Abstract
DNA damage induces the canonical p53 pathway including elevation of p21waf1 resulting in arrest of cell cycle progression. This can protect cells from subsequent Chk1 inhibition. Some p53 wild-type cancer cells such as HCT116 and U2OS exhibit attenuated p21waf1 induction upon DNA damage due to translational inhibition, and are incapable of maintaining arrest upon Chk1 inhibition. The purpose of this study was to determine whether this attenuated p21waf1 induction also occurred with the non-DNA damaging agent Nutlin-3 which induces p53 by disrupting binding to its negative regulator MDM2. We find that Nutlin-3 circumvented the attenuated induction of p21waf1 protein by increasing its half-life which led to G1 and G2 arrest in both cell lines. Interestingly, the p21waf1 protein half-life remained short on Nutlin-3 in p53 wild-type MCF10A cells; these cells achieve high p21waf1 levels through transcriptional upregulation. Consequently, all three p53 wild-type cells but not p53 mutant MDA-MB-231 cancer cells were protected from subsequent incubation with a combination of DNA damage plus a checkpoint inhibitor.
Keywords: p53, p21waf1, cell cycle arrest, DNA damage, Nutlin-3, Chk1
Introduction
The DNA integrity of a cell is constantly threatened by replication errors and DNA damage caused by radiation or chemical agents. In defense, the DNA damage checkpoints detect such mishaps and arrest the cells until the damage is repaired.1 If the DNA damage is beyond repair, the cells undergo apoptosis or senescence to eliminate the cells.2 Cancer cells often acquire mutations that disable these checkpoints and therefore allow them to continue proliferating despite having damaged DNA.3 These mutations include loss-of-function mutations, whereby the normal activity of tumor suppressors is lost.4 About 50% of cancers harbor mutations in the tumor suppressor p53. In many of the remaining 50%, the function of the retained wild-type p53 protein is compromised by deregulation of upstream or downstream components of the p53 pathway.5
Although these p53-defective cancer cells are more resistant to p53-induced apoptosis, they have a dysfunctional G1 checkpoint because of failure to activate the p53-p21waf1 pathway. Thus, these cells rely heavily on the S- and G2-phase checkpoints to arrest the cells, repair DNA damage and promote cell survival.2,6 This has inspired efforts to inhibit the S/G2 checkpoint as a potential strategy to sensitize cancer cells to radiation- or drug-induced DNA damage.3,7 One crucial S/G2 checkpoint regulator is Chk1 and selective Chk1 inhibitors are currently being tested to potentiate the efficacy of various DNA damaging therapies.6,8 Our previous studies using the topoisomerase I inhibitor SN38 have shown that Chk1 inhibition does not abrogate arrest in the non-tumorigenic p53 wild-type MCF10A cells, but abrogates arrest in p53 mutant cancer cells.9,10 However, we also observed that some p53 wild-type cancer cells such as HCT116 and U2OS were still sensitive to Chk1 inhibition as they have a very attenuated p21waf1 induction due to suppressed translation of the p21waf1 mRNA.11,12
An alternative means to increase the therapeutic index is to selectively protect the patient from anticancer drugs. One example is to activate the p53-p21waf1 pathway in normal cells thereby protecting them from S phase-dependent therapeutic drugs.13 p53-defective cancers would not be able to benefit from such treatment and would not be protected from the toxic drug treatments. In this study, we examined whether the attenuated p21waf1 induction observed in the p53 wild-type HCT116 and U2OS cancer cells also occurred with the non-genotoxic Nutlin-3 which induces p53 by disrupting binding to its negative regulator MDM2. If so, we could potentially extend the use of p53-mediated protection not only for patients with p53 mutant cancer but also for those patients with certain p53 wild-type cancers that have a defective p21waf1 induction. However, we find a much stronger p21waf1 induction by Nutlin-3 than SN38 due to a longer protein half-life, which effectively arrested both cell lines in G1 and G2. This arrest protected p53 wild-type cancer cells but not p53 mutant MDA-MB-231 cancer cells from subsequent incubation with a combination of DNA damage plus checkpoint inhibition.
Results
Nutlin-3 induces higher p21waf1 protein than SN38
We previously established that, when subjected to DNA damage, some p53 wild-type tumor cell lines have a highly attenuated induction of p21waf1 protein.12 This defect in the p53 pathway was not due to a defect in induction of p21waf1 mRNA but rather to a decrease in translation.11 In those experiments, p53 was activated via DNA damage induced by the topoisomerase I inhibitor SN38. Here, we investigated whether the non-DNA damaging agent Nutlin-3 would circumvent this attenuated p21waf1 induction.
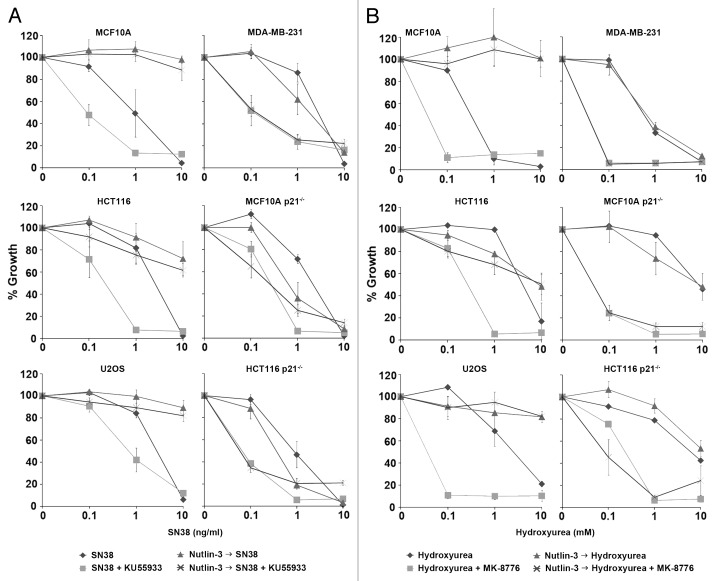
The concentration of SN38 used in these studies (10 ng/ml) was selected because of our previous experiments showing strong induction of S phase arrest in most cells after 24 h.9-12 Upon removal of SN38, there is complete inhibition of growth over the following 6 d. The concentrations that inhibit growth by 50% are in the range of 1–3 ng/ml for all the cell lines used here (see also Fig. 6). The concentration of Nutlin-3 was selected based on preliminary experiments demonstrating that this was the minimum concentration that induced G1 and G2 arrest and significant depletion of S phase cells following a 24 h incubation (Fig. S1A). This concentration also strongly induced p53 and p21waf1 (Fig S1B). However, this concentration was not cytotoxic as it was completely reversible upon removal of the drug (see below).
