Abstract
Purpose
To investigate symptom patterns and evaluate the relationship between patient characteristics and symptom severity before and after treatment for symptomatic children with convergence insufficiency (CI).
Methods
In a randomized clinical trial, the Convergence Insufficiency Symptom Survey (CISS) was administered pre- and post-treatment to 221 children 9 to <18 years with symptomatic CI. Frequency of symptom type was determined at baseline, mean change in performance-related versus eye-related symptoms for treatment responders was compared, and the relationship between patient characteristics and symptom severity at baseline for the entire cohort and after treatment for those who responded to treatment, was determined.
Results
At baseline, the score for performance-related symptoms was greater than that for eye-related symptoms (mean response of 2.3 vs. 1.8, p<0.001) regardless age, sex, race/ethnicity, or presence of parent-reported ADHD. Symptom severity increased with age for both the overall and eye-related subscale scores (p=0.048, p=0.022, respectively). Children with parent-reported ADHD were more symptomatic (p=0.005) than those without parent-reported ADHD because of a higher performance-related score (p<0.001). A significant and equal improvement (p<0.01) for the performance-related and eye-related symptoms was found in treatment responders. Girls had significantly lower performance-related symptoms than boys (p=0.014) and African-American children reported less eye-related symptoms than White children (p=0.022). Children without parent-reported ADHD had significantly less symptoms overall and less eye-related symptoms than children with parent-reported ADHD (p=0.019, p=0.011, respectively).
Conclusions
Because of a high frequency of both performance- and eye-related symptoms, clinicians should perform a targeted history that addresses both types of symptoms to help identify children with symptomatic CI. Future study regarding the relationship of CI and symptoms and their potential influence on ADHD, reading performance, and attention is warranted.
Keywords: convergence insufficiency, asthenopia, vision therapy, orthoptics, quality of life, vergence/accommodative therapy, exophoria, eyestrain, symptom survey, reading, ADHD
Convergence insufficiency (CI) is a common binocular vision disorder 1–4 that is often associated with symptoms that occur when a person reads or performs close work. Complaints such as eyestrain, headaches, blurred vision, diplopia, sleepiness, loss of place, difficulty concentrating, movement of print and poor comprehension after short periods of reading or performing near activities are often reported. 5–12 To quantify the frequency and severity of symptoms reported by individuals with symptomatic CI, the Convergence Insufficiency Symptom Survey (CISS) was developed.13–16 A self-report symptom inventory, the CISS has been shown to have good construct validity and reliability,14–16 and been used as an outcome measure for clinical trials evaluating treatment modalities for children and adults with symptomatic CI.17–20
The CISS uses a Likert-type scale with responses from 15 items summed to obtain an overall CISS score, with symptom severity ranging from 0 (best) to 60 (worst). Although it has been suggested13 that the CISS items are comprised of 2 categories of items – performance-related (e.g., difficulty concentrating when reading or studying) and eye-related (e.g., double vision) symptoms, the overall CISS score has been the only measure reported for the CI treatment trials.17–20
The CISS was used to quantify symptoms before and after treatment for 221 children with symptomatic CI enrolled into the Convergence Insufficiency Treatment Trial (CITT).20 While the overall CISS scores at baseline and outcome and after 1 year of follow-up have been reported,20–22 the frequency of occurrence of specific symptoms at baseline, and the relationship between patient characteristics and symptom severity have not been evaluated. The purpose of this report is to describe symptom patterns and to evaluate the relationship between patient characteristics and symptom severity before and after treatment.
METHODS
The study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, Department of Health and Human Services, and was conducted by the CITT Group. The protocol and Health Insurance Portability and Accountability Act (HIPAA) compliant informed consent forms were approved by the institutional review boards for participating sites and a parent or guardian of each study subject gave written informed consent. Each subject gave assent as required. An independent Data Safety Monitoring Committee provided study oversight. The study is registered at www.clinicaltrials.gov under identifier NCT0033861123 and the manual of procedures is available at http://optometry.osu.edu/research/CITT/index.cfm. The examination and treatment procedures have been reported previously.21 Major eligibility criteria for the trial and the procedure for the administration of the CISS are summarized below.
