Abstract
Methylthioadenosine phosphorylase (MTAP), a key enzyme in the catabolism of 5′-deoxy-5′-methylthioadenosine (MTA), catalyzes the formation of adenine and 5-methylthioribose-1-phosphate. MTAP is expressed in all cells throughout the body, but a significant percentage of human tumors have lost MTAP expression, thereby making MTAP-loss a potential therapeutic target. Here, we have tested an MTAP-targeting strategy based on the idea that MTAP-expressing cells can be protected from toxic purine and uracil analogs by addition of MTA, but MTAP-deleted tumor cells cannot. Addition of as little as 10 μM MTA could entirely protect isogenic MTAP+, but not MTAP-, HT1080 cells from toxicity caused by the chemotherapy agents 6-thioguanine (6TG) or 5-fluorouracil (5FU). Inhibitor studies showed that MTA protection requires functional MTAP activity. Addition of adenine protected both MTAP+ and MTAP- cells from 6TG and 5FU, consistent with the idea that adenine produced from the MTAP reaction competes with 6TG and 5FU for a rate limiting pool of phosphoribosyl-1-pyrophosphate (PRPP), which is required for the conversion of purine and uracil bases into nucleotides. Extracellular MTA can also protect mouse mesothelioma cells from killing by 6-TG or the drug L-alanosine in an MTAP-dependent manner. In addition, MTA can protect non-transformed MTAP+ mouse embryo fibroblasts from 6TG toxicity. Taken together, our data suggest that the addition of MTA to anti-purine-based chemotherapy may greatly increase the therapeutic index of this class of drugs if used specifically to treat MTAP- tumors.
Keywords: chemotherapy, mesothelioma, methionine, osteosarcoma, purine
Introduction
One of the earliest types of chemotherapy drugs developed was purine and pyrimidine analogs. These bases are converted to nucleotide analogs in vivo, disrupting DNA metabolism and thereby causing the death of rapidly dividing cells.1 Examples of these agents include 5-fluorouracil (5FU), used to treat colon and pancreatic cancer, and 6-thioguanine (6TG) and other anti-purines, used primarily to treat childhood leukemias.2 While these drugs kill cancer cells, they also kill proliferating healthy normal cells and thereby cause significant toxicity. Thus, the major problem with this class of drugs is that the therapeutic index is simply too low to be effective at treating the vast majority of tumors. Most modern research in chemotherapy has concentrated on increasing the therapeutic index of cancer drugs by finding ways to target agents to specific genetic and molecular changes present in cancer cells. However, an alternative approach would be to use molecular differences between tumor and normal cells to find ways to specifically “protect” normal tissue from a toxic non-targeted agent. If this could be done, higher doses of these non-targeted agents could be used which would likely increase their effectiveness at killing tumor cells.
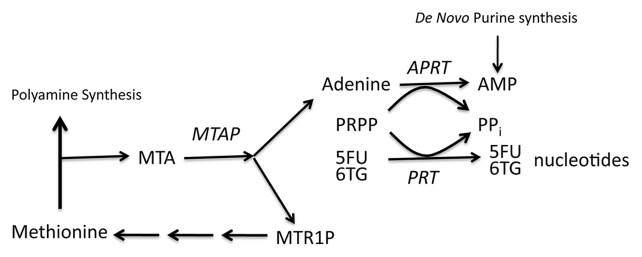
Methylthioadenosine phosphorylase (MTAP) is a key enzyme in the methionine salvage pathway that converts the polyamine byproduct 5′-deoxy-5′-methylthioadenosine (MTA) into adenine and 5-methylthioribose-1-phosphate (MTR-1-P) (Fig. 1). MTAP protein and activity are found in all human tissues, and as such, it has been dubbed a “housekeeping” enzyme.3 However, loss of MTAP is observed at a high frequency in a large number of different human malignancies including leukemias, lymphomas, mesothelioma, billiary tract cancer, glioblastoma, osteosarcoma, neuroendocrine tumors, as well as lung, breast, pancreatic, and squamous cell carcinomas.4-19 Loss rates range from 14–100% depending on the tumor type and the method used to assess MTAP status. The MTAP gene can be inactivated by either deletion or promoter hypermethylation.3,20 Initially, loss of MTAP in human tumor cells was thought to simply be a consequence of its proximity to the CDKN2A/ARF tumor suppressor locus; however, more recent studies clearly indicate that MTAP has tumor suppressor functions independent of CDKN2A/ARF loss.21,22
Figure 1. The role of MTAP in polyamine and purine metabolism. Methionine used for polyamine metabolism results in the production of 5′-deoxy-5′-methylthioadenosine (MTA) which is then phosphorylated by MTAP to produce methylthioribose 1-phosphate (MTR1P) and adenine. The adenine produced in this reaction is converted to AMP via the action of adenine phosphoribosyltransferase in a reaction that requires 5′-phosphoribosyl-1-pyrophospate (PRPP) as a co-substrate. This same co-factor is also required for the conversion of the base analogs 6TG and 5FU to their metabolically active nucleotide forms by various other cellular phosphoribosyltransferases (PRT).
The fact that MTAP is inactivated in a variety of tumor cell types, but is expressed in normal tissues, makes it an attractive target for the development of selective cancer therapy. A potential strategy to take advantage of MTAP loss in cancer cells was recently proposed by Lubin and Lubin,23 who demonstrated that addition of MTA to MTAP-expressing human fibroblasts could protect them from the toxic effects of certain purine analogs, whereas addition of MTA did not protect MTAP- tumor cell lines. The hypothesis put forward to explain these phenomena is centered on the observation that the conversion of purine and uracil base analogs to nucleotides requires the transfer of sugar and phosphate from phosphoribosyl-5-pyrophosphate (PRPP) by cellular phosphoribosyltransferases. Since PRPP levels are rate limiting in the conversion of purine and uracil analogs to form nucleotides,24 the adenine produced from MTA could compete with purine analogs, thereby resulting in decreased toxic nucleotide production (Fig. 1). Based on this hypothesis, it might be possible to add MTA to treatment with purine analogs and thus “protect” MTAP+ tissue from drug toxicity. This, in turn, would allow much higher doses of anti-purines to be given as chemotherapy.