Figure 6. Nutlin-3 protects only p53 wild-type cells from S phase-specific drug combinations. (A) Cells were incubated with 0–10 ng/ml SN38 alone or in combination with 10 µM KU55933 (ATM inhibitor) for 24 h. (B) Cells were incubated with 0–10 mM hydroxyurea alone or in combination with 1 µM MK-8776 (Chk1 inhibitor) for 24 h. Cells were also pretreated with 10 µM Nutlin-3 for 24 h, and this was left on during incubation with the other drugs. Drugs were removed 24 h later and cells were collected 5–7 d later when the untreated or Nutlin-3 only treated cells reached 70–80% confluency. The error bars represent SE of at least two independent experiments.
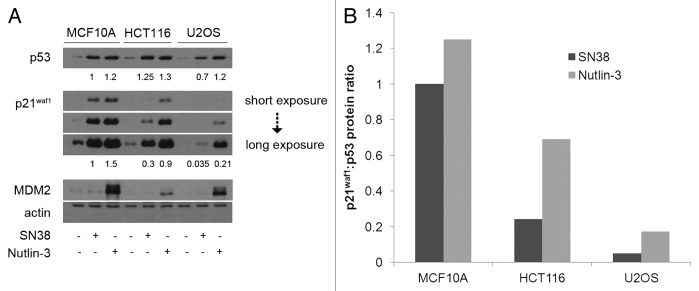
We first compared the absolute p21waf1 protein levels between MCF10A, HCT116 and U2OS cells after incubation with SN38 or Nutlin-3 (Fig. 1A). Both SN38 and Nutlin-3 induced p53 to comparable levels in MCF10A and HCT116 cells, whereas Nutlin-3 induced almost double the p53 induced by SN38 in U2OS cells. As previously shown, the level of p21waf1 protein was poorly induced by SN38 in HCT116 cells, and was hardly detectable in the U2OS cells.11 Nutlin-3 was a stronger inducer of p21waf1 protein than SN38 in all three cell lines. While the levels of p21waf1 induced in HCT116 and U2OS cells were still less than in MCF10A (60 and 14%, respectively), they were much greater than obtained upon incubation with SN38. These results were also expressed as the amount of p21waf1 protein induced for a given amount of p53 (Fig. 1B). While there is more p21waf1 protein in MCF10A cells, Nutlin-3 is much more efficient than SN38 at inducing p21waf1 in the other two cell lines.
Figure 1. p53 and p21waf1 protein levels induced by SN38 and Nutlin-3. (A) Cells were incubated with 10 ng/ml SN38 or 10 µM Nutlin-3 for 24 h. Protein levels were assessed by western blotting. (B) The ratio of p21waf1 to p53 protein levels were compared with that of MCF10A after 24 h of SN38.
Nutlin-3 is also a strong inducer of another p53 transcriptional target, MDM2, in MCF10A and U2OS cells but only a weak inducer in HCT116 cells (Fig. 1A). In contrast, no MDM2 was detected in cells incubated with SN38. As a possible explanation for the low MDM2 induction in HCT116 cells, we observed a much stronger band of ~60 kDa (Fig. S2A). We hypothesized that the low levels of full-length MDM2 protein in HCT116 could be due to caspase-dependent cleavage to the 60 kDa form.14-17 However, neither zVAD-fmk nor Q-VD-OPh caspase inhibitors prevented this cleavage (Fig. S2). The lower band does derive from MDM2 as siRNA targeted at MDM2 depletes not only the full-length band but also the lower band (Fig. S2C). On the other hand, the short MDM2 band may derive from a mRNA splice variant as suggested by others.18,19 If so, this splice variant seems to lack both N-terminal region and RING domain (C-terminal region) as primer sets directed at either region demonstrated much lower MDM2 transcript is induced in HCT116 cells compared with the other two cell lines (Fig. S2D).
