Abstract
Current landscape of treatment of castration-resistant prostate cancer (CRPC) has recently changed. Cabazitaxel, a new taxane with potential antineoplastic activity, has been approved by Food and Drug Administration (FDA) after docetaxel failure. In a phase III trial, cabazitaxel showed increased overall survival (OS) compared with mitoxantrone (15.1 vs. 12.7 mo, HR 0.70, 95% CI 0.59–0.83, p < 0.0001). Furthermore, chemotherapy is not the only strategy available: several studies have shown as CRPC remains dependent on androgen receptor function for growth. Abiraterone acetate, an irreversible inhibitor of CYP17, has also been approved by FDA after docetaxel failure. In a phase III trial comparing abiraterone acetate to placebo, abiraterone showed improvement in OS (14.8 vs. 10.4 mo, HR 0.65, 95% CI 0.54–0.77; p < 0.0001).
This review will discuss current options and the ongoing trials for second-line treatment of CRPC including chemotherapy, hormonal therapies, antiangiogenetic and immune strategies.
Keywords: CRPC, second-line therapy, antiangiogenic therapy, chemotherapy, hormone therapy, abiraterone acetate, cabazitaxel, MDV3100, TAK 700, sipuleucel-T
Introduction
In Europe, prostate cancer is the most common cancer among men with 382,000 new cases and 89,000 deaths annually.1 Androgen deprivation therapy (ADT) is the cornerstone for recurrent or metastatic prostate cancer, but almost all patients will develop progressive castration-resistant disease in 12–18 months.2
Mitoxantrone, estramustine and docetaxel are currently approved for first-line treatment of CRPC. Until 2004, mitoxantrone was the only therapy available because it showed improvement in quality of life, pain control and palliation of symptoms in two randomized trials, although not any benefit in OS over prednisone alone was reported.3,4
In 2004, two landmark phase III studies have established that docetaxel plus prednisone is the standard first-line therapy in CRPC. In TAX327 trial, docetaxel every three weeks demonstrated a survival benefit over mitoxantrone and weekly docetaxel (18.9 vs. 16.5 vs. 17.4 mo, respectively).5 An update analysis at three years confirmed an increased OS for docetaxel compared with mitoxantrone (19.2 mo vs. 16.3 mo, p = 0.004).6
The Southwest Oncology Group 99-16 study, comparing docetaxel plus estramustine to mitoxantrone plus prednisone, confirmed the increased OS for docetaxel arm (17.5 vs. 15.6 mo, p = 0.02).7 Furthermore, the addition of estramustine to docetaxel and prednisone did not provide any clinical advantage and more digestive and cardiovascular toxicities were reported.8
According to these data, docetaxel plus prednisone is considered the gold standard first-line chemotherapy for CRPC. Unfortunately, median OS for patients affected by metastatic prostate cancer is less than 20 months.
Recently, FDA approved cabazitaxel and abiraterone acetate as second-line therapies after docetaxel failure. Furthermore, other agents have been tested in this setting and interesting results have recently been published. In this article, we review the current options and the ongoing trials for second-line treatment of CRPC.
Methods
We performed a review of publications identified through searches of Medline/Pubmed from 2000 to the present, with particular focus on phase II/III trials. Abstracts from annual oncology meeting (e.g., American Society of Clinical Oncology [ASCO] and European Society of Medical Oncology [ESMO]) were taken into account. The ongoing phase II/III trials were searched from www.clinicaltrials.gov. Studies evaluating radiotherapy and systemic isotope therapy were not considered.
Chemotherapy
Satraplatin
Satraplatin is an oral platinum compound that forms platinum-DNA adducts and cross links but is not susceptible to some cisplatin resistance mechanisms. Phase I trials have tested satraplatin in different types of tumors and have explored several different dosing schedules, from daily dose for 5 d to a single dose every 3 weeks. Because of myelosuppression, the recommended dose and schedule is 100 and 120 mg/mq, for previously treated and untreated patients, for 5 d repeated every 4–5 weeks.9
A phase II trial evaluated safety and antitumor activity of satraplatin in 39 patients affected by CRPC.10 Satraplatin was administered at 120 mg/mq for 5 d every 4 weeks. 26% of patients had prostate-specific antigen (PSA) decline > 50% and 10% of patients with measurable disease had partial response (PR). Most frequent grade 3–4 toxicities were hematologic (54% thrombocytopenia, 52% neutropenia, 24% anemia) and gastrointestinal (28% diarrhea, 16% vomiting and 13% nausea).
