Abstract
The effects of oral treatment of rats with streptozotocin-induced diabetes with a range of vanadium dipicolinate complexes (Vdipic) and derivatives are reviewed. Structure-reactivity relationships are explored aiming to correlate properties such as stability, to their insulin-enhancing effects. Three types of modifications are investigated; first, substitutions on the aromatic ring, second, coordination of a hydroxylamido group to the vanadium, and third, changes in the oxidation state of the vanadium ion. These studies allowed us to address the importance of coordination chemistry, and redox chemistry, as modes of action. Dipicolinate was originally chosen as a ligand because the dipicolinatooxovanadium(V) complex (V5dipic), is a potent inhibitor of phosphatases. The effect of vanadium oxidation state (3, 4 or 5), on the insulin-enhancing properties was studied in both the Vdipic and VdipicCl series. Effects on blood glucose, body weight, serum lipids, alkaline phosphatase and aspartate transaminase were selectively monitored. Statistically distinct differences in activity were found, however, the trends observed were not the same in the Vdipic and VdipicCl series. Interperitoneal administration of the Vdipic series was used to compare the effect of administration mode. Correlations were observed for blood vanadium and plasma glucose levels after V5dipic treatment, but not after treatment with corresponding V4dipic and V3dipic complexes. Modifications of the aromatic ring structure with chloride, amine or hydroxyl groups had limited effects. Global gene expression was measured using Affymetrix oligonucleotide chips. All diabetic animals treated with hydroxyl substituted V5dipic (V5dipicOH) and some diabetic rats treated with vanadyl sulfate had normalized hyperlipidemia yet uncontrolled hyperglycemia and showed abnormal gene expression patterns. In contrast to the normal gene expression profiles previously reported for some diabetic rats treated with vanadyl sulfate, where both hyperlipidemia and hyperglycemia were normalized. Modification of the metal, changing the coordination chemistry to form a hydroxylamine ternary complex, had the most influence on the anti-diabetic action. Vanadium absorption into serum was determined by atomic absorption spectroscopy for selected vanadium complexes. Only diabetic rats treated with the ternary V5dipicOH hydroxylamine complex showed statistically significant increases in accumulation of vanadium into serum compared to diabetic rats treated with vanadyl sulfate. The chemistry and physical properties of the Vdipic complexes correlated with their anti-diabetic properties. Here, we propose that compound stability and ability to interact with cellular redox reactions are key components for the insulin-enhancing activity of vanadium compounds. Specifically, we found that the most overall effective anti-diabetic Vdipic compounds were obtained when the compound administered had an increased coordination number in the vanadium complex.
Keywords: Vanadium, dipicolinic acid, dipicolinate, dipicolinatooxovanadium(V), diabetes, streptozotocin, redox, coordination number, cellular oxidation of vanadium, gene expression, signal transduction
1.0 Introduction
1.1 Insulin-enhancing effects of V in rats with STZ-induced diabetes
The modern era of studying the anti-diabetic properties of vanadium (abbreviated here as V) was initiated in 1985 by John McNeill, who monitored the cardiac function of rats with streptozotocin (STZ)-induced diabetes after treatment with vanadyl sulfate [1]. Previously insulin-like effects of V salts in cell systems such as adipocytes had been reported [2]. The STZ diabetic rat model is widely used to study the in vivo effects of vanadium (abbreviated here as V) compounds. STZ-induced diabetes is considered a type 1 diabetic model since it arises from destruction of some, but not all, of the insulin-producing pancreatic β cells [3]. Rats with STZ-induced diabetes are not dependent upon insulin, and can survive for many months, even years, without any treatment. Interestingly many of the anti-diabetes effects of V treatment have been reported to last at least 3 months after the treatment has stopped [4]. In the Biobreeding (BB) rat, an inbred rat strain that spontaneously develops type 1 diabetes, treatment with V lowers the amount of insulin needed for treatment, although it cannot completely substitute for insulin [5]. In a similar way, serum insulin levels lower after administration of V-containing compounds to normal rats. Therefore, the anti-diabetic effect of V compounds on STZ-diabetic rats, is believed to be that of an ‘insulin-enhancer’ [6].