Comparison of p53 and p21waf1 protein kinetics induced by Nutlin-3
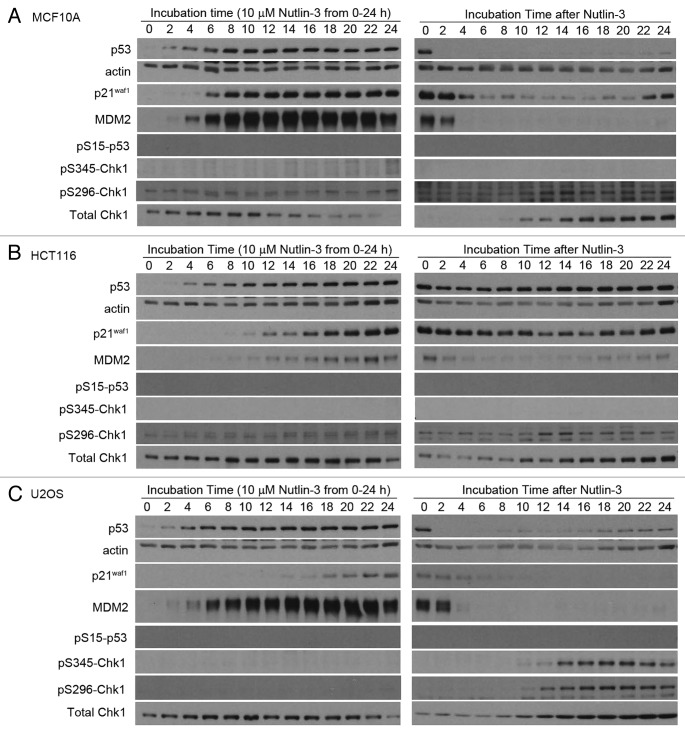
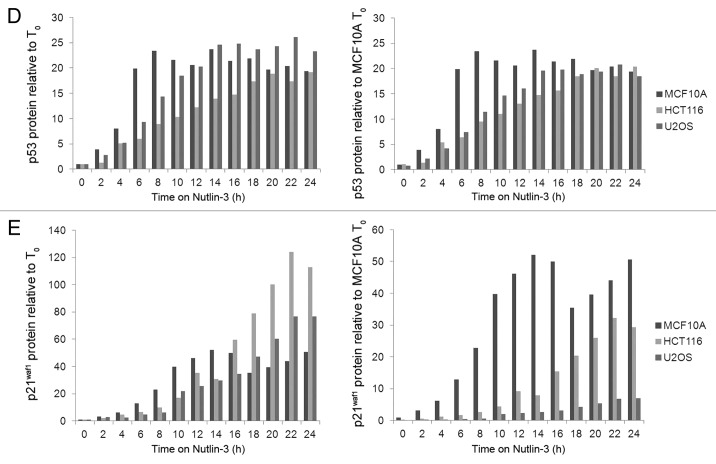
A more detailed kinetic analysis of the induction of p53 and p21waf1 by Nutlin-3 was performed (Fig. 2A–C, left panel). These results were quantified by densitometry of multiple exposures of the western blots to avoid analysis of over-exposed bands, and this was confirmed by comparison to a dilution series of each antigen. The results are expressed in two different ways: (1) relative to the protein level at 0 h for each cell line, or (2) relative to the protein level at 0 h for MCF10A cells (Fig. 2D–E). The latter expression provides a comparison of the absolute level of the protein. As the three cell lines have fairly similar basal levels of p53, these two methods of expressing the results show only small differences. Overall, the rate and level of p53 induction is fairly similar between the cell lines, suggesting similar Nutlin-3 uptake and ability to activate p53. In contrast, the level of p21waf1 protein induced by Nutlin-3 varies significantly depending on how the results are expressed. While the fold induction is fairly similar for the three cells lines, and is actually higher in HCT116 cells, the absolute level of protein produced is significantly less in U2OS cells. These results reiterate that the amount of p21waf1 protein induced, particularly in the U2OS cells is inconsistent with the level of p53 protein induced. The kinetics of induction of MDM2 and p21waf1 in MCF10A cells were both rapid while the induction of both proteins in HCT116 cells was much slower. However, in U2OS cells, the kinetics of induction of these two proteins differed markedly with MDM2 induction as rapid as in MCF10A cells, while the induction of p21waf1 was slow as in HCT116 cells.
Figure 2A–C. Protein kinetics during and following treatment with Nutlin-3. (A) MCF10A, (B) HCT116 and (C) U2OS were incubated with 10 µM Nutlin from 0–24 h. The drug was removed and cells were incubated for an additional 24 h in fresh medium. Cells were harvested at the indicated times and assayed for proteins using western blotting.
Figure 2D and E.
Protein kinetics during and following treatment with Nutlin-3. (D) p53 and E. p21waf1 protein levels were quantified by densitometry of multiple exposures of western blots. The left panels show protein induction compared with the untreated control of each cell line. The right panels show protein induction compared with untreated MCF10A cells.
Nutlin-3 did not induce DNA damage as no phosphorylation of Chk1 or p53 was observed. Hence the induction of p53 appears to be non-genotoxic which is consistent with the mechanism whereby Nutlin-3 disrupts p53:Mdm2 binding. In all three cell lines, total Chk1 decreases at later time points, presumably due to p21waf1 inhibition of Chk1 transcription.20 Additionally, as Chk1 is also cell cycle-regulated, the decrease in Chk1 may also be due to arrest.21
p21waf1 mRNA is insufficient to explain the higher p21waf1 protein levels induced by Nutlin-3
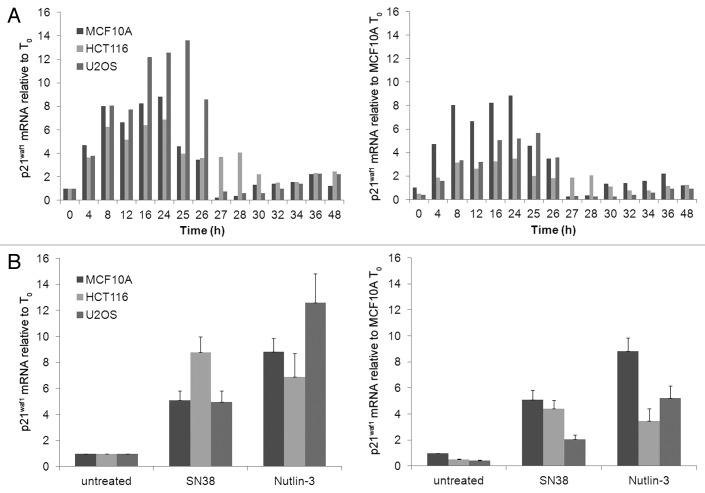
A detailed kinetic analysis of p21waf1 mRNA induction between 0 h and 24 h of incubation with Nutlin-3 is shown in Figure 3A. The results are expressed relative to the mRNA level at 0 h either for each cell line or for MCF10A cells. The basal level of mRNA in the HCT116 and U2OS cells was half as much as in MCF10A. The fold induction by Nutlin-3 was higher in U2OS than MCF10A, albeit the absolute level of mRNA was only 60% that of MCF10A. This should be contrasted to the protein levels in U2OS which was only 14% that of MCF10A. In HCT116 cells, absolute p21waf1 mRNA following Nutlin-3 treatment was 40% that of MCF10A, which more closely approximates to the protein level which is 60% that of MCF10A.