The important side effects reported in the phase II led to 2 phase III studies based on different dose and schedule. The first one was prematurely closed to further accrual by the sponsoring company.11 In this trial, satraplatin at dose of 100 mg/mq for 5 d every 5 weeks plus prednisone was compared with prednisone alone as first-line chemotherapy in CRPC. The ad hoc analysis of 50 enrolled patients showed improvement in progression free survival (PFS; 5.2 vs. 2.5 mo; HR 0.50, 95% CI 0.28–0.92, p = 0.02) and OS (14.9 vs. 11.9 mo) for satraplatin arm but the advantage in OS was not statistically significant due to the small sample size. These data led to the SPARC trial where satraplatin was compared with placebo on 950 patients affected by CRPC progressing after one prior chemotherapy. They were randomly assigned (2:1) to receive satraplatin 80 mg/mq for 5 d every 35 d or placebo, both plus prednisone 10 mg daily.12 Crossover between treatment arms was not allowed. Primary endpoints of the study were OS and PFS, secondary endpoint was time to pain progression (TPP), exploratory endpoints were PSA response rate (PSA RR), pain response rate (PRR) and objective tumor response rate (TRR). PFS was 11.1 vs. 9.7 weeks, p < 0.001, HR 0.67 (95% CI 0.57–0.77, p < 0.001) and median TPP was 66.1 vs. 22.3 weeks, p < 0.001, both higher in satraplatin arm. PSA RR (25.4% vs. 12.2%, p < 0.001), PRR (24.2% vs. 13.8%, p = 0.005) and TRR (8% vs. 0.7%, p = 0.002) were all in favor of satraplatin. Median OS was 61.3 weeks for satraplatin and 61.4 weeks for placebo (HR 0.98, 95% CI 0.84–1.15, p = 0.80). A quality-of-life assessment was not part of the trial, but satraplatin showed a positive effect on pain control.
Treatment was well tolerated: hematologic and gastrointestinal toxicities were more frequent adverse events in satraplatin group, but in contrast to other platinum analogs, no significant worsening of renal function or neuropathy occurred with satraplatin.
Because of the lack of survival benefit, the sponsor decided to not pursue on the drug’s development in prostate cancer.
Cabazitaxel
Cabazitaxel is a novel tubulin-binding taxane that showed antitumor activity in models resistant to docetaxel and paclitaxel. Cabazitaxel binds to and stabilizes tubulin and unlike other taxane compounds, this agent has decreased propensity for P-glycoprotein-mediated drug resistance. Cabazitaxel side effect profile is similar to that reported for other taxanes, with neuropathy and neutropenia being the most commonly reported toxicities.
A phase I trial evaluated cabazitaxel in 25 patients affected by advanced solid tumors refractory to conventional therapies.13 Patients were treated with a 3-weekly schedule at 4 dose levels (from 10 mg/mq to 25 mg/mq). Neutropenia was the principal dose-limiting toxicity and the recommended dose for further studies was 20 mg/mq. PR was documented in two patients, both affected by metastatic prostate cancer and in one case after docetaxel failure.
The lack of effective second-line therapies for CRPC and the encouraging results of the phase I trial conducted directly to a phase III study.
The TROPIC is a randomized, multicenter, open-label, phase III trial undertaken in 26 countries comparing cabazitaxel with mitoxantrone as second-line therapy in CRPC.14 In this trial 755 men affected by metastatic prostate cancer after progression with taxane-based chemotherapy were enrolled. Although the recommended dose was 20 mg/mq in the phase I study, 25 mg/mq of cabazitaxel was used in the phase III trial based on a previous phase II study conducted in younger women with metastatic breast cancer.15
Patients were randomly assigned to treatment groups: 378 received cabazitaxel 25 mg/mq every 3 weeks and 377 received mitoxantrone 12 mg/mq every 3 weeks, both plus prednisone 10 mg daily. Premedication consisting of corticosteroids, histamine H2-antagonist and antihistamine was administered 30 min before cabazitaxel. The primary endpoint of the study was OS, the secondary endpoints were PFS, PSA RR, time to PSA progression (TTPP), TRR, PRR, TPP, time to tumor progression (TTP) and safety. After a median follow-up of 12.8 mo, cabazitaxel showed an improvement in OS (15.1 vs. 12.7 mo) with a 30% reduction in relative risk of death (HR 0.70, 95% CI 0.59–0.83, p < 0.0001). PFS (2.8 vs. 1.4 mo, p < 0.0001), PSA RR (39.2% vs. 17.8%, p = 0.0002), TRR (14.4% vs. 4.4%, p = 0.0005), TTP (8.8 vs. 5.4 mo, p < 0.0001) and TTPP (6.4 vs. 3.1 mo, p = 0.001) were all in favor of cabazitaxel arm. PRR (9.2% vs. 7.7%, p = 0.63) and TPP were similar in the two groups.
Grade 3–4 related adverse events were: anemia, neutropenia (82% for cabazitaxel vs. 58% for mitoxantrone, febrile 8% vs. 1% respectively) and diarrhea (23% vs. < 1%). Death within 30 d from the last infusion was reported in 5% of patients treated with cabazitaxel and 2% treated with mitoxantrone, respectively. The most frequent cause of death in the cabazitaxel group was neutropenia and its clinical consequences. The higher risk of death within 1 mo from the last drug dose and high rates of neutropenia and diarrhea suggest that cabazitaxel treatment requires careful monitoring. Prophylactic use of granulocyte colony-stimulating growth factor and dose modifications can be considered correct strategies to manage side effects. To assess whether a lower dose of cabazitaxel has a better safety profile with the same efficacy, a phase III trial has been designed. The PROSELICA trial (NCT01308580) compares cabazitaxel at 25 mg/mq vs. cabazitaxel at 20 mg/mq, both every 3 weeks in combination with prednisone. The primary endpoint of the study is OS and the secondary endpoint is PFS.
Considering the improvement in OS and the manageable toxicity profile, cabazitaxel has been approved by FDA as second-line treatment for CRPC.