The effects of treatment with a wide variety of V compounds, including simple salts, have been studied in rats with STZ-induced diabetes. McNeill, Orvig and coworkers have extensively studied the effects of many V complexes, focusing on BMOV and its derivatives [7, 8]. These studies are of particular interest because BEOV, a close derivative, was selected for phase 1 and 2 clinical trials [7-9]. This selection was put forth once clinical studies using V-containing simple salts suggested the potential for success for using V in the treatment of diabetes[10-13]. The Sakurai group has also reported on a number of V picolinate complexes [14] and many other V(4)-containing complexes in multiple animal systems, including the STZ-diabetic rat [15]. Studies in the STZ-induced diabetic rat have been done using the dipicolinate series of V complexes by the Willsky and Ding groups [16-23]. The dipicolinate ligand is a tridentate ligand, and thus, different than the bidentate ligands (picolinate and maltol) listed above. This series distinguishes itself from other V-containing systems because the most effective compounds have the vanadium in oxidation state 5. In contrast, the other V compounds are generally most effective with the V in oxidation state 4. This fundamental difference suggests that studies on compounds within this class could be informative when the focus is compound optimization. This review focuses on work by Willsky, Crans, Ding and their collaborators investigating the effects of Vdipic complexes in rats with STZ-induced diabetes. The reader is referred elsewhere for animal studies using other V compounds [9, 14, 24-27]
The STZ-induced diabetic outbred Wistar rat was chosen as the diabetic animal model by Willsky and collaborators due to its prevalent use in studies with V as described above. In the outbred Wistar rat model of STZ induced diabetes, there is variability in the response of the animals with respect to blood glucose (BG) [6, 18]. The use of the Wistar outbred rat model, in part, accounts for the large deviations seen in the error bars for determination of blood and serum parameters in these experiments. In an effort to get a more uniform response, the Willsky lab has tried to study STZ-induced diabetes in various inbred rat models without finding a response to V to equal that of the outbred Wistar Rat. In addition to the published results with the inbred Wistar Kyoto and Wistar Furth strains [18], we have also looked at the Dahl, ACI, PVG, Buffalo, Lewis, Brown Norway, and F344 inbred rat strains (Willsky unpublished results).
The exact mechanism of the insulin-enhancing activity exerted by V compounds is not completely understood. These compounds are coordination complexes, and are inherently susceptible to hydrolysis (that is loss of metal also described as demetalation), such that the specific active species remains unclear. Administration of these V complexes is likely to result in loss of ligand, and extensive work has been carried out aimed at determining the active species [17, 19, 28-36]. Studies using BMOV and BEOV demonstrate that upon administration of the complex, the maltol ligand separates from the metal ion, and transport proteins such as transferrin are likely to play key roles in the distribution of vanadium intracellularly [8, 31, 36-40]. Due to transmetalation reactions and cellular compartmentation it is likely that other ligands in addition to transferrin are involved in the biological effects of V compounds. Transmetalation reactions are commonly seen with coordinated metal complexes where the tightly bound metal is transferred to other ligands, for example, as documented for Gadolinium chelates[41] Some studies have been performed using other vanadium complexes, such as in vivo observation of vanadium in the blood of an animal that had received the vanadium picolinate complex [24, 29, 42]. In summary, all these studies document that at some point, the vanadium complex decomposes after administration and that other complexes can form with cellular components.
Various mechanisms of action have been implicated in the anti-diabetic effects of V [7, 8, 12, 43]. The most widely accepted mode of action for V compounds thus far is attributed to the inhibition of protein tyrosine phosphatases [44-48]. Some V compounds are reversible inhibitors, whereas others are irreversible by modifying the protein through redox processes [46]. Our group has proposed that interactions of V complexes with cellular oxidation-reduction processes is important in the anti-diabetic effects of V compounds [19]. V causes increases in ROS and RNS via multiple mechanisms [49, 50]. Systematic studies such as those reviewed here are therefore important, allowing for evaluation of the observed effects when altering coordination geometry, ligand and oxidation state of the vanadium.
1.2 V dipicolinate complexes: Rationale for use and introduction of complexes reviewed
The insulin-enhancing effects of V were examined using a range of different dipicolinate complexes. In these studies alterations to the coordination chemistry around the V, the ligand, and the oxidation state were made. The common feature in this work was to maintain the dipic ligand coordinated to the V. These studies have investigated V compounds with diverse chemical properties, oxidation state, stability, redox potential, and lipophilicity. The dipicolinate ligand was originally chosen because Vdipic complexes are potent inhibitors of phosphatases [51]. At that time, this was considered the primary mode of action for the anti-diabetic effects observed with these compounds. Furthermore, the chemistry of the V(5) complex was not well characterized [16, 52, 53]. Several Vdipic complexes have been reported, including the V(5) complex with one dipic ligand. Vdipic also forms with V(4) however, two complexes can be present in solution; one that possesses a single dipic ligand and another that contains two [19, 54-56]. The crystalline material that has been structurally characterized and used for most studies is the 1:1 complex. Only trace amounts of the 1:2 complex forms under physiological conditions. As a result, it is not necessary to consider the 1:2 complex for use in biological studies. The V(3) complex, on the other hand, forms mainly a complex that has two dipic ligands coordinated to the V [19, 54-56].