Subjects
Major eligibility criteria for the trial included children aged 9 to <18 years with symptomatic CI defined as: an exodeviation at near at least 4 prism diopters (Δ) greater than at far, a receded near point of convergence (NPC) break (6 cm or greater), insufficient positive fusional vergence at near (PFV) (convergence amplitudes) (i.e., failing Sheard’s criterion [PFV less than twice the near phoria]24 or minimum PFV of ≤15Δ base-out blur or break), and a CISS (described below) score of ≥16. In addition, children were required to have best-corrected visual acuity at distance and near of 20/25 or better, no constant strabismus, no vertical phoria greater than 1Δ, and a monocular accommodative amplitude greater than 5 D. All testing was performed with the appropriate refractive correction in place. The complete eligibility and exclusion criteria have been reported previously.21
Convergence Insufficiency Symptom Survey (CISS) Administration
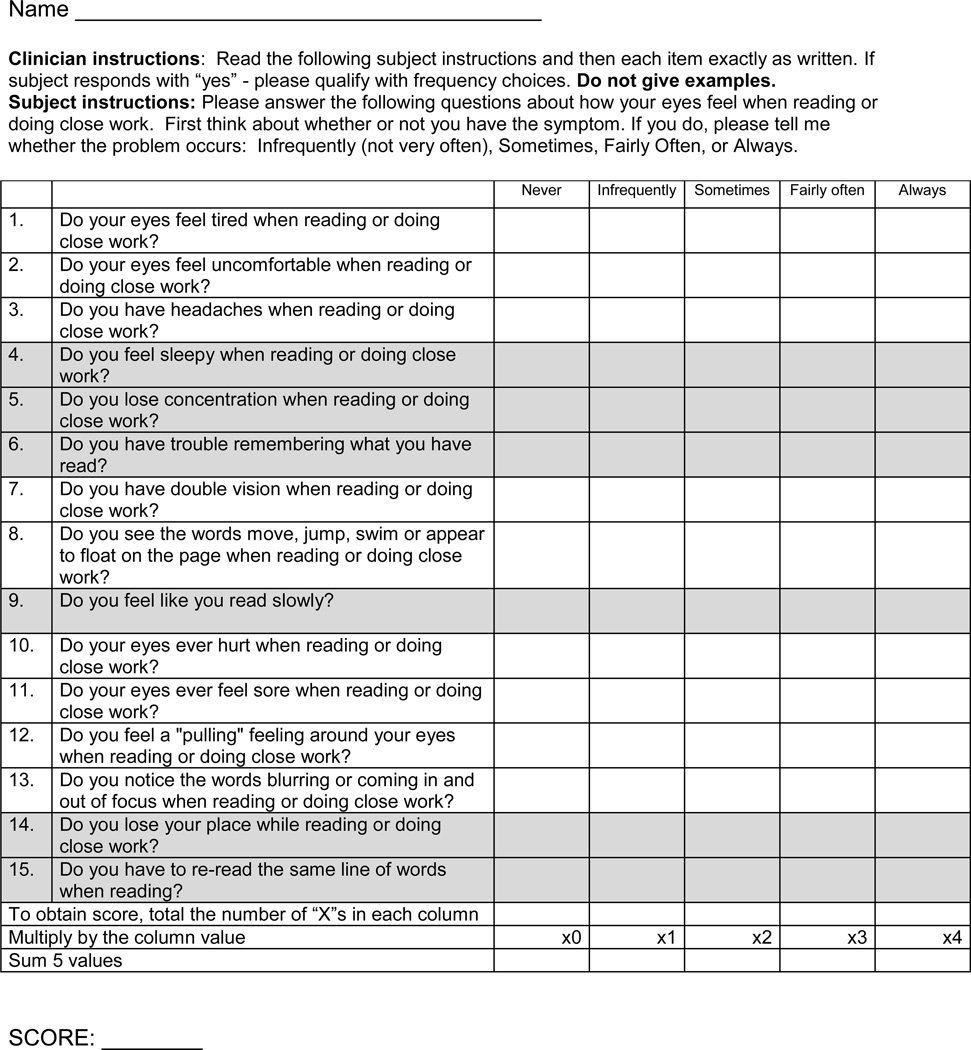
The CISS (Figure 1) was administered to each child by a trained and certified examiner who was masked to the child’s treatment assignment. The examiner sequentially read each of the 15 symptom questions aloud to the child while the child viewed a card with the 5 possible response options of never, infrequently, sometimes, fairly often, or always. Each response option corresponded to a numerical value ranging from 0 points for never to 4 points for always. The 15 items were summed to obtain the total CISS score. The score could range from 0 (least symptomatic) to 60 (most symptomatic; reporting always for all 15 symptoms). A CISS score ≥16 was considered symptomatic.14, 16
Figure 1.
Convergence Insufficiency Symptom Survey. Performance-related symptoms are shaded in grey. Eye-related symptoms are not shaded.
A priori, the 15 symptoms on the CISS were categorized into 2 subscales.13 The performance-related subscale consisted of 6 symptoms related to visual efficiency when reading or performing near work (e.g., loss of concentration, loss of place with reading, reading slowly) and the eye-related subscale consisted of 9 symptoms specific to visual function or asthenopic-type complaints (e.g., eyes hurt, diplopia, blurred vision, headaches) (Figure 1). The subscale score represents the average level (range: 0–4) for all the items that comprise that category. The CISS was administered at baseline and at the conclusion of treatment; at each of these visits, it was administered twice, once before the clinical examination and again after the clinical examination was completed. For this report, the first CISS administration at baseline and outcome was used to determine the frequency with which each symptom was reported. The average scores from the two administrations of the CISS at the particular study visit were used for all analyses of overall and subscale scores.
