteins. Toxicol Appl Pharmacol. 1977 Nov;42(2):319–327. doi: 10.1016/0041-008x(77)90008-4. [DOI] [PubMed] [Google Scholar]
- Karin M., Andersen R. D., Slater E., Smith K., Herschman H. R. Metallothionein mRNA induction in HeLa cells in response to zinc or dexamethasone is a primary induction response. Nature. 1980 Jul 17;286(5770):295–297. doi: 10.1038/286295a0. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D. Induction of metallothionein by adrenocortical steroids. Toxicology. 1981;20(4):275–279. doi: 10.1016/0300-483x(81)90034-2. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol. 1978 Mar;234(3):E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- Ohi S., Cardenosa G., Pine R., Huang P. C. Cadmium-induced accumulation of metallothionein messenger RNA in rat liver. J Biol Chem. 1981 Mar 10;256(5):2180–2184. [PubMed] [Google Scholar]
- Olafson R. W. Differential pulse polarographic determination of murine metallothionein induction kinetics. J Biol Chem. 1981 Feb 10;256(3):1263–1268. [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Isolation of an intestinal metallothionein induced by parenteral zinc. Biochem Biophys Res Commun. 1977 Mar 21;75(2):286–294. doi: 10.1016/0006-291x(77)91041-5. [DOI] [PubMed] [Google Scholar]
- Rózalski M., Kuziemska E., Wierzbicki R. Content of mercury in chromatin and level of metallothionein proteins in kidneys and liver of rats. Biochem Pharmacol. 1981 Aug 1;30(15):2177–2178. doi: 10.1016/0006-2952(81)90242-2. [DOI] [PubMed] [Google Scholar]
- Sandow M. J., Whitehead R. The Paneth cell. Gut. 1979 May;20(5):420–431. doi: 10.1136/gut.20.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh Z. A., Smith J. C. The biosynthesis of metallothionein rat liver and kidney after administration of cadmium. Chem Biol Interact. 1976 Dec;15(4):327–336. doi: 10.1016/0009-2797(76)90138-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sueda K., Onosaka S., Okahara K. Fate of 109Cd-labeled metallothionein in rats. Toxicol Appl Pharmacol. 1975 Aug;33(2):258–266. doi: 10.1016/0041-008x(75)90092-7. [DOI] [PubMed] [Google Scholar]
- Webb M. Biochemical effects of Cd 2+ -injury in the rat and mouse testis. J Reprod Fertil. 1972 Jul;30(1):83–98. doi: 10.1530/jrf.0.0300083. [DOI] [PubMed] [Google Scholar]