Hormonal Strategies
After an initial response to ADT, about 90% of patients with metastatic prostate cancer register an increase of serum PSA concentrations, with or without radiological progression of disease, and develop site-related symptoms which indicate the androgen-receptor (AR) reactivation after a time variable of length from months to several years.16 These events characterize a different phase of disease, referred as CRPC, with the development of a clone of cells insensible to the ADT.17
Over the past decade, several studies have shown that CRPC remains dependent on AR function for growth. The molecular basis for AR reactivation remains unclear, but some possible mechanisms include increased AR expression, AR mutations that enhance activation by weak androgens and AR antagonists, increased expression of transcriptional coactivator proteins and activation of signal transduction pathways that can enhance AR responses to low levels of androgens. Moreover AR may be activated by other endogenous steroids as those produced by adrenal gland.18,19
Several molecules with antiandrogen activity are currently under investigation in CRPC patients who have received or not docetaxel: abiraterone acetate, MDV3100 and TAK-700.
Abiraterone acetate
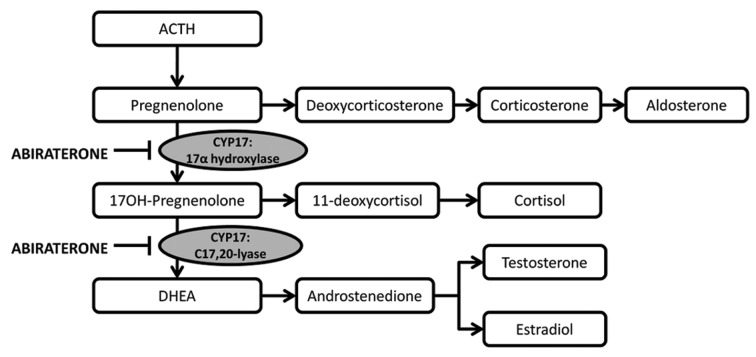
Abiraterone acetate is an orally active acetate salt of the steroidal compound abiraterone with antiandrogen activity (Fig. 1). Abiraterone inhibits the enzymatic activity of steroid 17alpha-monooxygenase (17alpha-hydrolase/C17,20 lyase complex), a member of the cytochrome P450 family that catalyzes the 17alpha-hydroxylation of steroid intermediates involved in testosterone synthesis. Administration of this agent may suppress testosterone production from testes and adrenal glands to castrate-range levels.
Figure 1. Mechanism of action of Abiraterone acetate.52
Preclinical data revealed as 17-(3-pyridyl)androsta-5,16-dien-3 β-ol (CB7598) is a potent steroidal inhibitors of cytochrome P450(17) α, a key enzyme for androgen biosynthesis.20
Two phase I studies demonstrated safety and activity of abiraterone acetate in patients with CRPC.21,22 A total of 54 patients were enrolled and received once daily orally escalating dose of abiraterone: from 250 to 1000 mg in patients previously treated with ketoconazole and from 250 to 2000 mg in ketoconazole-naïve patients. The most common adverse events were fatigue, hypertension, headache, nausea and diarrhea, which were predominantly grade 1–2. The most frequent treatment-related grade 3–4 toxicities were hypertension, hypokalemia, constipation, diarrhea, muscular weakness and arthralgia.
The pharmacokinetics analysis showed as abiraterone acetate was rapidly converted to abiraterone, confirming the intrapatient variability of metabolism and the influence of food. Particularly, the absorption was significantly extended after food intake and drug exposure was significantly increased (by 4.4-fold) by high-fat content food, compared with fasting administration. These studies did not show any cross-resistance between ketoconazole and abiraterone acetate and the recommended dose for subsequent phase II trials was 1000 mg daily, supporting the once daily administration based on the pharmacodinamics and pharmacokinetics data.
Three phase II studies have been conducted in CRPC patients. In a first study conducted in patients who did not receive first-line docetaxel chemotherapy, abiraterone acetate showed a significant clinical activity, with a ≥ 50% PSA reduction in 67% of patients and PR according to Response Evaluation Criteria In Solid Tumors (RECIST) Criteria in 37.5% of patients. Furthermore, the addition of dexamethasone at disease progression reversed resistance in 33% of patients.23
The other two studies tested the activity of abiraterone acetate in 105 patients with docetaxel-treated CRPC.24,25 The first trial reported a ≥ 50% PSA decline from baseline in 51% of patients. Moreover ≥ 30% or ≥ 90% PSA decline was reported in 68% and 15% of patients respectively; 27% of patients with measurable disease had PR defined by RECIST criteria.24
The second trial, evaluating the activity of abiraterone acetate plus prednisone, reported a ≥ 50% PSA decline in 36% of patients and a ≥ 30% or ≥ 90% PSA decline in 47% and 16% of patients respectively; PR was observed in 18% of patients with soft tissue measurable lesions. Both studies showed a period of 169 d TTPP.25
The overmentioned trials demonstrated that treatment with abiraterone is well tolerated and toxicities could be influenced by the concomitant administration of steroids. Abiraterone acetate alone caused hypokalemia, hypertension and fluid retention in 55%, 17% and 15% of patients, respectively; on the contrary, the combined modality caused hypokalemia and fluid retention in 5% and 7% of patients, respectively. Other reported toxicities in both studies such as fatigue, nausea, anorexia, headache and AST increase were not influenced by concomitant administration of steroids.