Determination of Treatment Responders (Post-treatment Cohort)
The post-treatment cohort was comprised of the children who responded to treatment during the CITT; they underwent different forms of treatment for CI, the details of which are reported elsewhere20. Treatment responders were determined using the Reliable Change Index (RCI), 25 which is a statistical method of determining the magnitude of change in a score (e.g., before and after intervention) necessary for a given self-reported measure to be considered statistically reliable. It represents the number of points necessary to determine if a change in score from pre- to post-treatment is from real change or from chance variation. The RCI takes into account both the population variance and the reliability of the test itself. Using CISS variability and reliability data from previous studies,17, 18 the RCI was calculated to be a within-subject change in symptom level of ≥ 8.0 points. This resulted in classifying 53% (116 of 218) of the children in the CITT as having a reliable decrease in symptoms after treatment. Thus, for the purposes of this report, children with a post-treatment CISS score at least 8 points less than their CISS score at baseline were considered treatment responders.
Data Analysis
Descriptive statistics are reported as the mean ± standard deviation (SD). The mean performance-related and eye-related subscale scores were compared using a paired t-test. The relationship between age and the overall CISS score and subscale scores at baseline were assessed using linear regression. The mean overall CISS score and subscale scores were compared between boys and girls, and children with and without parent-reported ADHD using two-sample t-tests. Analysis of variance (ANOVA) was used to investigate the effect of race/ethnicity at baseline. Linear regression and analysis of covariance (ANCOVA) techniques were used to determine the effect of patient characteristics on improvement in overall and subscale CISS scores at the completion of treatment. For these analyses, the CISS score at baseline (overall and subscales) was used as a covariate to adjust for any differences in baseline values. Tukey’s method was used to control the overall error rate when making post-hoc pair-wise comparisons. All data analyses were performed using SAS (version 9.1, SAS Institute, Cary, NC).
RESULTS
Of the 221 children enrolled into the CITT at 9 clinical sites, the mean age was 11.8 (±2.3) years and 59% were female. Self-reported Hispanic ethnicity was 34%; race was reported to be 54% White, 29% African American, and 17% other. Data analyses were performed after combining information on race and ethnicity as follows: Hispanics (any race), non-Hispanic Whites, and non-Hispanic African Americans. There were 34 children (15%) with parent-reported ADHD. Clinical characteristics for the CITT cohort have been reported previously.21 Of the 221 children enrolled, 218 (99%) completed their primary outcome examination.
Pre-treatment Symptom Frequency
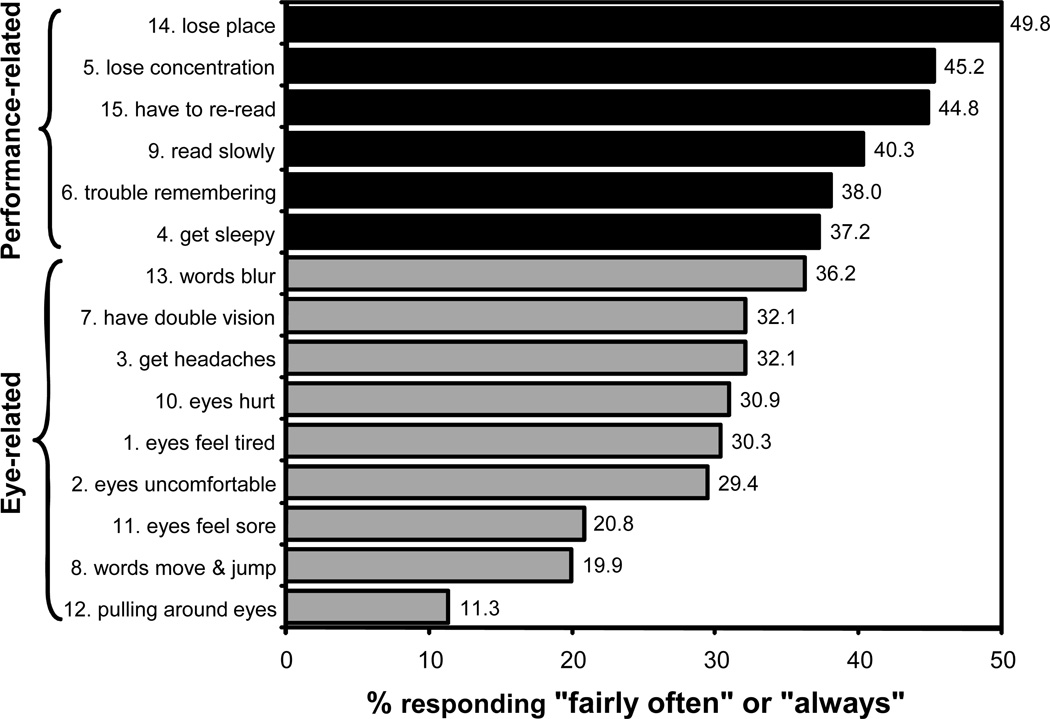
The mean overall CISS score at baseline was 29.8 (± 9.0) and the median score was 29.5. The mean response (range of never = 0 to always = 4) for the 15 symptoms ranged from a low of 1.12 (± 1.2) for “pulling around eyes” to a high of 2.55 (± 1.2) for “loses place.” The median response corresponded to “sometimes” for all symptoms except “words jump/move,” “eyes feel sore,” and “pulling around eyes,” which all had a median response of “infrequently.” The percentage of children who reported a particular symptom to occur “fairly often” or “always” is shown in Figure 2. The most frequently reported symptoms were the 6 performance-related items.