Figure 3. p21waf1 mRNA kinetics following Nutlin-3 treatment. (A) Cells were incubated with 10 µM Nutlin-3 from 0–24 h. The drug was removed and cells were incubated for an additional 24 h in fresh medium. Cells were harvested at the indicated times and assayed for p21waf1 mRNA levels using RT-qPCR. (B) Cells were incubated with 10 ng/ml SN38 or 10 µM Nutlin-3 for 24 h, harvested and assayed for p21waf1 mRNA levels using RT-qPCR. The error bars represent SE of at least three independent experiments.
We next determined whether the p21waf1 mRNA levels could explain the difference in SN38- and Nutlin-3-induced p21waf1 protein levels (Fig. 3B). In MCF10A cells, Nutlin-3 induced higher p21waf1 mRNA than SN38 and this correlates well with the higher p21waf1 protein induced (Fig. 1A). In HCT116 cells, both drugs induced similar p21waf1 mRNA, yet the protein induced by Nutlin-3 is three times higher than that induced by SN38. In U2OS cells, Nutlin-3-induced p21waf1 mRNA is about 2.5 times higher than induced by SN38, yet the protein induced is 6 times higher. Hence, the level of p21waf1 mRNA cannot adequately explain the higher protein induction by Nutlin-3 than SN38 in HCT116 and U2OS cells.
Nutlin-3 induces longer p21waf1 protein half-life
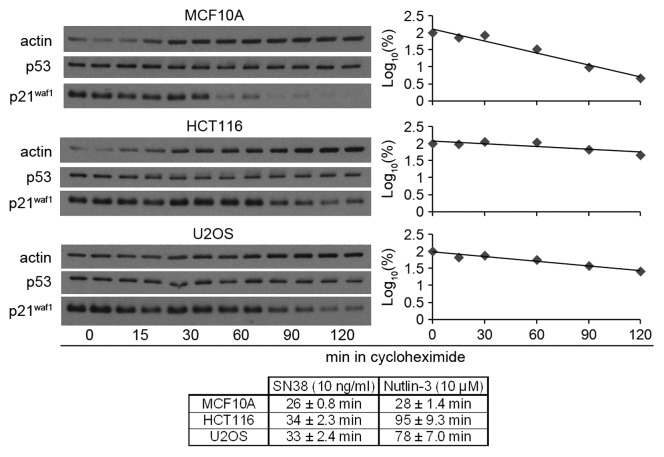
One possible explanation for the difference in p21waf1 protein levels could be differences in p21waf1 protein half-life. The p21waf1 protein half-life is about 30 min for MCF10A, and 95 and 80 min for HCT116 and U2OS, respectively (Fig. 4). In contrast, the p21waf1 protein half-life on SN38 was about 30 min in all three cell lines.11 This suggests that the higher p21waf1 protein induced by Nutlin-3 than SN38 in HCT116 and U2OS may be primarily due to the longer protein half-life.
Figure 4. Higher Nutlin-3-induced p21waf1 protein levels is due to longer p21waf1 protein half-life. Cells were incubated with 10 µM Nutlin-3 for 24 h and then incubated with the protein synthesis inhibitor cycloheximide (10 µg/ml) for the indicated times without removing nutlin-3. The densitometric scans of the western blots are graphed on the right. The half-lives were calculated from three such independent experiments and are presented as the means ± SE. The half-lives for SN38 are derived from our previous paper (11).
It has previously been suggested that MDM2 can target p21waf1 for degradation,22-24 and this is consistent with the shorter half-life in MCF10A in which MDM2 induction is the highest. However, siRNA knockdown of MDM2 in Nutlin-3-treated MCF10A cells did not increase p21waf1 protein half-life (Fig. S3). Thus, the difference in p21waf1 protein half-life in Nutlin-3-treated cells is unlikely due to MDM2-mediated degradation. We also looked at other candidates (MDMX, hSSB1 and cyclin D1) that have been proposed to affect p21waf1 protein half-life but the levels did not correlate with the half-life observed (Fig. S3C).25-27
Functional significance of p21waf1 induction
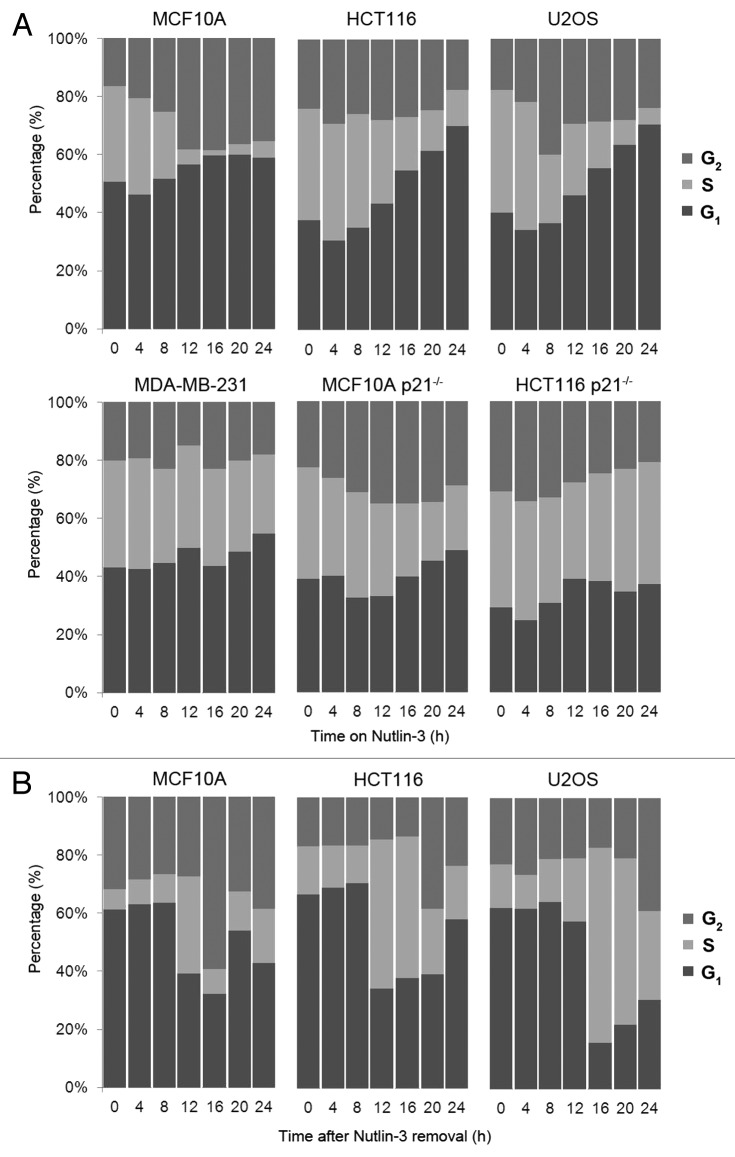
An important question to address is whether the different kinetics and levels of p21waf1 protein induction are functionally significant. We thus investigated whether the p21waf1 protein induced by Nutlin-3 in all three p53 wild-type cells is sufficient to induce arrest (Fig. 5A, top panel). In the MCF10A cells, Nutlin-3 caused loss of S phase cells by 12 h with accumulation in both G1 and G2. In contrast, both HCT116 and U2OS cells took 24 h to deplete the majority of S phase cells with accumulation primarily in G1 and little if any change in the G2 population. This arrest by Nutlin-3 was p53-mediated as no arrest was observed with the p53 mutant MDA-MB-231 cells (Fig. 5A, bottom panel). The importance of p21waf1 for arrest was confirmed using MCF10A and HCT116 cells deleted for p21waf1 (Fig. 5A, bottom panel). In both cases, Nutlin-3 was unable to induce arrest in either G1 or G2 phase.