Recently the results from a pre-specified interim analysis of the phase III study have been published.26 The COU-AA-301 is a multinational, randomized, double blind, placebo-controlled trial conducted in 13 countries. The trial enrolled 1195 patients whose metastatic CRPC had previously been treated with one or two chemotherapeutic agents, included docetaxel. Patients were randomized 2:1 to receive abiraterone acetate 1000 mg once daily or placebo, each with prednisone 5 mg twice daily continuously. OS was the primary endpoint of the study and the secondary were TTPP, PSA RR, radiographic progression-free survival (rPFS) and TRR.
The 797 patients in the abiraterone acetate arm reported a median OS of 14.8 mo compared with 10.4 mo of the 398 patients who received placebo (HR 0.65, 95% CI 0.54–0.77, p < 0.0001). Moreover, significant differences emerged between placebo and treatment groups for all secondary endpoints, including TTPP (10.2 vs. 6.6 mo, p < 0.0001), rPFS (5.6 vs. 3.6 mo, p < 0.0001), PSA RR (29% vs. 6%, p < 0.0001) and TRR (14% vs. 3%, p < 0.001).26 The most severe abiraterone-related adverse events were fluid retention (31% vs. 22% in placebo arm), hypokalemia (17% vs. 8%) and cardiac disorders (13% vs. 11%). Patients receiving placebo were permitted to cross over to the abiraterone arm. On the basis of these results, abiraterone acetate has been approved by FDA for CRPC after docetaxel failure on April 2011.
Given its efficacy and manageable toxicities, ongoing trials explore the effects of abiraterone in different setting. A second large phase III trial is ongoing involving comparison of abiraterone acetate 1000 mg with placebo, both with prednisone, in CRPC patients who have not yet received docetaxel (NCT00887198). A phase II trial is evaluating the role of abiraterone and prednisone with an LHRH agonist as neoadjuvant and concurrent therapy with external beam radiation in patients with intermediate-risk or high-risk localized prostate cancer (NCT01023061). The effect on androgen deprivation by LHRH and abiraterone combination is currently under investigation in patients with intermediate-risk or high-risk prostate cancer suitable for prostatectomy in a nonrandomized (NCT00924469) and in a randomized (NCT01088529) phase II trial, comparing the combined therapy with the LHRH alone.
Considering the feasibility and safety of combination with LHRH agonists, future combinations with new-generation antiandrogen molecules (MDV3100, TAK-700, etc.) may be feasible as well as the possible combination with chemotherapy.27,28
MDV3100
MDV3100 is an orally bioavailable, organic, non-steroidal small molecule targeting the androgen receptor with potential antineoplastic activity. MDV3100 inhibits the activity of prostate cancer cell ARs, which may result in prostate cancer cell proliferation reduction and, correspondingly, in PSA levels decline. This compound has been demonstrated to bind ARs in castration resistant xenograft prostate cancer cells with five to 8-fold greater affinity than bicalutamide, did not show agonist activity and antagonized the induction of PSA and other genes regulated by ARs, impairing DNA binding.29
A multicenter phase I-II trial enrolled patients with histologically proven prostate cancer and progressive castration-resistant disease, defined by the combination of castrate levels of testosterone (< 1.7 nmol/L), and a rising PSA with or without detectable metastases.30
A total of 140 patients were enrolled, with a median age of 68 y and radiological evidence of metastasis in the majority of patients. Both chemotherapy-naïve and previously treated patients were enrolled.
MDV3100 was associated with tumor regression and stable disease in 22% and 49% of patients with soft tissue disease, while stable disease was shown in 56% of patients with bone metastases. Median TTPP was 27 weeks and median TTP was 47 weeks. PSA decline ≥ 50% was observed in 62% of chemotherapy-naïve patients and in 51% of post-chemotherapy patients.
The most common adverse events were fatigue, nausea, constipation, diarrhea and anorexia. Considering that only 1% of patients treated at 240 mg or below discontinued treatment for an adverse event, it was chosen as maximum tolerated dose.
The AFFIRM trial is a randomized, double-blind, multinational phase III trial comparing MDV3100 (160 mg orally per day) vs. placebo in 1199 patients with CRPC previously treated with at least one docetaxel-based chemotherapy.31 Corticosteroids were not required but allowed. The primary endpoint of the study was OS, and secondary endpoints included PSA RR, Soft tissues objective response rate, circulating tumor cells (CTC) count conversion rate, rPFS, TTPP and time to first skeletal-related event. Recently, a planned interim analysis revealed that estimated median OS is 18.4 mo for men in the MDV3100 arm compared with 13.6 mo for men treated with placebo, with a reduction of the risk of death by 37% (p < 0.0001, HR 0.631). PSA RR (54% vs. 1.5%, p < 0.0001), TTPP (8.3 vs. 3.0 mo, p < 0.0001), Soft Tissue Response Rate (28.9% vs. 3.8%, p < 0.0001), rPFS (8.3 vs. 2.9 mo, p < 0.0001) were all in favor of MDV3100. MDV3100 was also well tolerated and the most frequent adverse events were fatigue (33.6% vs. 29.1% in the placebo arm), cardiac disorders (6.1% vs. 7.5%), liver function tests abnormalities (1% vs. 1.5%) and seizure (0.6% vs. 0%)
As a result, the trial’s Independent Data Monitoring Committee recommended that AFFIRM trial be stopped early and that men who were receiving placebo be offered MDV3100.31
Further ongoing trials evaluate MDV3100 in different setting. An ongoing trial will determine the effect of MDV3100 on the androgen-signaling pathway in order to identify potential predictors of response or resistance to therapy in patients affected by prostate cancer with bone metastasis, already treated with ADT (NCT01091103). MDV3100 is compared with placebo in patients with metastatic CRPC who have failed ADT but not yet received chemotherapy (PREVAIL trial, NCT01212991). Furthermore, a phase II trial is testing the efficacy and safety of MDV3100 in patients with prostate cancer for whom ADT is indicated (except when indicated in a neoadjuvant/adjuvant therapy), with non-castrate level of testosterone (≥ 8 nmol/L) and PSA ≥ 2 ng/mL (NCT01302041). Another phase II trial evaluates MDV3100 vs. bicalutamide in men with metastatic prostate cancer who have failed medical or surgical castration (NCT01288911).