Although this strategy appears promising, the experimental data23 were rather incomplete. In particular, the study only utilized two MTAP+ and two MTAP- cell lines, none of which were genetically related, leaving open the distinct possibility that the response to the toxic purine analogs and the differential response of the cells to MTA protection was due to some other genetic factor. In addition, little data were presented supporting the idea that the mechanism for MTA protection was due to competition between adenine and purine analogs for rate limiting pools of PRPP. In the work described here, we have performed a series of experiments designed to extend and validate various aspects of the Lubins’ hypothesis using both isogenic cell lines and pharmacologic approaches. Our findings support their proposal and provide significant preclinical data suggesting that high dose purine analogs in combination with MTA might be a useful therapeutic approach in the treatment of MTAP- cancers.
Results
Effect of MTA on 6TG and 5FU toxicity in isogenic MTAP+ and MTAP- HT1080 cells
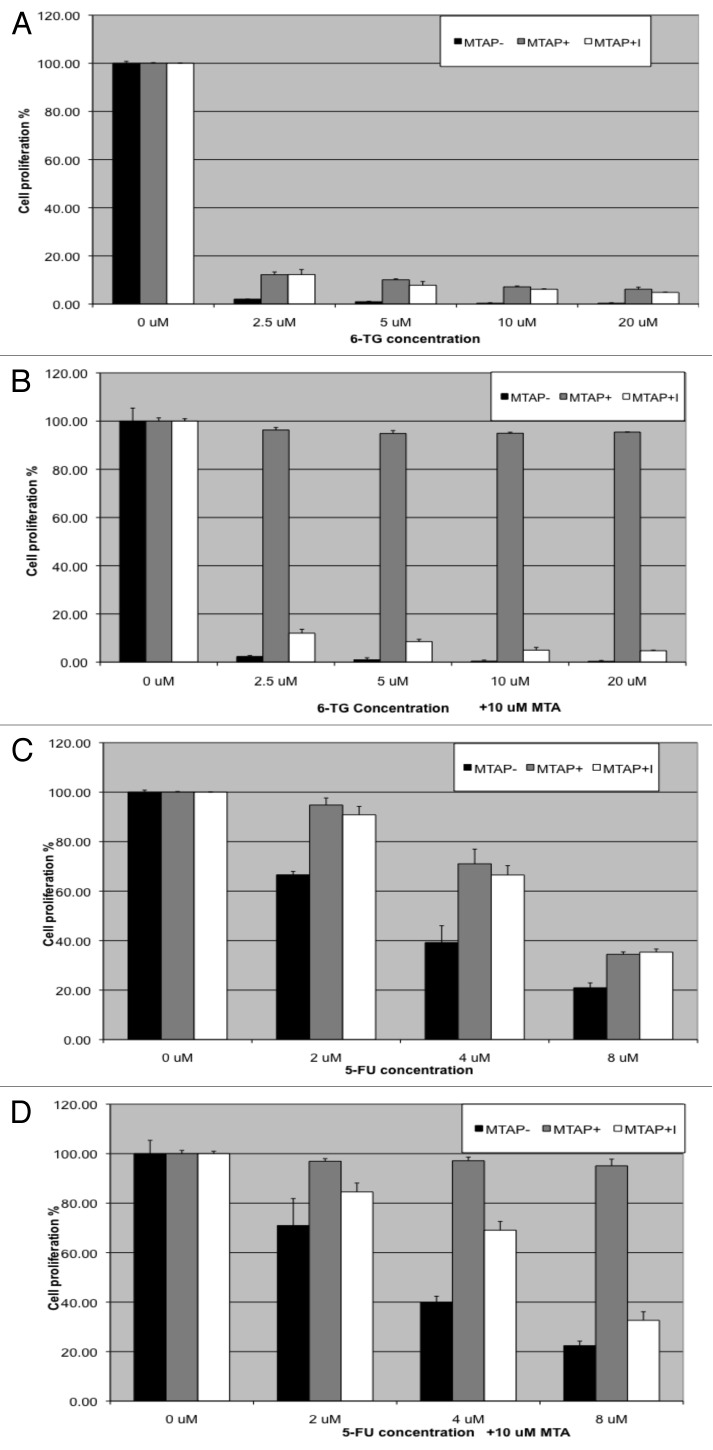
HT1080 is fibrosarcoma cell line that has no detectable expression of the MTAP gene.25 To create isogenic MTAP+ and MTAP- cells, we stably transfected HT1080 cells with either an empty expression vector (pTRE2) or a vector that expresses MTAP at high levels (pTRE2:MTAP). To control for any possible position effect variability, we pooled 12 expressing clones and 12 non-expressing clones to form two composite cell lines, MTAP+ or MTAP- (Fig. 2A and B). The amount of MTAP expressed was similar to that observed in non-MTAP-deleted HeLa cells, indicating that we are expressing MTAP at near physiological levels. Each of these pooled cell lines was then exposed to several different concentrations of 6TG and 5FU, in the presence of variable MTA levels for 48 h (Fig. 2C and D). In the absence of MTA, we found that as little as 5 μM 6TG resulted in > 85% growth inhibition in both MTAP+ and MTAP- cells (Fig. 2C, first cluster). In contrast, when 10 μM MTA was added, we observed nearly complete rescue of the MTAP+ cells but no rescue of MTAP- cells (Fig. 2C, second cluster). Interestingly, 10 μM MTA was sufficient for complete rescue even when doses of 6TG as high as 40 μM were used. We also performed identical studies using 5FU (Fig. 2D). Although 5FU was somewhat less efficient than 6TG at inhibiting proliferation of HT1080 cells, we observed the same behavior with respect to MTA protection, i.e., protection of MTAP+, but not MTAP-, cell lines.