Figure 2.
Percentage of patients responding fairly often or always to each item on the Convergence Insufficiency Symptom Survey before treatment. Black color indicates performance-related symptoms; grey color indicates eye-related symptoms.
Performance-related symptoms were reported to occur more frequently than eye-related symptoms, with mean subscale scores (i.e., average level of all items in that category) of 2.3 (± 0.8) and 1.8 (± 0.7), respectively (p<0.001). This was consistent within age (p-values ≤ 0.012) and racial/ethnic groups (p-values ≤ 0.005), for both boys and girls (p-values <0.0001), and regardless of the presence or absence of parent-reported ADHD (p-values ≤ 0.001).
Association of Pre-treatment Symptom Severity with Patient Characteristics
At baseline, the mean overall CISS score and the performance- and eye-related subscale scores (Table 1) did not differ for boys and girls (p-values ≥ 0.40) or based on race/ethnicity (p-values ≥ 0.56). There was a slight increase in the overall CISS score with age (model R2 = 0.018, p = 0.048), indicating that a 1-year increase in age is associated with an increase of 0.52 in overall CISS score. This increase was driven by an increase in the eye-related subscale score (model R2 = 0.024, p = 0.022). The mean overall CISS and performance-related subscale scores were higher for the children with parent-reported ADHD (p = 0.005, p < 0.0001, respectively), but not for the eye-related subscale score (p = 0.31) (Table 1).
Table 1.
Convergence Insufficiency Symptom Survey overall score* and mean subscale score** for performance- and eye-related items prior to treatment.
| Patient Characteristic | n | Overall score: mean (SD) |
Performance- related subscale score: mean (SD) |
Eye-related subscale score: mean (SD) |
|---|---|---|---|---|
| Sex | ||||
| Female | 131 | 30.3 (9.2) | 2.3 (0.7) | 1.8 (0.8) |
| Male | 90 | 29.2 (8.6) | 2.3 (0.8) | 1.7 (0.5) |
| p-value (t-test) | 0.40 | 0.58 | 0.44 | |
| Age | ||||
| 9–10 years | 76 | 28.7 (7.8) | 2.3 (0.7) | 1.7 (0.7) |
| 11–12 years | 69 | 29.4 (8.6) | 2.3 (0.8) | 1.8 (0.7) |
| 13–14 years | 39 | 31.2 (10.7) | 2.4 (1.0) | 1.9 (0.8) |
| 15–17 years | 37 | 31.7 (9.3) | 2.3 (0.7) | 2.0 (0.8) |
| p-value (regression) | 0.048 | 0.48 | 0.022 | |
| Race/ethnicity† | ||||
| African American | 59 | 30.5 (8.7) | 2.4 (0.7) | 1.8 (0.7) |
| Hispanic | 75 | 29.4 (9.9) | 2.3 (0.8) | 1.7 (0.8) |
| White | 78 | 30.4 (8.1) | 2.3 (0.7) | 1.9 (0.7) |
| p-value (ANOVA) | 0.69 | 0.91 | 0.56 | |
| Parent-reported ADHD status†† | ||||
| No ADHD | 178 | 29.5 (8.7) | 2.2 (0.8) | 1.8 (0.7) |
| ADHD | 34 | 34.1 (8.6) | 2.8 (0.5) | 1.9 (0.8) |
| p-value (t-test) | 0.005 | <0.0001 | 0.31 | |
Sum of item responses (range: 0–60)
Average level of all items (range: 0–4)
n=212 (9 subjects categorized as “other” excluded from analysis)
n=212 (Missing ADHD status for 9 children)
Post-Treatment Symptoms
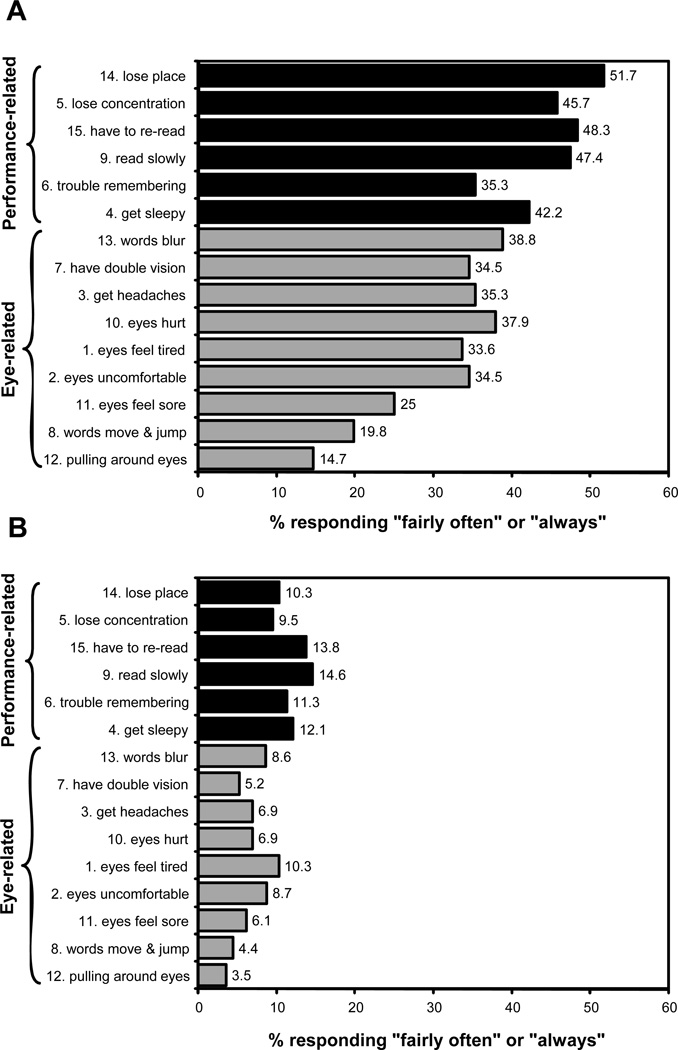
Among treatment responders, the median change was a 1-point decrease (lessening in severity by one level) for each symptom except for “pulling around the eyes” which had a median change of 0. The mean improvement was the same for both the performance-related (1.1 ±0.7) and eye-related subscales (1.1 ±0.6). The percentage of treatment responders reporting “fairly often” or “always” for each of the 15 symptoms before and after treatment are shown in Figures 3A and 3B, respectively. As found at baseline, performance-related symptoms were reported to occur more often than eye-related symptoms after treatment (p <0.0001).