TAK-700
TAK-700 is an orally bioavailable non-steroidal androgen synthesis inhibitor of steroid 17alpha-monooxygenase (17,20 lyase) with potential antiandrogen activity. TAK-700 binds to and inhibits the steroid 17alpha-monooxygenase in both the testes and adrenal glands, thereby inhibiting androgen production.
TAK-700 has shown impressive activity in a phase I/II study.32,33 In the first portion of the study,32 26 patients affected by metastatic CRPC received TAK-700 at five doses levels (from 100 to 600 mg BID, plus prednisone 10 mg daily). All patients receiving at least 300 mg BID had a PSA decrease. The most common adverse events were fatigue, nausea, constipation, anorexia and vomiting.
The second portion of the study33 is a open-label, multicenter, phase II trial evaluating four additional dose cohorts (300 mg BID, 400 and 600 mg BID plus prednisone 10 mg daily, 600 mg QD). Patients enrolled have not received prior chemotherapy, have baseline testosterone < 50 ng/dL and PSA ≥ 5 ng/ml. TAK-700 appears well tolerated and active in all groups. PSA decline ≥ 50% at 12 weeks was observed in 63%, 50%, 41%, and 60% of patients in the 300 mg BID, 400 and 600 mg BID plus prednisone and 600 mg QD groups. At 12 weeks, median dehydroepiandrosterone sulfate, testosterone levels and CTC numbers decreased from baseline in all groups.
An ongoing phase III trial (NCT01193257) evaluates TAK-700 vs. placebo, both with prednisone, in patients with CRPC who have progressed following taxanes-based therapy.
Antiangiogenic and Immune Strategies
Antiangiogenic therapies
Tumor angiogenesis is a well-known, crucial target in many solid tumors such as colon, lung, breast and renal cancer. Antiangiogenic therapy represents as well a fashionable approach in advanced prostate cancer treatment. Main markers of angiogenesis such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and transforming growth factor (TGF) are highly expressed in prostate carcinoma compared with nonmalignant prostate cells.34 Moreover, angiogenesis (measured as microvessel density; MVD) seems to be correlated with tumor stage, grade and clinical course in prostate cancer.35,36
Some antiangiogenic strategies have been studied and are currently under investigation in patients with CRPC. Tyrosine kinases inhibitors including sorafenib, sunitinib and cediranib or monoclonal antibodies against circulating VEGF have been tested in phase II-III trials.
Sorafenib is a synthetic compound targeting RAF kinase, a critical component of the RAF/MEK/ERK signaling pathway that controls cell division and proliferation. In addition, sorafenib inhibits vascular endothelial growth factor receptor 2/platelet derived growth factor receptor β (VEGFR-2/PDGFR-β) signaling cascade, thereby blocking tumor angiogenesis. In prostate cancer cell lines, sorafenib inhibits ERK phosphorylation and induces apoptosis.37
Three phase II38-40 tested sorafenib at the dose of 400 mg orally twice daily in CRPC patients, but only one included patients that have received a previous line of chemotherapy.38 In this trial the primary endpoint was disease progression, defined as either the appearance of new lesions or unidimensional or bidimensional tumor measurements increasing > 50% or increase of PSA. Results showed reduction of bone lesions despite PSA raising, reporting manageable side effects. In the study’s conclusions, it is hypothesized an activity only in patients presenting primarily with metastatic bone lesions whereas PSA seems to be a poor marker of disease progression.
Sunitinib is a tyrosine kinase inhibitor with potential antineoplastic activity in prostate cancer. It acts blocking the tyrosine kinase activity of VEGFR2, PDGFR-b and c-kit, thereby inhibiting angiogenesis and cell proliferation. Sunitinib is currently considered the best therapeutic approach for patient with advanced renal cell carcinoma and for the treatment of gastrointestinal stromal tumors after disease progression or intolerance to imatinib mesylate.
Preclinical evidence of sunitinib activity in prostate cancer cells41,42 leaded to a phase II trial in patients with CRPC after a docetaxel-based chemotherapy.
The primary end-point of the study was reached, with a PFS at 12 weeks of 75.8% and a median PFS of 19.4 weeks. 12% of patients had a ≥ 50% PSA decline and 21% had a ≥ 30% PSA decline compared with baseline; decrease of measurable disease and of pain score were also reported. A favorable safety profile characterized by fatigue, anemia, nausea, anorexia and neutropenia as the most common toxic effects was also demonstrated.43 Despite these encouraging results, the subsequent phase III trial was prematurely closed due to lack of any advantage compared with placebo.