Figure 2. MTA protection in isogenic MTAP+ and MTAP- HT1080 cells. (A) Western analysis of MTAP+ and MTAP- HT1080 cells. HeLa cells have been included to show that MTAP levels are not significantly different than those observed in endogenous MTAP+ cells. (B) MTAP enzyme activity. MTAP enzyme activity was measured in same cell lysates used in the western. (C) Inhibition of cell viability by 6TG in combination with MTA. MTAP- (black bars), or MTAP+ cells (gray bars) were exposed to 6TG concentrations ranging from 0–40 μM for 48 h. The experiment was performed in either the absence or presence of the indicated amounts of MTA. Cell viability was assessed using an MTS assay, and all results are given as percent cell viability compared with the untreated parental cell line. (D) Same as (C), except 5FU doses ranging between 0–4 μM were used instead of 6TG.
MTAP enzymatic activity is required for MTA rescue
To confirm that MTAP enzymatic activity was required for MTA rescue, we performed a series of experiments using a pharmacologic inhibitor of MTAP called MT-DADMe-ImmA. This compound mimics the transition-state of bound MTA and binds extremely tightly to the active site with a Ki of 86 pM, thereby inhibiting MTAP’s enzymatic activity.26 For these experiments, we examined the effect of 6TG and 5FU in the absence and presence of MTA on MTAP- cells, MTAP+ cells, and MTAP+ cells treated with DADMe-ImmA. As expected from our previous experiment, we found that treatment with MTA protected only MTAP+ cells from cell killing by 6TG and 5FU (Fig. 3). Treatment with MT-DADMe-ImmA had no effect on cell proliferation by itself and no effect on the sensitivity of cells treated with either 5FU or 6TG. However, the key finding was that MTAP+ cells were no longer protected by MTA if MT-DADMe-ImmA was added to the media. These results imply that MTAP’s enzymatic activity is required for the protective effect of MTA against 6TG and 5FU.
Figure 3. MTAP enzymatic activity is required for MTA rescue. (A) Inhibition of cell viability by 6TG of MTAP- (black bars), MTAP+ cells (gray bars), or MTAP+ cells treated with 1 μM of the MTAP inhibitor MT-DADMe-ImmA for 48 h was assessed. Cell viability was measured using an MTS assay, and all results are given as percent cell viability compared with that of the untreated parental cell line. (B) Same as (A)., except that 10 μM MTA was added to each well. (C) Growth inhibition by 5FU of same three cell lines as used in (A). (D) Same as (C), exempt that 10 μM MTA was added to each well.