Figure 5. Nutlin-3-induced p21waf1 protein leads to reversible G1/G2 arrest. (A) p53 wild-type cells (top panel) and p53- and p21waf1-defective cells (bottom panel) were incubated with 10 µM Nutlin-3 for 0–24 h. Cells were harvested and assayed for DNA content by flow cytometry. (B) After 24 h of 10 µM Nutlin-3, the drug was removed and cells were incubated for an additional 24 h in fresh medium before assaying by flow cytometry.
Recovery from Nutlin-3
One of the goals of these experiments was to assess the ability of Nutlin-3 to arrest p53 wild-type cells and then to determine whether this would protect the cells from DNA damaging agents. As p53 activation can also lead to senescence, it is critical to determine whether these cells re-enter the cell cycle upon removal of Nutlin-3. Cell cycle perturbation was assessed following release of cells from Nutlin-3 (Fig. 5B). Both MCF10A and HCT116 cells recovered within 12 h as reflected in an increase of cells in S phase. U2OS cells took about 16 h for the majority of cells to enter S phase. Overall, this shows that all three cell lines recover from Nutlin-3.
Next we assessed the reversibility of p53 and p21waf1 expression following removal of Nutlin-3 (Fig. 2A–C, right panel). In both MCF10A and U2OS cells, the p53 protein disappeared within 2 h of removing Nutlin-3, presumably due to the high levels of MDM2 targeting p53 for degradation. This results in a rapid decrease in p21waf1 mRNA and protein as well as a decrease in MDM2 protein (Figs. 2A–C and 3A). Similar results were obtained in HCT116 cells, although the decrease in p53 and p21waf1 was much less in magnitude. This is probably due to the low level of MDM2 induced, and therefore the decrease in p53 and subsequently p21waf1 is not as dramatic (Fig. 1A). Overall, the results show that the impact of Nutlin-3 is rapidly reversible upon removal of drug.
In all three cell lines, total Chk1 started to recover as p21waf1 protein decreased, presumably due to relief of p21waf1-mediated inhibition of transcription or relief of p21waf1-mediated arrest.20,21 As expected, there was no significant induction of phosphorylation of p53 or Chk1 in either MCF10A or HCT116 cells. In contrast, increased phosphorylation of Chk1 at serine 345 and 296 was observed in U2OS cells, suggesting the presence of some DNA damage in these cells. Despite this, no phosphorylation of p53 at serine 15 was detected and these cells still progressed through the cell cycle (Fig. 5B).
Nutlin-3 protects p53 wild-type cells from S phase-specific chemotherapeutic combinations
We have previously shown that KU55933 (ATM inhibitor) and MK-8776 (Chk1 inhibitor) in combination with SN38 and hydroxyurea, respectively, can dramatically sensitize both p53 wild-type and mutant cells.28 We thus asked if Nutlin-3 could be used to protect p53 wild-type cells and not p53 mutant cells from these combinations (Fig. 6). In p53 wild-type cells (MCF10A, HCT116, U2OS) and p53 mutant cells (MDA-MB-231), SN38 and hydroxyurea alone only killed the cells at the highest concentration used. KU55933 and MK-8776 dramatically sensitized these cells to SN38 and hydroxyurea, respectively. Pretreatment with Nutlin-3 protected all three p53 wild-type cell lines from the DNA damaging drugs alone or in combination with the checkpoint inhibitors. In contrast, Nutlin-3 did not protect the p53 mutant MDA-MB-231 cells (Fig. 6), consistent with its failure to induce a G1/G2 arrest in these cells (Fig. 5A, bottom panel).
To confirm that this Nutlin-3-mediated protection is p21waf1-dependent, we performed parallel experiments in MCF10A and HCT116 cells that have been deleted for p21waf1 (Fig. 6). While SN38 killed the cells at the highest concentration, 10 mM hydroxyurea failed to kill the cells. KU55933 and MK-8776 dramatically sensitized these cells to SN38 and hydroxyurea, respectively. Pretreatment with Nutlin-3 did not protect these cells from either of the DNA damaging drugs alone or in combination with a checkpoint inhibitor. This supports the importance of p21waf1 protein in Nutlin-3-mediated protection from these S phase-specific chemotherapeutic drugs and combinations.