The dipic ligand is a potent metal chelator, but structural modification changes the effectiveness of the chelator, as evidenced from studies with different dipicolinate ligands [23, 52, 55, 57, 58]. In particular, the dipic ligand forms ternary complexes. Ternary complexes significantly change the electronic properties of the V complex [59, 60]. In addition, it is a natural metabolite in humans, making it a very promising system for the study of the anti-diabetic effects.
The chemistry of V-picolinate derivatives and their insulin-enhancing effects in cells and animals had been described[61-63]. Sakurai and colleagues have studied ‘insulin-like’ effects in V complexes by extensive structure-function experiments [14] in part employing a cell model in which the release of free fatty acids (FFA) from adipocytes is monitored and also utilizing real time monitoring of V in the blood of living rats [62].
Redox properties of these V dipic complexes are irreversible in aqueous media [64] and (Crans et al unpublished). Recently, studies using the parent complex have demonstrated, that this class of compounds are readily dissolved in interfaces [65, 66]. Additionally it has been found that the dipic ligand itself follows this trend, which may be important to the action of these compounds[67].
The V dipic and dipicCl complexes and ligands used in the studies that are reviewed here are shown in Figure 1. V5dipic is the parent complex, and was one of the most effective compounds examined [16, 19]. The aromatic moiety of the dipic ligand was perturbed and resulted in studies with dipicOH [58], dipicCl [21, 22], and dipicNH2 [20]. The coordination chemistry of V was perturbed by addition of a hydroxylamine (HA) group to the metal. This modification with the dipicolinate derivatives was studied [20, 59, 60] and some of these compounds were selected for testing in animals. Specifically the complexes used were V5dipic(MeHA) and V5dipicOH(HA). The chemistry and pharmacology of a range of V compounds including Vdipic complexes have been described in recent books [68, 69].
Fig 1.
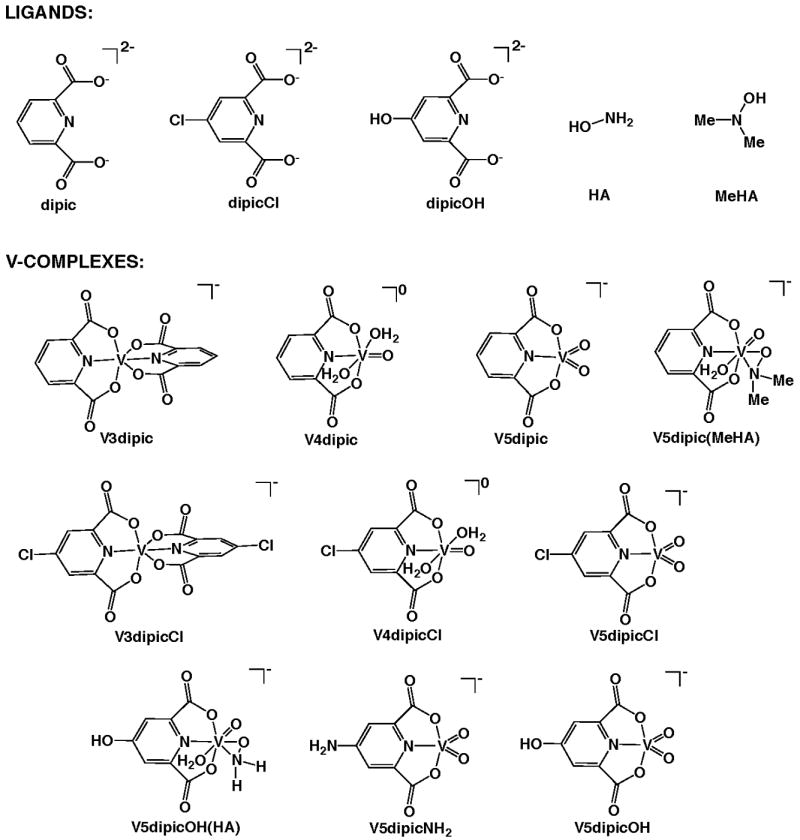
Structures of compounds used.