Figure 3.
Percentage of patients classified as treatment responders who responded fairly often or always to each item on the Convergence Insufficiency Symptom Survey before (A) and after (B) treatment. Black color indicates performance-related symptoms; grey color indicates eye-related symptoms.
Association of Post-treatment Symptom Severity and Patient Characteristics
Post-hoc comparisons were performed to assess the relationship of age, sex, race/ethnicity, and presence of parent-reported ADHD with improvements in symptoms as measured by the overall and subscale CISS scores for children classified as treatment responders (Table 2). There were no differences in the post-treatment symptom scores for the overall and the 2 subscale CISS scores based on age (p-values≥0.34). For race/ethnicity, there was only a difference for the eye-related subscale (p=0.026), African-American children reported fewer eye-related symptoms than White children (p=0.022). Children without parent-reported ADHD had significantly lower overall CISS and eye-related scores than children with parent-reported ADHD (p=0.019, p=0.011, respectively) but not in their performance-related subscale score (p=0.13). After treatment, girls had significantly lower performance-related symptoms than boys (p=0.014); this was not found for the overall CISS and eye-related subscale scores (p-values≥0.17).
Table 2.
Convergence Insufficiency Symptom Survey overall score* and mean subscale score** for performance- and eye-related items among children classified as “treatment responders” using Reliable Change Index after treatment.
| Patient Characteristic | n | Overall score: mean (SD) |
Performance- related subscale score: mean (SD) |
Eye-related subscale score: mean (SD) |
|---|---|---|---|---|
| Sex | ||||
| Female | 72 | 14.1 (10.2) | 1.2 (0.8) | 0.8 (0.7) |
| Male | 44 | 15.1 (9.2) | 1.4 (0.8) | 0.8 (0.6) |
| p-value (ANCOVA) | 0.17 | 0.014 | 0.85 | |
| Age | ||||
| 9–10 years | 36 | 14.6 (9.9) | 1.3 (0.9) | 0.7 (0.7) |
| 11–12 years | 38 | 14.3 (8.4) | 1.2 (0.8) | 0.8 (0.6) |
| 3–14 years | 21 | 15.2 (12.4) | 1.3 (1.0) | 0.8 (0.9) |
| 15–17 years | 21 | 13.9 (9.7) | 1.1 (0.6) | 0.8 (0.7) |
| p-value (Regression) | 0.34 | 0.62 | 0.35 | |
| Race/ethnicity† | ||||
| African American | 30 | 14.3 (9.1) | 1.4 (0.9) | 0.6 (0.6) |
| Hispanic | 43 | 13.4 (9.4) | 1.2 (0.7) | 0.7 (0.7) |
| White | 37 | 16.7 (10.6) | 1.2 (0.8) | 1.0 (0.7) |
| p-value (ANCOVA) | 0.29 | 0.87 | 0.026 | |
| Parent-reported ADHD status†† | ||||
| No ADHD | 97 | 13.8 (9.2) | 1.2 (0.8) | 0.7 (0.7) |
| ADHD | 13 | 22.2 (11.0) | 1.9 (0.8) | 1.2 (0.8) |
| p-value (ANCOVA) | 0.019 | 0.13 | 0.011 | |
Sum of item responses (range: 0–60)
Average level of all items (range: 0–4)
n=110 (6 subjects categorized as “other” excluded from analysis)
n=110 (Missing ADHD status for 6 children)
DISCUSSION
Using the CISS, we compared the frequency of specific symptoms at baseline and post-treatment as reported by children with symptomatic CI who participated in the CITT.20 Performance-related symptoms (e.g., difficulty concentrating when reading or studying) were reported more frequently than eye-related symptoms prior to treatment. In fact, the 6 most frequently reported symptoms were all performance-related items, regardless of age, sex, race/ethnicity, and presence of parent-reported ADHD. This finding is consistent with data reported from our pilot study which found the top 4 symptoms reported at a fairly often or always level were performance-related items.14
Pre-treatment, older children reported an increased frequency of eye-related symptoms and a greater overall CISS. This may have occurred for several reasons. Younger children may find it difficult to explain how their eyes feel or they may not report symptoms because they consider them to be normal. Larger print size and less time spent performing sustained near work may result in fewer symptoms. It also is possible that as children get older the condition progresses with a subsequent increase in eye-related symptoms. Or lastly, older children may spend more time with concentrated near tasks than their younger cohorts.