Discussion
As a consequence of defects in cell cycle checkpoints, cancer cells may become “addicted” to alternative survival mechanisms that are dispensable in normal cells.1 This provides a means to selectively increase the efficacy of chemotherapy agents against cancer and thus enhance the therapeutic index (defined as the toxic dose divided by the therapeutic dose).2,29 Enhancement of the therapeutic index can also be accomplished by selectively protecting a patient’s normal cells from drugs that would otherwise kill all cells. For example, Nutlin-3 can induce a G1/G2 arrest and protect normal cells but not p53 mutant cancer cells from S-phase specific drugs.30 As expected, Nutlin-3 can also induce a p21waf1-dependent G1/G2 arrest in some cancer cells that retain wild-type p53.31 However, we did not anticipate this to occur in HCT116 and U2OS cells because of their attenuated p21waf1 protein production when incubated with another p53 activating drug, SN38.11 Here, we studied the mechanism of how Nutlin-3 is able to induce functional levels of p21waf1 that lead to G1/G2 arrest in these two cell lines.
We found that Nutlin-3 was able to induce a much higher level of p21waf1 protein than SN38 in HCT116 and U2OS cells. This did not seem to be due to greater p21waf1 protein production but rather to longer p21waf1 protein half-life. As previously shown, the p21waf1 protein half-life is about 30 min upon treatment with SN38, which causes double-strand breaks in DNA.11 Others have also shown that the p21waf1 protein half-life is less than 30 min in cells with DNA damage.32,33 On the other hand, nongenotoxic agents, such as heregulin, compound C (AMPK inhibitor), ritonavir (HIV protease inhibitor), lactacystin (proteasome inhibitor), and grifolin (a secondary metabolite isolated from the fresh fruiting bodies of the mushroom Albatrellus confluens), have been shown to increase p21waf1 protein half-life to more than 1 h.34-37 Indeed, we found the p21waf1 protein half-life to be about 95 and 78 min for HCT116 and U2OS cells, respectively, upon treatment with the nongenotoxic MDM2 inhibitor Nutlin-3. Surprisingly though, the p21waf1 protein half-life remained short (~30 min) in Nutlin-3-treated MCF10A cells. Further experiments are needed to understand what is targeting p21waf1 protein for degradation in both treatments.
We also looked at another p53 transcriptional target, MDM2. Interestingly, MDM2 protein induction was undetectable in SN38-treated cells, especially when compared with Nutlin-3-treated cells. We found that this difference correlated with a lack of MDM2 mRNA induction in SN38-treated cells (data not shown). Thus, it is apparent that not all p53-activating stimuli result in the same repertoire of transcribed p53 target genes. Studies have shown that post-translational modifications of p53 such as phosphorylation can alter its DNA binding affinities.38 This may be a feasible explanation as SN38 and Nutlin-3 induce different phosphorylation status of p53. SN38-induced DNA damage leads to phosphorylation of p53, whereas Nutlin-3 induces p53 by disrupting the p53:MDM2 binding. Others have found that the lack of MDM2 but not p21waf1 mRNA induction by the topoisomerase I inhibitor camptothecin correlates with phosphorylation of p53 on both serines 15 and 20.39
Despite the observation that Nutlin-3 induces G1/G2 arrest by 24 h in all three cell lines, there are differences that perhaps highlight the defective p53 pathway in HCT116 and U2OS compared with MCF10A cells. For example, it takes HCT116 and U2OS cells a longer time to attain the same extent of arrest observed in MCF10A. Additionally, HCT116 and U2OS cells preferentially arrest in G1 rather than G2 on Nutlin-3. This may be explained by the kinetics of p21waf1 protein. In MCF10A cells, the more rapid p21waf1 protein induction results in accumulation in both G1 and G2. In HCT116 and U2OS cells, the slower p21waf1 protein induction does not appear to arrest cells in G2 so the increase observed is in G1. Interestingly the level of p21waf1 protein in U2OS cells is only 14% of that in MCF10A, yet it is still sufficient to arrest these cells. The ability of this relatively low level of p21waf1 to arrest U2OS cells may depend on differences in the ratio of p21waf1 to cyclin-cdk complexes between the cell lines.40
As p53 activation can lead to cell cycle arrest, apoptosis or senescence (permanent arrest), it is important to note that the latter two events were not observed following 24 h Nutlin-3 treatment. Nutlin-3 has been reported to induce a DNA damage response in some cells, including serine-15-phosphorylation of p53, yet this was not seen in this study.41 Comparing several reports, all using HCT116 cells, we note that our results are consistent with those of Thompson et al.42 and Verma et al.,43 but at variance with Valentine et al.41 We note the latter paper reports a different source of HCT116 cells which may explain their discrepancy. We observed no increase in the sub-G1 population and the cells continued to cycle upon removal of Nutlin-3 (Fig. 5). Due to this reversibility, the cells must be maintained on Nutlin-3 during subsequent treatments with the S phase-specific drug combinations if protection is to be observed (Fig. 6).
To conclude, translation of p21waf1 mRNA is inhibited in both HCT116 and U2OS cells treated with SN38.11 This is circumvented by the significant increase in p21waf1 protein half-life when these cells are incubated with Nutlin-3. The higher p21waf1 protein induced by Nutlin-3 allows HCT116 and U2OS to reach the same extent of cell cycle arrest observed in MCF10A within 24 h. This arrest protects all three p53 wild-type cells, but not the p53 mutant MDA-MB-231 cells, from the combination of SN38 plus KU55933 or hydroxyurea plus MK-8776. Importantly, it has been shown that mice tolerated oral administration of MI-219 (another MDM2 inhibitor) at doses that achieve robust systemic upregulation of p21waf1 in a p53-dependent manner.44 This demonstrates the therapeutic potential of using a MDM2 inhibitor to protect patients with p53 mutant cancers from the potentially toxic combinations of SN38 with an ATM inhibitor or hydroxyurea with a Chk1 inhibitor.
Materials and Methods
Chemicals
Drugs were obtained from the following sources: SN38, Pfizer; Nutlin-3, hydroxyurea and cycloheximide, Sigma-Aldrich; KU55933, Tocris Biosciences. MK-8776 (previously known as SCH900776) was provided by Merck. Hoechst 33258 was obtained from Hoefer Scientific.