The animal work reviewed here was conducted by G. R. Willsky and W. Ding in separate, parallel, repeated experimental studies, in collaboration with D. C. Crans and J.H. McNeill [17-19, 32, 53, 70]. In addition, previously unpublished results are included from the Willsky laboratory involving the anti-diabetic effects of treatment with the V5dipicOH complex with hydroxylamine modifications and the effects of treatment with V5dipicOH on global gene expression. These studies were carried out as part of a larger study and were presented at the V7 Conference in Toyama Japan in October of 2010.
1.3 Effects of ligands and V5dipic on diabetic hyperglycemia, diabetic hyperlipidemia, and toxicity
The effect of treatment with V5dipic on BG in rats with STZ-induced diabetes has been previously reported [19] and is shown in Fig. 2. These results are compared with the effect of treatment with the simple salt VS, and treatment with the dipic and dipicOH ligands [18, 19, 32]. Lowering of BG upon V5dipic treatment yielded similar effects when compared to treatment with VS. Administration of the ligands (dipic and dipicOH) to diabetic rats did not cause a statistically significant difference in BG levels when compared to untreated diabetic animals. Lower BG levels were seen after treatment with dipic, while dipicOH treatment showed a tendency to raise BG. Treatment with another ligand, dipicCl, also raised BG levels (Fig 5).
Fig 2.
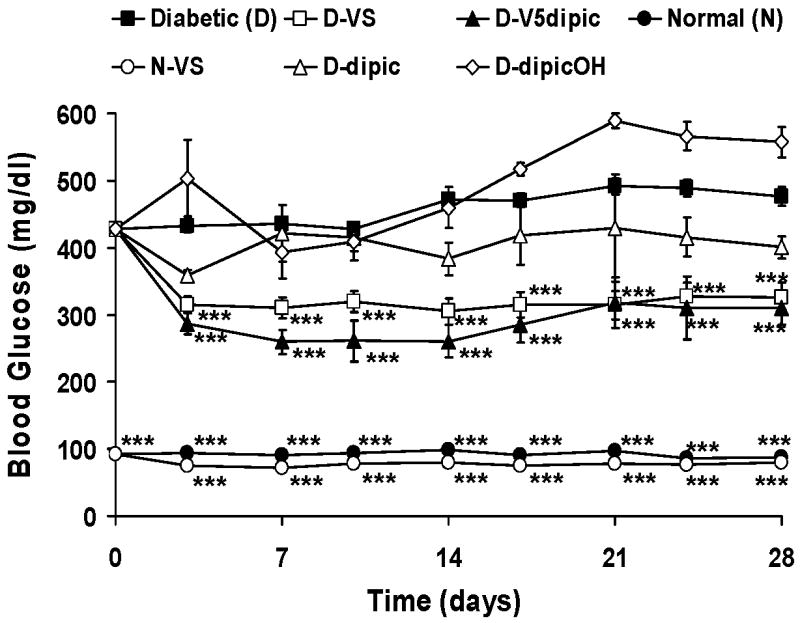
Effect of oral chronic administration of V5dipic and dipic ligands on BG in rats with STZ-induced diabetes. Normal untreated (N, ●); Diabetic untreated (D, ■); N treated with VS(○), D treated with VS (□); D treated with dipic(△), D treated with dipicOH(◊), D treated with V5dipic(▲) Data redrawn from [18, 19, 32]. Data analyzed by one way ANOVA with multiple means testing. *** represents p < .001 vs diabetic rats.
Fig 5.