Bevacizumab, a recombinant humanized monoclonal antibody directed against VEGF, was also tested in CRPC patients. Particularly, at the 2010 ASCO Annual Meeting was reported that bevacizumab plus docetaxel/prednisone prolongs PFS as first-line therapy but is unable to prolong OS compared with docetaxel/prednisone in a phase III trial.44
Moreover, a combined therapy with docetaxel and bevacizumab was administered to a group of highly pretreated patients with CRPC in a non randomized phase II trial.45 The combination showed a favorable safety profile with grade 4 toxicity limited to neutropenia and thrombocytopenia and a ≥ 50% PSA decline in 55% of patients. Objective responses were observed in 37.5% of patients with measurable disease.
In conclusion, angiogenesis remain an attractive target in prostate cancer although clinical trials did not show any encouraging result. Current data are extremely confused due to heterogeneity of studied populations and of endpoints used. Probably, deeper insights in prostate cancer biology and a better understanding of the optimal timing of the angiogenic switch may improve the clinical activity of these therapeutic strategies in CRPC patients.
Immune strategies
The IMPACT study showed first as therapy with the autologous vaccine sipuleucel-T yielded an improvement in OS compared with placebo in CRPC patients.
Sipuleucel-T is an active cellular immunotherapy, a type of therapeutic cancer vaccine, consisting of autologous peripheral-blood mononuclear cells, including antigen-presenting cells, that have been activated ex vivo with a recombinant fusion protein (PA2024). PA2024 consists of a prostate antigen, prostatic acid phosphatase, that is fused to granulocyte-macrophage colony-stimulating factor, an immune cell activator.
Although the IMPACT study was designated to assess Sipuleucel-T in first-line setting, the trial enrolled patients who had received docetaxel (15%). The results confirmed an improvement in OS (25.8 vs. 21.7 mo, HR 0.78, 95% CI 0.61–0-98, p = 0.03) for the whole group.46
Conclusions
All these data show as the traditional approach to CRPC progressed after docetaxel in first-line therapy is changing. Cabazitaxel, abiraterone acetate, Sipuleucel-T and MDV3100 have demonstrated improvement in OS in second-line setting (Table 1).
Table 1. Clinical trials in CRPC after docetaxel progression.
| Class of Drug | Molecule | Phase study | Patients | Comparator | PSA-RR (%) |
ORR (%) |
PFS (months) | OS (months) |
HR |
|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy |
Satraplatin + prednisonea |
III |
950 (2:1) |
Placebo + prednisone |
25.4 vs. 12.4 p < 0.001 |
8.0 vs. 0.7 p = 0.002 |
11.1 vs. 9.7 weeks p = 0.001 |
66.1 vs. 62.9 weeks p = 0.8 |
0.91 (95% CI, 0.72–1.14) |
| Cabazitaxel + prednisone |
III |
755 (1:1) |
Mitoxantrone + prednisone |
39.2 vs. 17.8 p = 0.0002 |
14.4 vs. 4.4 p = 0.0005 |
2.8 vs. 1.4 p = 0.0001 |
15.1 vs. 12.17 p < 0.0001 |
0.70 (95% CI, 0.59–0.83) |
|
| Immunotherapy |
Sipuleucelb |
III |
512 (2:1) |
Placebo |
2.6 vs. 1.3 |
N.A. |
3.7 vs. 3.6 p = 0.63 |
25.8 vs. 21.7 p = 0.02 |
0.76 (95% CI, 0.61–0.95) |
| Hormonal | Abiraterone + prednisone |
III |
1195 (2:1) |
Placebo + prednisone |
29.0 vs. 6.0 p < 0.001 |
14.0 vs. 3.0 p < 0.001 |
5.6 vs. 3.6 p < 0.001 |
14.8 vs. 10.9 p < 0.001 |
0.67, (95% CI, 0.58–0.78) |
| MDV3100 ± prednisone |
III | 1199 (2:1) |
Placebo ± prednisone |
54.0 vs. 1.5 p < 0.0001 |
28.9 vs. 3.8 p < 0.0001 |
8.3 vs. 2.9 p < 0.0001 |
18.4 vs. 13.6 p < 0.0001 |
0.63 (95% CI, 0.53-0.75) |
Abbreviations: CRPC, castration resistant prostate cancer; PSA-RR, PSA response rate (≥ 50%); ORR, overall response rate; PFS, progression free survival; OS, overall survival; HR, hazard ratio (for OS); CI, confidence interval; aonly 51% of patients have received docetaxel; bonly 14.4% of patients have received docetaxel.
Although new drugs are available, many questions should be resolved as the best sequence after docetaxel failure or the selection of appropriate predictive factors of response to second-line therapies.