Before treatment, children with parent-reported ADHD had a significantly higher overall CISS score than children without parent-reported ADHD. This difference was almost entirely attributed to an increase in the frequency and severity of performance-related symptoms. While CI undoubtedly contributes to the presence of performance-related symptoms, there may be an additional contribution from the ADHD condition itself. For example, loss of concentration and trouble remembering when reading and doing close work are symptoms that are similar to behaviors such as “difficulty sustaining attention” and “forgetful in daily activities” that are often observed in individuals with inattentive ADHD.26 It would be of interest to investigate CISS performance- and eye-related subscale scores in children who have normal binocular vision and a primary diagnosis of ADHD.
Children who responded to treatment typically reported a decrease in symptoms for both performance-related and eye-related symptoms. This trend was similar across all age groups. The post-treatment profile of symptoms showed that performance related-symptoms still occurred with more frequency and severity than eye-related symptoms; this is similar to the profile reported in children with normal binocular vision.14 At this time we do not have plausible hypotheses to explain why girls showed a greater reduction in their performance-related symptoms than boys and why eye-related symptoms decreased more in African-American children than in White children. It could be spurious findings due to multiple statistical tests.
Among children who were treatment responders, those without parent-reported ADHD reported a lower overall CISS score as compared to children with parent-reported ADHD. The decrease in performance-related symptoms was similar between the two groups, despite the presence of higher performance-related symptoms at baseline among the children with parent-reported ADHD. The larger decrease in symptoms in the children without parent-reported ADHD is primarily attributed to a decrease in eye-related symptoms. We speculate that children without parent-reported ADHD can attend better and longer, which may allow them to better notice improvement in their eye-related symptoms.
Our cohort of children reported performance-related symptoms with a higher frequency than eye-related symptoms. These symptoms, which include loss of place while reading, having to reread, reading slowly, loss of concentration, trouble remembering what was read may be obstacles to the reading process. In turn, performance-related symptoms may affect specific aspects of reading. Because successfully treated children report a significant decrease in performance-related symptoms, it is possible that the treatment of symptomatic CI may have a positive effect on reading performance and attention. These findings support the need for a randomized clinical trial to investigate whether the successful treatment of CI leads to improvements in specific measures of reading performance and attention.
Potential limitations of the study are that some children may experience symptoms that are not included on the CISS. In addition, there may be other conditions that may or may not have any relationship to CI or the eye, which could cause some of the same symptoms. Our previous finding that children with normal binocular vision have a mean CISS score of 8.1 illustrates that even these children are not without symptoms.14 However, clinicians less commonly recommend CI treatment for patients who do not have associated clinical signs, and the CISS cut-off of ≥16 differentiates between children with symptomatic CI and those with normal binocular vision.14, 16 It is important to note, that by design, we only treated symptomatic children who scored ≥16 on the CISS. Thus, some children with CI may report fewer symptoms and some may not be symptomatic at all. Only a population-based study can determine the frequency of symptomatic CI. Because all children were not tested for ADHD, and the classification of ADHD was based on a parental report that this diagnosis had been previously made by a medical professional, there are likely to be some misclassifications.
Particular strengths of this study are that a reliable and valid symptom survey was administered in a standardized fashion by examiners masked to treatment assignment. The study population was a large, well-defined group of children with symptomatic CI randomized to therapies administered by trained and certified therapists. Retention in the trial was excellent at 99%.20
Children in our study of symptomatic CI reported performance-related symptoms more frequently than eye-related symptoms. With increasing age, the severity of performance-related symptoms remains constant while eye-related symptoms increase. Children with parent-reported ADHD can be expected to score higher on performance-based symptoms and overall. Although both performance-related and eye-related symptoms decrease in children after successful treatment, eye-related symptoms are still more frequently reported in those without parent-reported ADHD. More research is needed to determine whether a decrease in performance-related symptoms after treatment affects specific measures of reading performance and attention in children with symptomatic CI.
Clinical Application.
Because children with symptomatic CI report performance-related symptoms more frequently than eye-related symptoms, a targeted history such as the CISS that addresses both performance- and eye-related symptoms is recommended.
ACKNOWLEDGMENTS
Supported by National Eye Institute/National Institute of Health, DHHS U10 grants: EY014713, EY014659, EY014716, EY014715, EY014709, EY014710, EY014676, EY014706, EY014712.