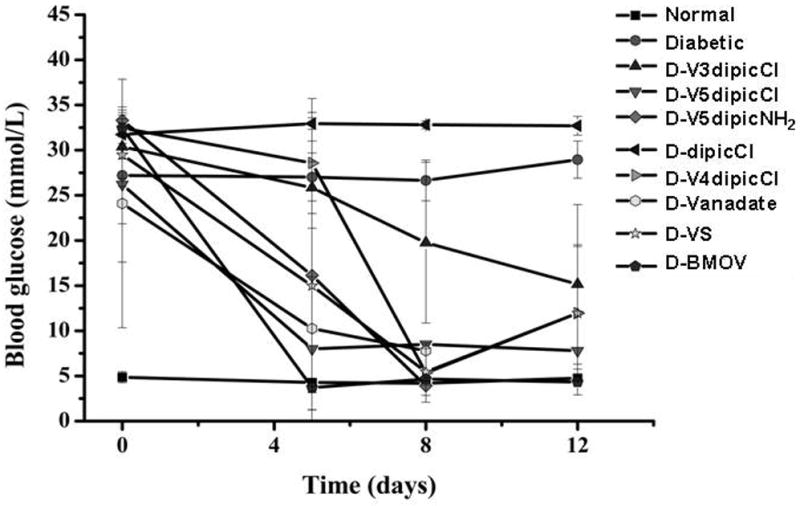
Effect of oral chronic administration of V3dipicCl, V4dipicCl, V5dipicCl, V5dipicNH2, BMOV, VS, and Vanadate on BG levels in normal rats and rats with STZ-induced diabetes. Data analyzed by one way ANOVA with multiple means testing. Symbols for treatment group indicated in Figure. Data redrawn from previous publications [21, 22]
The effects of treatment with ligands and Vdipic complexes on diabetic hyperlipidemia was previously reported by Willsky [19, 58, 71] and results are shown in Table 1. Treatment with dipic did not significantly lower serum lipid levels in diabetic animals, while treatment with dipicOH lowered diabetic hyperlipidemia. Note, treatment with the specific ligand showing a tendency to lower diabetic hyperglcemia did not lower diabetic hyperlidemia, and vice versa. Administration of both the parent Vdipic complex (V5dipic) or simple salt (VS) significantly lowered diabetic hyperlipidemia. In another study, administration of another modified verison of the dipic ligand, dipicCl, [21, 22] statistically lowered serum cholesterol (chol) with no effect on serum triglyceride TG (see below).
Table 1. Effect of treatment with VS and V dipicolinic acid Complexes on serum lipids in rats with STZ-induced diabetesb.
| Treatment | n | TG (mg/dl) | Chol(mg/dl) | FFA (mg/dl) |
|---|---|---|---|---|
| Normal (N) a | 18 | 134 ± 15.3 *** | 78 ± 3.8 *** | 78 ± 3.8 *** |
| N-VSa | 15 | 111 ± 11.8 *** | 80 ± 4.2 *** | 80 ± 4.2 *** |
| Diabetic (D) a | 32 | 1427 ± 124.2 ### | 219 ± 15.0 ### | 219 ± 15.0 ### |
| D-Oral VS Ra | 5 | 173 ± 12.9 *** | 79 ± 6.0 *** | 79 ± 6.0 *** |
| D-Oral VS NR | 7 | 239 ± 51 (3)**,# | 91 ± 5.7 (3) * | 91 ± 5.7 (3) * |
| D-VS | 27 | 245 ± 33 *** | 104 ± 8 *** | 104 ± 8 *** |
| D-V3Dipica | 8 | 154 ± 22 *** | 114 ± 5 *** | 114 ± 5 *** |
| D-V4Dipica | 5 | 121 ± 12.7 *** | 101 ± 5.8 *** | 101 ± 5.8 *** |
| D-V5Dipica | 5 | 161 ± 20.5 *** | 92.5 ± 5.77 *** | 92.5 ± 5.77 *** |
| D-V5Dipic-OHa | 12 | 76.7 ± 20.5 *** | 97.5 ± 4.31 *** | 97.5 ± 4.31 *** |
| D-V5Dipic-OH(HA) | 5 | 141 ± 20 *** | 106 ± 4.3 *** | 106 ± 4.3 *** |
| D-V5Dipic(MeHA) | 5 | 1755 ± 483 ### | 195 ± 21.3 ### | 195 ± 21.3 ### |
Data previously published [19,58.71].
Symbols used:
significance compared to D,
significance compared to N one symbol p< 0.05, two symbols p< 0.01, three symbols P< 0,001
The toxicity of the ligands on the diabetic animals has been examined using weight loss, need for rehydration, elevated serum parameters such as alkaline phosphatase (ALP) and aspartate animotransferase (AST) that are signs of liver dysfunction, and survival in various studies [21, 22]. Animals treated with the ligand alone did not show any significant differences in the parameters observed in the diabetic animals. Treatment with most of the Vdipic complexes reported in these studies show some toxic effects while monitoring the parameters, and a few deaths did occur in a limited number of groups.