Adenine, but not thymidine, protects both MTAP+ and MTAP- cells from 6TG and 5FU
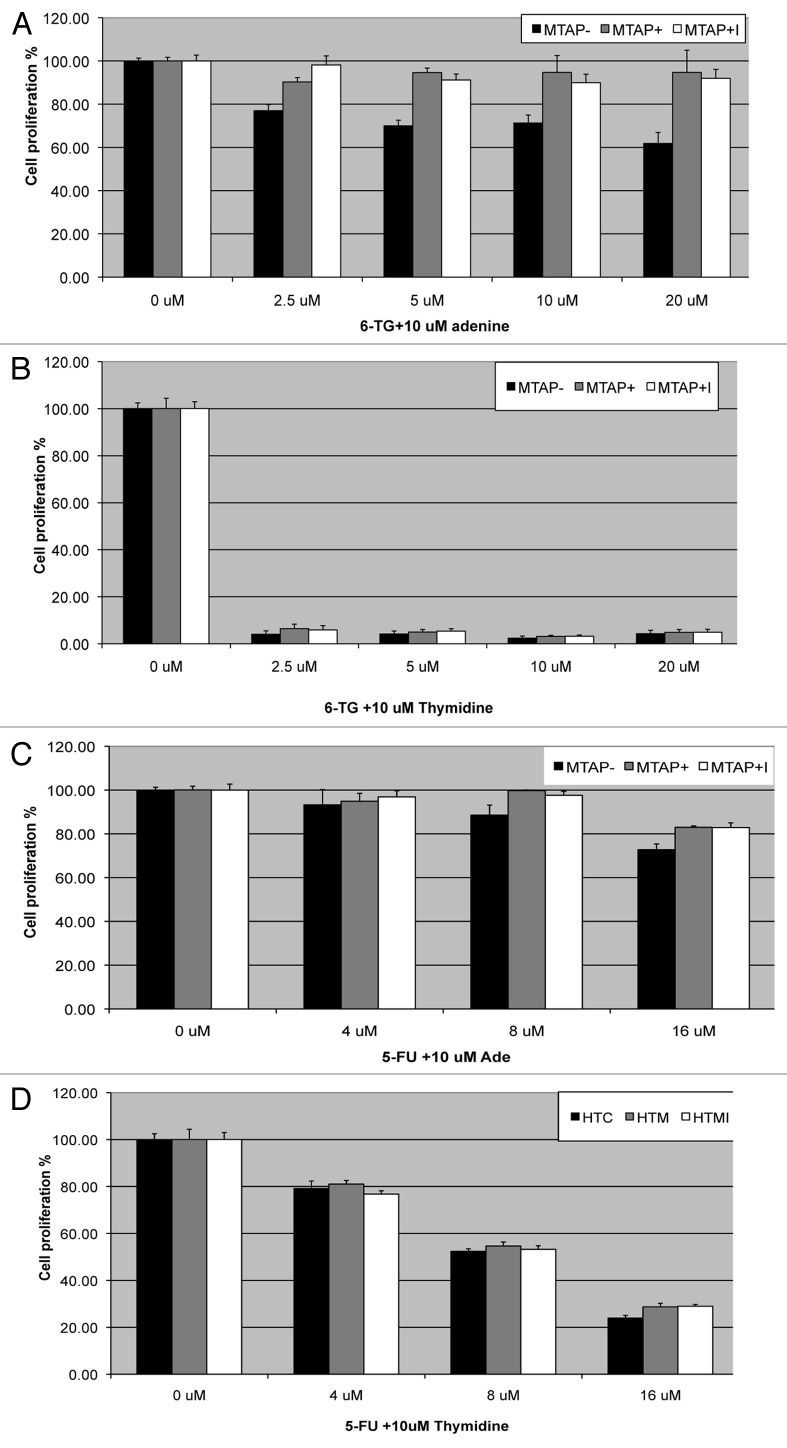
Based on Lubins’ hypothesis, the protection by MTA in MTAP+ cells is due to the production of adenine from MTA. If correct, then addition of adenine should directly be able to protect cells from both 6TG and 5-FU independent of MTAP status. Therefore, we examined how the addition of extracellular adenine affected response to 6TG and 5FU. As predicted, we found that addition of adenine could protect both MTAP+ and MTAP- cells, supporting the idea that adenine inhibits the conversion of 6TG and 5FU to nucleosides via competition with PRPP (Fig. 4A and C). As a control, we also treated cells with thymidine, which is not a substrate for a PRPP based phosphoribosylation reaction (Fig. 4B and D). As expected, thymidine had no ability to protect either MTAP+ and MTAP- cells from 6TG and 5FU toxicity.
Figure 4. Adenine rescue of MTAP+ and MTAP- cells. (A) Inhibition of cell viability by 6TG of MTAP- (black bars), MTAP+ cells (gray bars), or MTAP+ cells treated with the indicated amount of 6TG in combination with 10 μM adenine for 48 h. Cell viability was assessed using an MTS assay, and all results are given as percent viability compared with that of the untreated parental cell line. (B) Identical to (A), except cells were treated with 10 μM thymidine instead of adenine. (C) Inhibition of cell viability by the indicated amount of 5FU in combination with 10 μM adenine. (D) Same as (C), except cells were treated with 10 μM thymidine instead of adenine.
MTA protection of MTAP+ and MTAP- mouse mesothelioma cell lines
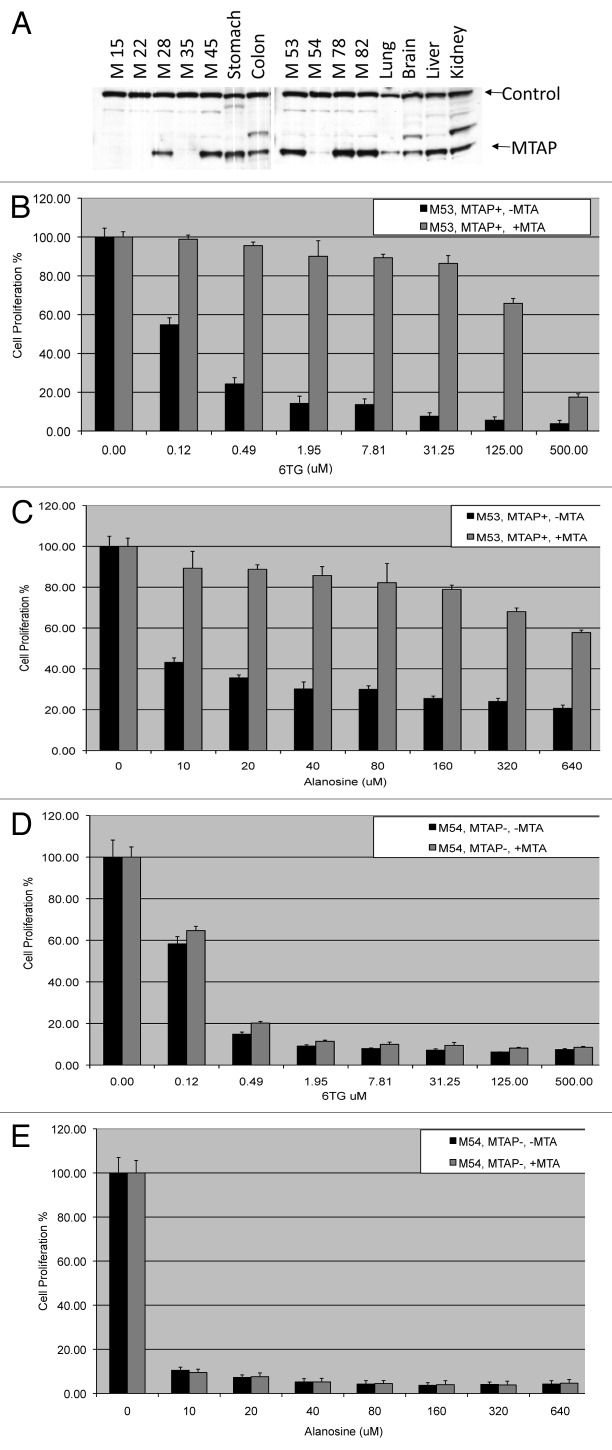
We next examined MTAP status and MTA protection in a series of congenic mouse mesothelioma cell lines. These cell lines were derived from malignant effusions obtained from a series of Nf2+/− mice that developed mesotheliomas following multiple intraperitoneal injections of asbestos.27 Tumors from these mice faithfully recapitulate many of the molecular features of human malignant mesothelioma, including frequent homologous deletions encompassing the murine Cdkn2a/Arf locus and nearby Cdkn2b tumor suppressor gene. Altogether, 6 of 9 MM cell lines from Nf2+/− mice tested showed loss of Cdkn2a/Arf expression, and 4 of these 9 MM lines also showed loss of MTAP expression (Fig. 5A and Fig. S1).