Cell culture
The origin and maintenance of MCF10A, HCT116, U2OS and MDA-MB-231 cells have been described previously.11 MCF10A p21−/− cells were obtained from Dr. Ben Park (Johns Hopkins University).45 HCT116 p21−/− colorectal carcinoma cell lines were obtained from Dr. Bert Vogelstein (Johns Hopkins University).46
Cell cycle analysis
Cell cycle analysis was performed as described previously, whereby cells were harvested, fixed in ethanol, incubated with ribonuclease and stained with propidium iodide.47 DNA content was determined on a flow cytometer (FACScan, Becton Dickinson).
Cell growth assay
Each well of a 96-well plate was seeded with 500–1,000 cells and plates incubated overnight to allow cells to attach. After 24 h incubation with drugs, the cells were incubated in media for 5–7 d by which time the untreated cells reached 70–80% confluency. Cells were washed, frozen at -80°C, then thawed and stained with Hoechst 33258 as described previously.48 Fluorescently-stained DNA was then measured at excitation λ 350 nm and emission λ 460 nm on an automated 96-well plate reader using the SOFTmax 4.8 software. The plate reader was blanked to wells with no cells present. The fluorescent reading for untreated cells was set as 100% and the fluorescent readings for the treated cells were compared with the untreated cells.
Western blot analysis
Cells were harvested and analyzed by immunoblotting as previously detailed11 with the following additional antibodies: MDM2 (SMP14) and cyclin D1 (M-20) from Santa Cruz Biotechnology.
Quantification of p21waf1 protein half-life
Cells were incubated with the protein synthesis inhibitor cycloheximide (10 µg/ml) for the indicated times. p21waf1 protein levels were detected by western blotting and protein half-lives were calculated as previously described.11
Real-time PCR for mRNA
Total RNA was isolated using RNeasy Mini Kit (Qiagen). CDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). Primer sequences for p21waf1 and GAPDH are as previously described.11 Real-time PCR was performed with CFX96 real-time system (Bio-Rad) using iQSYBR Green PCR Supermix (Bio-Rad). The fold change of p21waf1 mRNA was determined by the equation of Pfaffl using GAPDH as the endogenous control.49
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Health (CA117874; A. E.) and Cancer Center Support grant to the Norris Cotton Cancer Center (CA23108).
Disclosure of Potential Conflicts of Interest
The authors declare they have no conflicts of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/21047
References
- 1.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ejeh F, Kumar R, Wiegmans A, Lakhani SR, Brown MP, Khanna KK. Harnessing the complexity of DNA-damage response pathways to improve cancer treatment outcomes. Oncogene. 2010;29:6085–98. doi: 10.1038/onc.2010.407. [DOI] [PubMed] [Google Scholar]
- 3.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph J. Inhibiting transient protein-protein interactions: lessons from the Cdc25 protein tyrosine phosphatases. Nat Rev Cancer. 2007;7:202–11. doi: 10.1038/nrc2087. [DOI] [PubMed] [Google Scholar]
- 5.Aylon Y, Oren M. New plays in the p53 theater. Curr Opin Genet Dev. 2011;21:86–92. doi: 10.1016/j.gde.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98:523–8. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuntz K, O’Connell MJ. The G(2) DNA damage checkpoint: could this ancient regulator be the Achilles heel of cancer? Cancer Biol Ther. 2009;8:1433–9. doi: 10.4161/cbt.8.15.9081. [DOI] [PubMed] [Google Scholar]
- 8.Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res. 2007;13:1955–60. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- 9.Eastman A, Kohn EA, Brown MK, Rathman J, Livingstone M, Blank DH, et al. A novel indolocarbazole, ICP-1, abrogates DNA damage-induced cell cycle arrest and enhances cytotoxicity: similarities and differences to the cell cycle checkpoint abrogator UCN-01. Mol Cancer Ther. 2002;1:1067–78. [PubMed] [Google Scholar]
- 10.Kohn EA, Ruth ND, Brown MK, Livingstone M, Eastman A. Abrogation of the S phase DNA damage checkpoint results in S phase progression or premature mitosis depending on the concentration of 7-hydroxystaurosporine and the kinetics of Cdc25C activation. J Biol Chem. 2002;277:26553–64. doi: 10.1074/jbc.M202040200. [DOI] [PubMed] [Google Scholar]
- 11.Chang L-J, Eastman A. Decreased translation of p21waf1 mRNA causes attenuated p53 signaling in some p53 wild-type tumors. Cell Cycle. 2012;11:1818–26. doi: 10.4161/cc20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levesque AA, Fanous AA, Poh A, Eastman A. Defective p53 signaling in p53 wild-type tumors attenuates p21waf1 induction and cyclin B repression rendering them sensitive to Chk1 inhibitors that abrogate DNA damage-induced S and G2 arrest. Mol Cancer Ther. 2008;7:252–62. doi: 10.1158/1535-7163.MCT-07-2066. [DOI] [PubMed] [Google Scholar]
- 13.Eastman A. The importance of p53 signaling in the response of cells to checkpoint inhibitors. In: Siddik ZH (ed). Checkpoint Controls and Targets in Cancer Therapy. Humana Press, 2010 pp. 189-98. [Google Scholar]
- 14.Oliver TG, Meylan E, Chang GP, Xue W, Burke JR, Humpton TJ, et al. Caspase-2-mediated cleavage of Mdm2 creates a p53-induced positive feedback loop. Mol Cell. 2011;43:57–71. doi: 10.1016/j.molcel.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pochampally R, Fodera B, Chen L, Lu W, Chen J. Activation of an MDM2-specific caspase by p53 in the absence of apoptosis. J Biol Chem. 1999;274:15271–7. doi: 10.1074/jbc.274.21.15271. [DOI] [PubMed] [Google Scholar]
- 16.Pochampally R, Fodera B, Chen L, Shao W, Levine EA, Chen J. A 60 kd MDM2 isoform is produced by caspase cleavage in non-apoptotic tumor cells. Oncogene. 1998;17:2629–36. doi: 10.1038/sj.onc.1202206. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Marechal V, Moreau J, Levine AJ, Chen J. Proteolytic cleavage of the mdm2 oncoprotein during apoptosis. J Biol Chem. 1997;272:22966–73. doi: 10.1074/jbc.272.36.22966. [DOI] [PubMed] [Google Scholar]