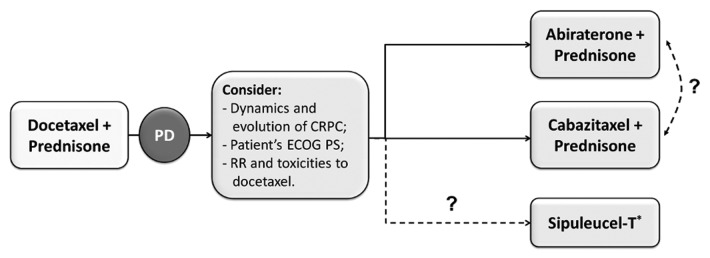
Further clinical trials are needed to better understand which patients will most benefit from a second-line cytotoxic chemotherapy rather than hormonal approaches. Probably, patient’s conditions, response and toxicities reported with previous therapies and the history of disease may be the most useful parameters in clinical practice before that new guidelines will be published. In asymptomatic patients, timing of treatment is not clear and it should be tailored individually (Fig. 2).47 Symptomatic patients need a rapid response rate to improve pain and disease related symptoms. For them, both cabazitaxel and abiraterone should be considered an appropriate treatment, reporting the same TRR (14.4% for cabazitaxel and 14% for abiraterone). Patients with an ECOG Performance Status of 2 represent only a small part (about 10%) of the study population of the main phase III trials. For them, best supportive care is still a feasible option. Recent data have demonstrated that a previous duration of prostate cancer sensitivity to ADT ≥ 16 mo is a predictive factor for efficacy of subsequent endocrine manipulations in patients with CRPC.48
Figure 2. Possible algorithm for treatment of docetaxel progressed CRPC.52 PD = progression of disease; CRPC = castration resistant prostate cancer. ECOG PS = Eastern Cooperative Oncology Group. Performance Status; RR = Response Rate. * Unlike other drugs approved for CRPC patients progressing during or after docetaxel-based chemotherapy, Sipuleucel-T has been approved for patients with metastatic, asymptomatic or minimally symptomatic, hormone-refractory prostate cancer.
Furthermore, new biomarkers predictive of efficacy have to be evaluated. Until now, PSA has been the most studied biomarker of disease evolution for prostate cancer, but some data suggest that it is not a surrogate of survival in all phases of disease.49 Recently, there is a strong interest for CTC and it has been demonstrated that pre-treatment CTC count is an independent predictor of OS in CRPC.50 The role of CTC count in second-line setting is still under investigation, but it appears promising.51
Further trials have to be designed to better understand how to use the new agents in sequence or in combination for treating CRPC. Results of the ongoing trials evaluating hormonal treatments after ADT failure (NCT00887198 for abiraterone and PREVAIL trial for MDV3100) are awaited to better define the role of docetaxel in CRPC.
Nowadays we have more opportunities than in the past, so next challenge should be to find out the best sequential therapy after docetaxel failure and a possible third-line for selected patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- CRPC
castration-resistant prostate cancer
- FDA
Food and Drug Administration
- OS
overall survival
- HR
hazard ratio
- ADT
androgen deprivation therapy
- ASCO
American Society of Clinical Oncology
- ESMO
European Society of Medical Oncology
- PSA
prostate-specific antigen
- PR
partial response
- PFS
progression-free survival
- CI
confidence interval
- TPP
time to pain progression
- PSA RR
PSA response rate
- PRR
pain response rate
- TRR
objective tumor response rate
- TTPP
time to PSA progression
- TTP
time to tumor progression
- AR
androgen-receptor
- RECIST
Response Evaluation Criteria In Solid Tumors
- rPFS
radiographic progression-free survival
- LHRHa
luteinizing hormone-releasing hormone agonist
- CTC
circulating tumor cells
- BID
bis in die
- QD
quoque die
- VEGF
vascular endothelial growth factor
- PDGF
platelet derived growth factor
- TGF
transforming growth factor
- MVD
microvessel density
- ECOG
Eastern Cooperative Oncology Group
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/21188
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Lassi K, Dawson NA. Emerging therapies in castrate-resistant prostate cancer. Curr Opin Oncol. 2009;21:260–5. doi: 10.1097/CCO.0b013e32832a1868. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17:2506–13. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 7.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 8.Machiels JP, Mazzeo F, Clausse M, Filleul B, Marcelis L, Honhon B, et al. Prospective randomized study comparing docetaxel, estramustine, and prednisone with docetaxel and prednisone in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2008;26:5261–8. doi: 10.1200/JCO.2008.16.9524. [DOI] [PubMed] [Google Scholar]
- 9.McKeage MJ, Raynaud F, Ward J, Berry C, O’Dell D, Kelland LR, et al. Phase I and pharmacokinetic study of an oral platinum complex given daily for 5 days in patients with cancer. J Clin Oncol. 1997;15:2691–700. doi: 10.1200/JCO.1997.15.7.2691. [DOI] [PubMed] [Google Scholar]
- 10.Latif T, Wood L, Connell C, Smith DC, Vaughn D, Lebwohl D, et al. Phase II study of oral bis (aceto) ammine dichloro (cyclohexamine) platinum (IV) (JM-216, BMS-182751) given daily x 5 in hormone refractory prostate cancer (HRPC) Invest New Drugs. 2005;23:79–84. doi: 10.1023/B:DRUG.0000047109.76766.84. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg CN, Whelan P, Hetherington J, Paluchowska B, Slee PH, Vekemans K, et al. Genitourinary Tract Group of the EORTC Phase III trial of satraplatin, an oral platinum plus prednisone vs. prednisone alone in patients with hormone-refractory prostate cancer. Oncology. 2005;68:2–9. doi: 10.1159/000084201. [DOI] [PubMed] [Google Scholar]