Figure 5. MTAP status and MTA protection in mouse mesothelioma cell lines. (A) Mouse mesothelioma cell lines, which were derived from mesotheliomas induced by treating Nf2 +/− animals with asbestos,27 were examined for MTAP expression by western analysis. Control band is 50 Kd protein of unknown identity that cross-reacts with MTAP anti-serum. (B) MTAP+ mesothelioma cell line M53 was treated with the indicated levels of 6TG for 48 h in the presence or absence of 10 μM MTA. Cell viability was assessed using an MTS assay, and all results are given as percent change compared with that of the untreated parental cell line. (C) Identical to (B) except MTAP- M54 cell line was used. (D) MTAP+ mesothelioma cell line M53 was treated with the indicated levels of L-alanosine for 48 h in the presence or absence of 10 μM MTA. (E) MTAP- mesothelioma cell line M54 was treated with the indicated levels of L-alanosine for 48 h in the presence or absence of 10 μM MTA.
We treated 7 of our 9 MM lines from Nf2+/− mice (M15, M28, M45, M53, M54, M78, M82) with increasing concentrations of 6TG in the presence or absence of 10 μM MTA. We observed significant protection with 6TG in all five of the MTAP positive lines, and no protection of the two MTAP-null lines (Fig. 5B and C and Fig. S2). In MTAP+ cells, we found that the IC50 of 6TG increased 34–1400 fold in the presence of MTA (Table 1). These findings show that MTAP is required for MTA protection from 6TG in murine mesothelioma cells and that MTA addition can increase the IC50 of 6TG in MTAP+ cells by several orders of magnitude.
Table 1. IC50 values for L-alanosine and 6-TG.
| |
L-alanosine IC50 (μM) |
6TG uM IC50 |
||||
|---|---|---|---|---|---|---|
| 0 μM MTA | 10 μM MTA | Fold change | 0 μM MTA | 10 μM MTA | Fold change | |
|
M28 (MTAP+) |
24.9 |
1085 |
43.6 |
0.33 |
167.1 |
509.0 |
|
M45 (MTAP+) |
117.4 |
1569 |
13.4 |
0.18 |
248.9 |
1419.9 |
|
M54 (MTAP-) |
0.27 |
0.21 |
0.8 |
0.12 |
0.16 |
1.3 |
|
M78 (MTAP+) |
11.4 |
870.2 |
76.3 |
16.3 |
565.7 |
34.7 |
|
M5 (MTAP-) |
11.9 |
17.5 |
1.5 |
0.22 |
0.38 |
1.7 |
|
M53 (MTAP+) |
14.9 |
966.2 |
65.0 |
0.14 |
192.8 |
1368.3 |
| M82 (MTAP+) | 147.5 | 470.9 | 3.2 | 0.56 | 57.5 | 102.1 |
We also examined these lines for sensitivity and protection from the drug L-alanosine, a potent inhibitor of AMP biosynthesis.28 We reasoned that MTAP catalyzed conversion of MTA to adenine in MTAP+ cells could restore adenine and AMP levels in L-alanosine treated cells, and thus counteract its growth inhibitory effects. We found that all of the mesothelioma lines were sensitive to L-alanosine, but only the MTAP+ lines had L-alanosine induced growth inhibition reversed by the addition of MTA (Fig. 5D and E and Fig. S3). In MTAP+ cells, we found that the IC50 of L-alanosine increased 3.2- to 76.3- fold in the presence of MTA (Table 1). These findings show that MTAP catalyzed conversion of MTA to adenine can also protect cells from drugs that inhibit AMP biosynthesis.
Since both 6TG and L-alanosine were effective in inhibiting mesothelioma cell, we examined the effects of combining these two agents in the presence or absence of MTA in two different MTAP+ cell lines. Somewhat surprisingly, we did not observe any increased inhibition of cell viability by combining these two agents and actually observed less overall inhibition when the agents were combined (Fig. S4), suggesting these two agents are somehow antagonistic. Nonetheless, we did still observe significantly less growth inhibition in MTAP+ cells treated with MTA.
MTA protection of MEF cells
Our findings above show that it is possible to protect MTAP+ tumor cells from the harmful effects of 6TG and 5FU by addition of MTA. Since all normal tissues seem to have significant MTAP activity levels, we next wanted to examine if non-transformed MTAP+ cells could also be protected from these agents. Therefore, we exposed two different MTAP+ early passage primary MEF lines to 6TG and 5FU in the presence or absence of MTA (Fig. S5). For 6TG, we observed significant inhibition of cell viability of MEFs at 20 μM or greater concentration, and this inhibition was entirely reversible by addition of 10 μM MTA. Unexpectedly, the MEFs were not very sensitive to 5FU (only 40% decrease in cell viability observed at 20 μM), and this modest inhibition was not able to be rescued by MTA.
Discussion
The experiments in this paper explore the feasibility of developing a targeted chemotherapeutic strategy by taking advantage of the loss of MTAP observed in a wide assortment of tumor types. The strategy involves combining either a toxic purine analog or an inhibitor of purine biosynthesis with the MTAP substrate MTA. The logic behind the strategy is that in MTAP+ cells, MTA will be converted to adenine, which then combines with PRPP to form AMP by the action of adenine phosphoribosyltransferases. Because cellular PRPP levels are low,24 this would deplete PRPP, leaving none available for the conversion of toxic base analogs such as 6TG or 5FU to their respective nucleotide forms. Without this conversion, these analogs cannot be inserted into DNA and therefore are not toxic. In contrast, MTAP- cells lack the ability to convert MTA to adenine and therefore PRPP is available for toxic nucleotide formation.