The Convergence Insufficiency Treatment Trial Investigator Group
Clinical Sites
Sites are listed in order of the number of patients enrolled in the study with the number of patients enrolled is listed in parentheses preceded by the site name and location. Personnel are listed as (PI) for principal investigator, (SC) for coordinator, (E) for examiner, and (VT) for therapist.
Study Center: Bascom Palmer Eye Institute (35)
Susanna Tamkins, OD (PI); Hilda Capo, MD (E); Mark Dunbar, OD (E); Craig McKeown, MD (CO-PI); Arlanna Moshfeghi, MD (E); Kathryn Nelson, OD (E); Vicky Fischer, OD (VT); Adam Perlman, OD (VT); Ronda Singh, OD (VT); Eva Olivares (SC); Ana Rosa (SC); Nidia Rosado (SC); Elias Silverman (SC)
Study Center: SUNY College of Optometry (28)
Jeffrey Cooper, MS, OD (PI); Audra Steiner, OD (E, Co-PI); Marta Brunelli (VT); Stacy Friedman, OD (VT); Steven Ritter, OD (E); Lily Zhu, OD (E); Lyndon Wong, OD (E); Ida Chung, OD (E); Kaity Colon (SC); Ashley Fazarry (SC)
Study Center: UAB School of Optometry (28)
Kristine Hopkins, OD, MSPH (PI); Marcela Frazier, OD, MSPH (E); Janene Sims, OD, PhD (E); Marsha Swanson, OD (E); Katherine Weise, OD, MBA (E); Adrienne Broadfoot, MS, OTR/L (VT, SC); Michelle Anderson, OD (VT); Catherine Baldwin (SC); Leslie Simms, MS, OTR/L (SC)
Study Center: NOVA Southeastern University (27)
Rachel Coulter, OD (PI); Deborah Amster, OD (E); Gregory Fecho, OD (E); Tanya Mahaphon, OD (E); Jacqueline Rodena, OD (E); Mary Bartuccio, OD (VT); Yin Tea, OD (VT); Annette Bade, OD (SC)
Study Center: Pennsylvania College of Optometry (25)
Michael Gallaway, OD (PI); Brandy Scombordi, OD (E); Mark Boas, OD (VT); Tomohiko Yamada, OD (VT); Ryan Langan (SC), Ruth Shoge, OD (E); Lily Zhu, OD (E)
Study Center - The Ohio State University College of Optometry (24)
Marjean Kulp, OD, MS (PI); Michelle Buckland, OD (E); Michael Earley, OD, PhD (E); Gina Gabriel, OD, MS (E); Aaron Zimmerman, OD (E); Kathleen Reuter, OD (VT); Andrew Toole, OD, PhD (VT); Molly Biddle, MEd (SC); Nancy Stevens, MS, RD, LD (SC)
Study Center: Southern California College of Optometry (23)
Susan Cotter, OD, MS (PI); Eric Borsting, OD, MS (E); Michael Rouse, OD, MSEd (E); Carmen Barnhardt, OD, MS (VT); Raymond Chu, OD (VT); Susan Parker (SC); Rebecca Bridgeford (SC); Jamie Morris (SC); Javier Villalobos (SC)
Study Center: University of CA San Diego: Ratner Children's Eye Center (17)
David Granet, MD (PI); Lara Hustana, OD (E); Shira Robbins, MD (E); Erica Castro, OC(c) (VT); Cintia Gomi, MD (SC)
Study Center: Mayo Clinic (14)
Brian G. Mohney, MD (PI); Jonathan Holmes, MD (E); Melissa Rice, OD (VT); Virginia Karlsson, BS, CO (VT); Becky Nielsen (SC); Jan Sease, COMT/BS (SC); Tracee Shevlin (SC)
CITT Study Chair
Mitchell Scheiman, OD (Study Chair); Karen Pollack (Study Coordinator); Susan Cotter, OD, MS (Vice Chair); Richard Hertle, MD (Vice Chair); Michael Rouse, OD, MSEd (Consultant)
CITT Data Coordinating Center
G. Lynn Mitchell, MAS, (PI); Tracy Kitts, (Project Coordinator); Melanie Bacher (Programmer); Linda Barrett (Data Entry); Loraine Sinnott, PhD (Biostatistician); Kelly Watson (student worker); Pam Wessel (Office Associate)
National Eye Institute, Bethesda, MD
Maryann Redford, DDS, MPH
CITT Executive Committee
Mitchell Scheiman, OD; G. Lynn Mitchell, MAS; Susan Cotter, OD, MS; Richard Hertle, MD; Marjean Kulp, OD, MS; Maryann Redford, DDS, MPH; Michael Rouse, OD, MSEd
Data and Safety Monitoring Committee
Marie Diener-West, PhD, Chair; Rev. Andrew Costello, CSsR; William V. Good, MD; Ron D. Hays, PhD; Argye Hillis, PhD (Through March 2006); Ruth Manny, OD, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented as a poster at AAO October 2007 in Tampa, FL, and a paper at ARVO in 2008, Ft. Lauderdale, FL.