- 12.Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431–8. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 13.Mita AC, Denis LJ, Rowinsky EK, Debono JS, Goetz AD, Ochoa L, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–30. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 14.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. TROPIC Investigators Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 15.Pivot X, Koralewski P, Hidalgo JL, Chan A, Gonçalves A, Schwartsmann G, et al. A multicenter phase II study of XRP6258 administered as a 1-h i.v. infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann Oncol. 2008;19:1547–52. doi: 10.1093/annonc/mdn171. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61:332–53. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Steineck G, Kelly WK. Hormone-refractory (D3) prostate cancer: refining the concept. Urology. 1995;46:142–8. doi: 10.1016/S0090-4295(99)80182-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol. 2009;10:981–91. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–73. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 21.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 22.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–8. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. COU-AA-301 Investigators Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Veldhuizen PJ, Reed G, Aggarwal A, Baranda J, Zulfiqar M, Williamson S. Docetaxel and ketoconazole in advanced hormone-refractory prostate carcinoma: a phase I and pharmacokinetic study. Cancer. 2003;98:1855–62. doi: 10.1002/cncr.11733. [DOI] [PubMed] [Google Scholar]
- 28.Figg WD, Woo S, Zhu W, Chen X, Ajiboye AS, Steinberg SM, et al. A phase I clinical study of high dose ketoconazole plus weekly docetaxel for metastatic castration resistant prostate cancer. J Urol. 2010;183:2219–26. doi: 10.1016/j.juro.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. J Clin Oncol. 2012;30 [Google Scholar]
- 32.Dreicer R, Agus DB, MacVicar GR, Wang J, MacLean D, Stadler WM. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostate cancer: A phase I/II, open-label study. J Clin Oncol. 2010; 28 [Google Scholar]
- 33.Agus DB, Stadler WM, Shevrin DH, Hart L, Mac Vicar GR, Hamid O, et al. Safety, efficacy, and pharmacodynamics of the investigational agent ortonel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): updated data from a phase I/II study. J Clin Oncol. 2012;30 [Google Scholar]
- 34.Ryan CJ, Lin AM, Small EJ. Angiogenesis inhibition plus chemotherapy for metastatic hormone refractory prostate cancer: history and rationale. Urol Oncol. 2006;24:250–3. doi: 10.1016/j.urolonc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Strohmeyer D, Rössing C, Strauss F, Bauerfeind A, Kaufmann O, Loening S. Tumor angiogenesis is associated with progression after radical prostatectomy in pT2/pT3 prostate cancer. Prostate. 2000;42:26–33. doi: 10.1002/(SICI)1097-0045(20000101)42:1<26::AID-PROS4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Ullén A, Farnebo M, Thyrell L, Mahmoudi S, Kharaziha P, Lennartsson L, et al. Sorafenib induces apoptosis and autophagy in prostate cancer cells in vitro. Int J Oncol. 2010;37:15–20. doi: 10.3892/ijo_00000648. [DOI] [PubMed] [Google Scholar]
- 38.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–14. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 39.Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br J Cancer. 2007;97:1480–5. doi: 10.1038/sj.bjc.6604064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chi KN, Ellard SL, Hotte SJ, Czaykowski P, Moore M, Ruether JD, et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann Oncol. 2007;19:746–51. doi: 10.1093/annonc/mdm554. [DOI] [PubMed] [Google Scholar]
- 41.Cumashi A, Tinari N, Rossi C, Lattanzio R, Natoli C, Piantelli M, et al. Sunitinib malate (SU-11248) alone or in combination with low-dose docetaxel inhibits the growth of DU-145 prostate cancer xenografts. Cancer Lett. 2008;270:229–33. doi: 10.1016/j.canlet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 43.Sonpavde G, Periman PO, Bernold D, Weckstein D, Fleming MT, Galsky MD, et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol. 2010;21:319–24. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- 44.Kelly WK, Halabi S, Carducci MA, George DJ, Mahoney JF, Stadler WM, et al. A randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): Survival results of CALGB 90401. J Clin Oncol. 2010;28 doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Lorenzo G, Figg WD, Fossa SD, Mirone V, Autorino R, Longo N, et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol. 2008;54:1089–1096. doi: 10.1016/j.eururo.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 46.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 47.Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–83. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 48.Loriot Y, Massard C, Albiges L, Di Palma M, Blanchard P, Bossi A, et al. Personalizing treatment in patients with castrate-resistant prostate cancer: A study of predictive factors for secondary endocrine therapies activity. J Clin Oncol. 2012;30 [Google Scholar]
- 49.Collette L, Burzykowski T, Schröder FH. Prostate-specific antigen (PSA) alone is not an appropriate surrogate marker of long-term therapeutic benefit in prostate cancer trials. Eur J Cancer. 2006;42:1344–50. doi: 10.1016/j.ejca.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 50.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 51.Scher HI, Heller G, Molina A, Kheoh TS, Attard G, Moreira J, et al. Evaluation of circulating tumor cell (CTC) enumeration as an efficacy response biomarker of overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC): Planned final analysis (FA) of COU-AA-301, a randomized double-blind, placebo-controlled phase III study of abiraterone acetate (AA) plus low-dose prednisone (P) post docetaxel. J Clin Oncol. 2011;29 [Google Scholar]
- 52.Iacovelli R, Palazzo A, Procopio G, Gazzaniga P, Cortesi E. Abiraterone acetate in castration-resistant prostate cancer. Anticancer Drugs. 2011 doi: 10.1097/CAD.0b013e32834e696c. [DOI] [PubMed] [Google Scholar]