- 18.Bartel F, Harris LC, Würl P, Taubert H. MDM2 and its splice variant messenger RNAs: expression in tumors and down-regulation using antisense oligonucleotides. Mol Cancer Res. 2004;2:29–35. [PubMed] [Google Scholar]
- 19.Bartel F, Taubert H, Harris LC. Alternative and aberrant splicing of MDM2 mRNA in human cancer. Cancer Cell. 2002;2:9–15. doi: 10.1016/S1535-6108(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 20.Gottifredi V, Karni-Schmidt O, Shieh SS, Prives C. p53 down-regulates CHK1 through p21 and the retinoblastoma protein. Mol Cell Biol. 2001;21:1066–76. doi: 10.1128/MCB.21.4.1066-1076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci USA. 2002;99:14795–800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y, Lee H, Zeng SX, Dai MS, Lu H. MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J. 2003;22:6365–77. doi: 10.1093/emboj/cdg600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Zhang Z, Li M, Zhang R. MDM2 promotes proteasomal degradation of p21Waf1 via a conformation change. J Biol Chem. 2010;285:18407–14. doi: 10.1074/jbc.M109.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem. 2004;279:16000–6. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- 25.Coleman ML, Marshall CJ, Olson MF. Ras promotes p21(Waf1/Cip1) protein stability via a cyclin D1-imposed block in proteasome-mediated degradation. EMBO J. 2003;22:2036–46. doi: 10.1093/emboj/cdg189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Y, Zeng SX, Sun XX, Lee H, Blattner C, Xiao Z, et al. MDMX promotes proteasomal turnover of p21 at G1 and early S phases independently of, but in cooperation with, MDM2. Mol Cell Biol. 2008;28:1218–29. doi: 10.1128/MCB.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu S, Feng Z, Zhang M, Wu Y, Sang Y, Xu H, et al. hSSB1 binds and protects p21 from ubiquitin-mediated degradation and positively correlates with p21 in human hepatocellular carcinomas. Oncogene. 2011;30:2219–29. doi: 10.1038/onc.2010.596. [DOI] [PubMed] [Google Scholar]
- 28.Montano R, Chung I, Garner KM, Parry D, Eastman A. Preclinical development of the novel Chk1 inhibitor SCH900776 in combination with DNA-damaging agents and antimetabolites. Mol Cancer Ther. 2012;11:427–38. doi: 10.1158/1535-7163.MCT-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4:216–25. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- 30.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–41. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA. 2006;103:1888–93. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Zeng SX, Lu H. UV Induces p21 rapid turnover independently of ubiquitin and Skp2. J Biol Chem. 2006;281:26876–83. doi: 10.1074/jbc.M605366200. [DOI] [PubMed] [Google Scholar]
- 33.Stuart SA, Wang JY. Ionizing radiation induces ATM-independent degradation of p21Cip1 in transformed cells. J Biol Chem. 2009;284:15061–70. doi: 10.1074/jbc.M808810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menendez JA, Mehmi I, Lupu R. Heregulin-triggered Her-2/neu signaling enhances nuclear accumulation of p21WAF1/CIP1 and protects breast cancer cells from cisplatin-induced genotoxic damage. Int J Oncol. 2005;26:649–59. [PubMed] [Google Scholar]
- 35.Nam M, Lee WH, Bae EJ, Kim SG. Compound C inhibits clonal expansion of preadipocytes by increasing p21 level irrespectively of AMPK inhibition. Arch Biochem Biophys. 2008;479:74–81. doi: 10.1016/j.abb.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 36.Gaedicke S, Firat-Geier E, Constantiniu O, Lucchiari-Hartz M, Freudenberg M, Galanos C, et al. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002;62:6901–8. [PubMed] [Google Scholar]
- 37.Luo XJ, Li W, Yang LF, Yu XF, Xiao LB, Tang M, et al. DAPK1 mediates the G1 phase arrest in human nasopharyngeal carcinoma cells induced by grifolin, a potential antitumor natural product. Eur J Pharmacol. 2011;670:427–34. doi: 10.1016/j.ejphar.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 39.Ashcroft M, Taya Y, Vousden KH. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–33. doi: 10.1128/MCB.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–49. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentine JM, Kumar S, Moumen A. A p53-independent role for the MDM2 antagonist Nutlin-3 in DNA damage response initiation. BMC Cancer. 2011;11:79. doi: 10.1186/1471-2407-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson T, Tovar C, Yang H, Carvajal D, Vu BT, Xu Q, et al. Phosphorylation of p53 on key serines is dispensable for transcriptional activation and apoptosis. J Biol Chem. 2004;279:53015–22. doi: 10.1074/jbc.M410233200. [DOI] [PubMed] [Google Scholar]
- 43.Verma R, Rigatti MJ, Belinsky GS, Godman CA, Giardina C. DNA damage response to the Mdm2 inhibitor nutlin-3. Biochem Pharmacol. 2010;79:565–74. doi: 10.1016/j.bcp.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA. 2008;105:3933–8. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachman KE, Blair BG, Brenner K, Bardelli A, Arena S, Zhou S, et al. p21(WAF1/CIP1) mediates the growth response to TGF-beta in human epithelial cells. Cancer Biol Ther. 2004;3:221–5. doi: 10.4161/cbt.3.2.666. [DOI] [PubMed] [Google Scholar]
- 46.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–90. [PubMed] [Google Scholar]
- 47.Demarcq C, Bunch RT, Creswell D, Eastman A. The role of cell cycle progression in cisplatin-induced apoptosis in Chinese hamster ovary cells. Cell Growth Differ. 1994;5:983–93. [PubMed] [Google Scholar]
- 48.Rago R, Mitchen J, Wilding G. DNA fluorometric assay in 96-well tissue culture plates using Hoechst 33258 after cell lysis by freezing in distilled water. Anal Biochem. 1990;191:31–4. doi: 10.1016/0003-2697(90)90382-J. [DOI] [PubMed] [Google Scholar]
- 49.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.