REFERENCES
- 1.Letourneau JE, Lapierre N, Lamont A. The relationship between convergence insufficiency and school achievement. Am J Optom Physiol Opt. 1979;56:18–22. doi: 10.1097/00006324-197901000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Letourneau J, Ducic S. Prevalence of convergence insufficiency among elementary school children. Can J Optom. 1988;50:194–197. [Google Scholar]
- 3.Porcar E, Martinez-Palomera A. Prevalence of general binocular dysfunctions in a population of university students. Optom Vis Sci. 1997;74:111–113. doi: 10.1097/00006324-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, Scheiman M, Gallaway M, De Land PN, the Convergence Insufficiency and Reading Study (CIRS) group. Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, Scheiman M, Gallaway M, De Land PN. Frequency of convergence insufficiency among fifth and sixth graders. Optom Vis Sci. 1999;76:643–649. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Daum KM. Convergence insufficiency. Am J Optom Physiol Opt. 1984;61:16–22. doi: 10.1097/00006324-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Cooper J, Duckman R. Convergence insufficiency: incidence, diagnosis, and treatment. J Am Optom Assoc. 1978;49:673–680. [PubMed] [Google Scholar]
- 7.Kent PR, Steeve JH. Convergence insufficiency, incidence among military personnel and relief by orthoptic methods. Mil Surg. 1953;112:202–205. [PubMed] [Google Scholar]
- 8.Poynter HL, Schor C, Haynes HM, Hirsch J. Oculomotor functions in reading disability. Am J Optom Physiol Opt. 1982;59:116–127. doi: 10.1097/00006324-198202000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Mazow ML. The convergence insufficiency syndrome. J Pediatr Ophthalmol Strabismus. 1971;8:243–244. [Google Scholar]
- 10.Duke-Elder S, Wybar K. Ocular motility and strabismus. In: Duke-Elder S, editor. System of Ophthalmology. vol.6. St. Louis: Mosby; 1973. pp. 204–206. [Google Scholar]
- 11.Pickwell LD, Hampshire R. The significance of inadequate convergence. Ophthalmic Physiol Opt. 1981;1:13–18. [PubMed] [Google Scholar]
- 12.Borsting E, Rouse MW, Deland PN, Hovett S, Kimura D, Park M, Stephens B. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74:25–34. [PubMed] [Google Scholar]
- 13.Borsting E, Rouse MW, De Land PN the Convergence Insufficiency and Reading Study (CIRS) Group. Prospective comparison of convergence insufficiency and normal binocular children on CIRS symptom surveys. Optom Vis Sci. 1999;76:221–228. doi: 10.1097/00006324-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Borsting EJ, Rouse MW, Mitchell GL, Scheiman M, Cotter SA, Cooper J, Kulp MT, London R. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003;80:832–838. doi: 10.1097/00006324-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Rouse MW, Borsting EJ, Mitchell GL, Scheiman M, Cotter SA, Cooper J, Kulp MT, London R, Wensveen J. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalmic Physiol Opt. 2004;24:384–390. doi: 10.1111/j.1475-1313.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 16.Rouse M, Borsting E, Mitchell GL, Cotter SA, Kulp M, Scheiman M, Barnhardt C, Bade A, Yamada T. Validity of the convergence insufficiency symptom survey: a confirmatory study. Optom Vis Sci. 2009;86:357–363. doi: 10.1097/OPX.0b013e3181989252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheiman M, Mitchell GL, Cotter S, Cooper J, Kulp M, Rouse M, Borsting E, London R, Wensveen J. A randomized clinical trial of treatments for convergence insufficiency in children. Arch Ophthalmol. 2005;123:14–24. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Scheiman M, Cotter S, Rouse M, Mitchell GL, Kulp M, Cooper J, Borsting E. Randomised clinical trial of the effectiveness of base-in prism reading glasses versus placebo reading glasses for symptomatic convergence insufficiency in children. Br J Ophthalmol. 2005;89:1318–1323. doi: 10.1136/bjo.2005.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheiman M, Mitchell GL, Cotter S, Kulp MT, Cooper J, Rouse M, Borsting E, London R, Wensveen J. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optom Vis Sci. 2005;82:583–595. doi: 10.1097/01.opx.0000171331.36871.2f. [DOI] [PubMed] [Google Scholar]
- 20.Convergence Insufficiency Treatment Trial (CITT) Study Group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008;126:1336–1349. doi: 10.1001/archopht.126.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Convergence Insufficiency Treatment Trial (CITT) Study Group. The convergence insufficiency treatment trial: design, methods, and baseline data. Ophthalmic Epidemiol. 2008;15:24–36. doi: 10.1080/09286580701772037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Convergence Insufficiency Treatment Trial Study Group. Long-term effectiveness of treatments for symptomatic convergence insufficiency in children. Optom Vis Sci. 2009;86:1096–1103. doi: 10.1097/OPX.0b013e3181b6210f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrials.gov. [Accessed: January 25, 2012];Convergence Insufficiency Treatment Trial (CITT). 2006 [updated 2010] Available at: www.ClinicalTrials.gov.
- 24.Sheard C. Zones of ocular comfort. Am J Optom. 1930;7:9–25. [Google Scholar]
- 25.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: The Association; 2000. [Google Scholar]