In the experiments reported here, we have verified and extended several key components of this strategy. First, using isogenic MTAP+ and MTAP- cell lines as well as a pharmacological inhibitor of MTAP, we demonstrated that MTAP expression is required for MTA protection against toxicity from the base analogs 6TG and 5FU. Second, we have shown that the reason for MTAP’s ability to potentiate protection by MTA is because of the production of adenine. Unlike MTA addition, adenine protects both MTAP+ and MTAP- cells equally well. Third, we have shown that MTA protection by MTAP occurs in at least three different cell types, human fibrosarcoma cells, mouse mesothelioma cells, and MEFs. Finally, we have shown that MTA can also protect against toxicity to L-alanosine, a drug that specifically inhibits the de novo production of inosine monophosphate (IMP) and downstream AMP biosynthesis.
Our results support the idea that a combination of purine analogs and MTA might be useful in treating MTAP-deficient tumors. It should be noted that purine analogs, such as 6TG and 5FU, are already used in the clinic. 6TG is used to treat childhood leukemia, while 5FU is used to treat primarily colon and pancreatic cancer. MTA is not currently used in the clinic, but there are data indicating that the drug would be safe. In rats, MTA has been reported to have protective effects against liver damage and, when given intraperitoneally to rodents over extended periods, showed no toxicity.29,30 In addition, a patent application claims that oral MTA given at 1600 mg/day to volunteers for one month did not exhibit toxicity.31 By combining MTA with 6TG, 5FU, or other purine anti-metabolites, it may be possible to use far higher doses of these purine analogs than have been previously used. Based our in vitro data, we see that MTA protection is quite remarkable, with at least 10- to 100-fold changes in IC50 levels (Table 1). These findings imply that potentially much higher levels of 6TG and 5FU could be given than is currently possible. There is some limited in vivo data already suggesting that MTA can protect against 5FU and 6TG toxicity. Bertino et al. reported that co-administration of 100 mg/Kg MTA protected nude mice from a lethal dose of 5FU, although the number of animals used in these studies were very small.32 In addition, Collins and colleagues reported that six mice exposed to three treatments of 75 mg/Kg 6TG+MTA survived to 12 d of age (when the experiment was terminated), while 0/6 control animals treated with 75 mg/Kg 6TG alone were alive at this time point.33 These data, along with the data presented here, indicate that additional animal studies should be performed to determine more precisely how much the therapeutic window could be expanded.
One potential caveat to this approach is that there is no guarantee that even high dose 5FU or 6TG will be effective in killing a particular tumor type. Historically, 6TG as well as other purine analogs have not been successful in treating solid tumors.34 However the potential order of magnitude increase in 6TG that could potentially by achieved by combining with MTA could entirely change the equation. In addition, for MTAP- tumors that are already known to be responsive to 6TG or 5FU (or potentially anti-folates), the addition of MTA might also be useful in ameliorating side effects even at currently used dosages.
In summary, the work presented here demonstrates that a variety of different MTAP+, but not MTAP-, cells can be protected from toxic base analogs by simultaneous incubation with MTA. We have shown that this protection is due to the enzymatic activity of MTAP, which converts MTA to adenine. These findings suggest that it may be possible to selectively kill tumor cells lacking MTAP expression by using high concentrations of toxic base analogs such as 6TG or 5FU in combination with MTA.
Methods and Methods
Plasmid construction and cell lines
Isogenic MTAP+ and MTAP- cell lines were made as follows. Plasmid pTRE2:MTAP was created by inserting a hMTAP containing BamHI/EcoRV fragment from pCR:sMTAP25 into pTRE2hyg (Clontech). Plasmid pTRE2:MTAP and pTRE2 vector alone were then used to stably transfect HT1080 cells (Clontech) using Fugene6 reagent (Roche) according to the manufacturer's instructions. Cells were cultured in DMEM medium supplemented with 2 mM glutamine, 100 μg/ml penicillin, 100 μg/ml streptomycin, 10% fetal bovine serum (FBS), and 250 μg/ml G418. Clones were selected using 250 μg/ml Hygromycin from a 50 mg/ml stock solution in PBS (Sigma). All media, serum, and antibiotics were obtained from the Tissue Culture Core Facility at Fox Chase Cancer Center (FCCC). Twelve clones from each transfection were isolated using a cloning cylinder, and each of these clones was then expanded for subsequent expression analysis. All 12 of the clones expressed MTAP at similar levels as judged by both western blot and enzyme activity analysis. MTAP immunoblot and enzyme activity analysis were performed as previously described.22
Mouse embryonic fibroblast (MEF) cell lines were derived from C57B6 14-d-old embryos by the FCCC Tissue Culture Facility. Congenic mouse mesothelioma cell lines were created as previously described.27
Viability assays
Cells were seeded onto a 96-well cell culture plate in the medium mentioned above, at 2,000 cells/0.2 mL per well. After seeding for 24 h, testing agents were added at the indicated concentrations to a total volume of 10 μl. Each treatment was performed in triplicate. Cell proliferation was determined by MTS assay with CellTiter 96 Aqueous One Solution (Promega) following the manufacturer's instructions. The optical absorbance of the MTS metabolite formazin was measured by reading absorbance at 492 nm wavelength using an automated plate reader, ELx808 (BioTek Instruments). 6TG and MTA were obtained from Sigma and were dissolved in DMSO. DADMe-ImmA was obtained from Dr Vern Schramm, Albert Einstein College of Medicine. L-alanosine was a generous gift from Dr Dennis Carson, University of California at San Diego. Dose response curve and IC50 were created and calculated with nonlinear regression analysis by using GraphPad Prism 4.0 software (GraphPad Software, Inc.). All graphs show standard deviations.
Supplementary Material
Acknowledgments
This research was supported by CA06927 and CA131024 from the NIH and an appropriation from the Commonwealth of Pennsylvania. J.R.T. was supported in part by CA-114047. We thank Fang Jin in the FCCC Tissue Culture Facility for the preparation of the MEF lines used in this study and Deborah Altomare for assistance with the culture and expansion of mouse mesothelioma cell lines. We thank Dr. Schramm and Dr. Carson for providing drugs used in the study. We also thank Burt and Adam Lubin for critical reading of the manuscript.
Glossary
Abbreviations:
- 5FU
5-fluorouracil
- 6TG
6-thioguanine
- MTAP
Methylthioadenosine phosphorylase
- MTA
5′-deoxy-5′-methylthioadenosine
- MTR-1-P
5-methylthioribose-1-phosphate
- PRPP
phosphoribosyl-5-pyrophosphate
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/21115
References
- 1.Robins RK, Revankar GR. Purine analogs and related nucleosides and nucleotides as antitumor agents. Med Res Rev. 1985;5:273–96. doi: 10.1002/med.2610050302. [DOI] [PubMed] [Google Scholar]
- 2.Elgemeie GH. Thioguanine, mercaptopurine: their analogs and nucleosides as antimetabolites. Curr Pharm Des. 2003;9:2627–42. doi: 10.2174/1381612033453677. [DOI] [PubMed] [Google Scholar]
- 3.Nobori T, Takabayashi K, Tran P, Orvis L, Batova A, Yu AL, et al. Genomic cloning of methylthioadenosine phosphorylase: a purine metabolic enzyme deficient in multiple different cancers. Proc Natl Acad Sci U S A. 1996;93:6203–8. doi: 10.1073/pnas.93.12.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subhi AL, Tang B, Balsara BR, Altomare DA, Testa JR, Cooper HS, et al. Loss of methylthioadenosine phosphorylase and elevated ornithine decarboxylase is common in pancreatic cancer. Clin Cancer Res. 2004;10:7290–6. doi: 10.1158/1078-0432.CCR-04-0972. [DOI] [PubMed] [Google Scholar]
- 5.Hustinx SR, Hruban RH, Leoni LM, Iacobuzio-Donahue C, Cameron JL, Yeo CJ, et al. Homozygous deletion of the MTAP gene in invasive adenocarcinoma of the pancreas and in periampullary cancer: a potential new target for therapy. Cancer Biol Ther. 2005;4:83–6. doi: 10.4161/cbt.4.1.1380. [DOI] [PubMed] [Google Scholar]
- 6.Schmid M, Malicki D, Nobori T, Rosenbach MD, Campbell K, Carson DA, et al. Homozygous deletions of methylthioadenosine phosphorylase (MTAP) are more frequent than p16INK4A (CDKN2) homozygous deletions in primary non-small cell lung cancers (NSCLC) Oncogene. 1998;17:2669–75. doi: 10.1038/sj.onc.1202205. [DOI] [PubMed] [Google Scholar]
- 7.Brat DJ, James CD, Jedlicka AE, Connolly DC, Chang E, Castellani RJ, et al. Molecular genetic alterations in radiation-induced astrocytomas. Am J Pathol. 1999;154:1431–8. doi: 10.1016/S0002-9440(10)65397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T, Maruno M, Wada K, Kagawa N, Fujimoto Y, Hashimoto N, et al. Genetic analysis of human glioblastomas using a genomic microarray system. Brain Tumor Pathol. 2004;21:27–34. doi: 10.1007/BF02482174. [DOI] [PubMed] [Google Scholar]
- 9.Stadler WM, Olopade OI. The 9p21 region in bladder cancer cell lines: large homozygous deletion inactivate the CDKN2, CDKN2B and MTAP genes. Urol Res. 1996;24:239–44. doi: 10.1007/BF00295899. [DOI] [PubMed] [Google Scholar]
- 10.García-Castellano JM, Villanueva A, Healey JH, Sowers R, Cordon-Cardo C, Huvos A, et al. Methylthioadenosine phosphorylase gene deletions are common in osteosarcoma. Clin Cancer Res. 2002;8:782–7. [PubMed] [Google Scholar]
- 11.Dreyling MH, Roulston D, Bohlander SK, Vardiman J, Olopade OI. Codeletion of CDKN2 and MTAP genes in a subset of non-Hodgkin’s lymphoma may be associated with histologic transformation from low-grade to diffuse large-cell lymphoma. Genes Chromosomes Cancer. 1998;22:72–8. doi: 10.1002/(SICI)1098-2264(199805)22:1<72::AID-GCC10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.M’soka TJ, Nishioka J, Taga A, Kato K, Kawasaki H, Yamada Y, et al. Detection of methylthioadenosine phosphorylase (MTAP) and p16 gene deletion in T cell acute lymphoblastic leukemia by real-time quantitative PCR assay. Leukemia. 2000;14:935–40. doi: 10.1038/sj.leu.2401771. [DOI] [PubMed] [Google Scholar]
- 13.Mirebeau D, Acquaviva C, Suciu S, Bertin R, Dastugue N, Robert A, et al. EORTC-CLG The prognostic significance of CDKN2A, CDKN2B and MTAP inactivation in B-lineage acute lymphoblastic leukemia of childhood. Results of the EORTC studies 58881 and 58951. Haematologica. 2006;91:881–5. [PubMed] [Google Scholar]
- 14.Batova A, Diccianni MB, Nobori T, Vu T, Yu J, Bridgeman L, et al. Frequent deletion in the methylthioadenosine phosphorylase gene in T-cell acute lymphoblastic leukemia: strategies for enzyme-targeted therapy. Blood. 1996;88:3083–90. [PubMed] [Google Scholar]
- 15.Hori Y, Hori H, Yamada Y, Carrera CJ, Tomonaga M, Kamihira S, et al. The methylthioadenosine phosphorylase gene is frequently co-deleted with the p16INK4a gene in acute type adult T-cell leukemia. Int J Cancer. 1998;75:51–6. doi: 10.1002/(SICI)1097-0215(19980105)75:1<51::AID-IJC9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Karikari CA, Mullendore M, Eshleman JR, Argani P, Leoni LM, Chattopadhyay S, et al. Homozygous deletions of methylthioadenosine phosphorylase in human biliary tract cancers. Mol Cancer Ther. 2005;4:1860–6. doi: 10.1158/1535-7163.MCT-05-0103. [DOI] [PubMed] [Google Scholar]
- 17.Chen YJ, Lin SC, Kao T, Chang CS, Hong PS, Shieh TM, et al. Genome-wide profiling of oral squamous cell carcinoma. J Pathol. 2004;204:326–32. doi: 10.1002/path.1640. [DOI] [PubMed] [Google Scholar]
- 18.Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–13. [PubMed] [Google Scholar]
- 19.Wong YF, Chung TK, Cheung TH, Nobori T, Chang AM. MTAP gene deletion in endometrial cancer. Gynecol Obstet Invest. 1998;45:272–6. doi: 10.1159/000009983. [DOI] [PubMed] [Google Scholar]
- 20.Berasain C, Hevia H, Fernández-Irigoyen J, Larrea E, Caballería J, Mato JM, et al. Methylthioadenosine phosphorylase gene expression is impaired in human liver cirrhosis and hepatocarcinoma. Biochim Biophys Acta. 2004;1690:276–84. doi: 10.1016/j.bbadis.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Christopher SA, Diegelman P, Porter CW, Kruger WD. Methylthioadenosine phosphorylase, a gene frequently codeleted with p16(cdkN2a/ARF), acts as a tumor suppressor in a breast cancer cell line. Cancer Res. 2002;62:6639–44. [PubMed] [Google Scholar]
- 22.Kadariya Y, Yin B, Tang B, Shinton SA, Quinlivan EP, Hua X, et al. Mice heterozygous for germ-line mutations in methylthioadenosine phosphorylase (MTAP) die prematurely of T-cell lymphoma. Cancer Res. 2009;69:5961–9. doi: 10.1158/0008-5472.CAN-09-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubin M, Lubin A. Selective killing of tumors deficient in methylthioadenosine phosphorylase: a novel strategy. PLoS One. 2009;4:e5735. doi: 10.1371/journal.pone.0005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardalan B, Villacorte D, Heck D, Corbett T. Phosphoribosyl pyrophosphate, pool size and tissue levels as a determinant of 5-fluorouracil response in murine colonic adenocarcinomas. Biochem Pharmacol. 1982;31:1989–92. doi: 10.1016/0006-2952(82)90410-5. [DOI] [PubMed] [Google Scholar]
- 25.Tang B, Li YN, Kruger WD. Defects in methylthioadenosine phosphorylase are associated with but not responsible for methionine-dependent tumor cell growth. Cancer Res. 2000;60:5543–7. [PubMed] [Google Scholar]
- 26.Singh V, Shi W, Evans GB, Tyler PC, Furneaux RH, Almo SC, et al. Picomolar transition state analogue inhibitors of human 5′-methylthioadenosine phosphorylase and X-ray structure with MT-immucillin-A. Biochemistry. 2004;43:9–18. doi: 10.1021/bi0358420. [DOI] [PubMed] [Google Scholar]
- 27.Altomare DA, Vaslet CA, Skele KL, De Rienzo A, Devarajan K, Jhanwar SC, et al. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005;65:8090–5. doi: 10.1158/0008-5472.CAN-05-2312. [DOI] [PubMed] [Google Scholar]
- 28.Kindler HL, Burris HA, 3rd, Sandler AB, Oliff IA. A phase II multicenter study of L-alanosine, a potent inhibitor of adenine biosynthesis, in patients with MTAP-deficient cancer. Invest New Drugs. 2009;27:75–81. doi: 10.1007/s10637-008-9160-1. [DOI] [PubMed] [Google Scholar]
- 29.Simile MM, Banni S, Angioni E, Carta G, De Miglio MR, Muroni MR, et al. 5′-Methylthioadenosine administration prevents lipid peroxidation and fibrogenesis induced in rat liver by carbon-tetrachloride intoxication. J Hepatol. 2001;34:386–94. doi: 10.1016/S0168-8278(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 30.Wolford RW, Riscoe MK, Johnson L, Ferro AJ, Fitchen JH. Effect of 5′-methylthioadenosine (a naturally occurring nucleoside) on murine hematopoiesis. Exp Hematol. 1984;12:867–71. [PubMed] [Google Scholar]
- 31.Moratti EM. Inventor. Pharmaceutical compositions containing 5′-deoxy-5′-methylthioadenosine S-adenosylmethionine and their salts for reducing seborrhea USA. 1990. U.S. Patent No. 5753213.
- 32.Bertino JR, Waud WR, Parker WB, Lubin M. Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: current strategies. Cancer Biol Ther. 2011;11:627–32. doi: 10.4161/cbt.11.7.14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins CC, Volik SV, Lapuk AV, Wang Y, Gout PW, Wu C, et al. Next generation sequencing of prostate cancer from a patient identifies a deficiency of methylthioadenosine phosphorylase, an exploitable tumor target. Mol Cancer Ther. 2012;11:775–83. doi: 10.1158/1535-7163.MCT-11-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters GJ, Schornagel JH, Milano GA. Clinical pharmacokinetics of anti-metabolites. Cancer Surv. 1993;17:123–56. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.