2.0 Effect of oxidation state modification of Vdipic and VdipicCl complexes on insulin-enhancing activity
2.1 Effects of oxidation state modification of Vdipic on diabetic hyperglycemia, hyperlipidemia and absorption into serum
Treatment of the diabetic rats with Vdipic complexes in the 3, 4 or 5 oxidation states lowered BG significantly after day 3[19] and results are shown in Fig. 3. The BG lowering effect of administration of the Vdipic complexes may be additive with the non-statistically significant trend of treatment with dipic alone to lower BG (Fig. 2). Of the V dipic complexes, only treatment with the V5dipic complex lowered BG significantly compared to treatment with the dipic ligand alone by ANOVA analysis. These results show that there are oxidation state differences in the effect of treatment with Vdipic complexes in rats with STZ-induced diabetes.
Fig 3.
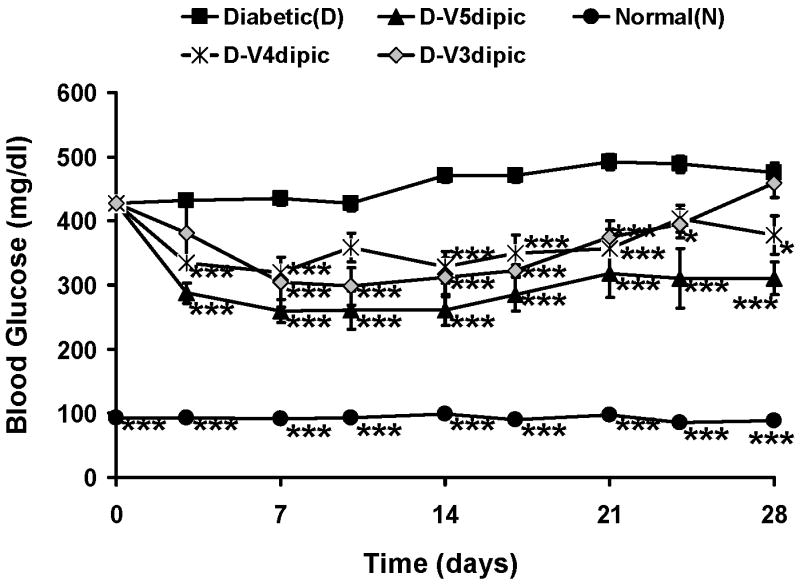
Effect of oral chronic administration of V3dipic, V4dipic, or V5dipic on BG in rats with STZ-induced diabetes. Normal untreated (N, ●); Diabetic untreated (D, ■); D treated with V3dipic (◊); D treated with V4dipic(*); D treated with V5dipic(▲). The data for 14 days of treatment were previously published [19] and have been extended to 28 days. Data analyzed by one way ANOVA with multiple means testing *** represents p < .001 vs diabetic rats.
The effects of varying the oxidation state on diabetic hyperlipidemia of the Vdipic complexes are seen in Table 1[32]. Treatment with V3dipic, V4dipic, or V5dipic significantly lowered elevated TG, chol and FFA in the diabetic animals. Interestingly, the serum lipid levels for most of the treated diabetic rats were not statistically different from lipid levels observed in normal animals.
The effect of oxidation state on V absorption into serum was examined by comparing the dose ingested to the amount of V found in serum by atomic absorption Table 2 [19]. In this analysis, only the animals treated with V4dipic showed a correlation of dose ingested to serum V using Spearman correlation analysis. Although the animals treated with VS and V3dipic showed Spearman correlation values close to significance p=(0.055 and 0.058 respectively), the relationship of serum V to dose ingested for animals taking V5dipic was clearly not correlated (p=0.20). Since the V complexes were present in the drinking water and all animals did not drink the same amount of V, the results for the amount of V in the serum was normalized to the dose ingested. Diabetic animals dosed with VS or V3dipic had significantly lowered ratios for serum V to average dose ingested compared to that see in the normal animal dosed with VS. Treatment of diabetic rats with VS, one of the well studied anti-diabetic V compounds, resulted in significantly less V per dose ingested to be found in serum compared to that seen in Normal animals treated with VS. This is another example of the differences in metabolism and response to V of normal and diabetic animals.
Table 2. Effect of treatment with VS and V dipicolinate acid complexes on V absorption into serum.
| Treatment group | n | Average Dose a b (mmolV/kg/Day) | Average Serum V a (nmol/ml) | Spearman Correlation of average Dose to Serum V (pvalue) | Ratio of Serum V to Average Dose a b |
|---|---|---|---|---|---|
| D-VSc | 13 | 0.95 ± 0.14# | 11.5 ± 1.1 | 0.544 (p=0.055) | 13.6 ± 1.3### |
| D-V3dipicc | 8 | 1.25 ± 0.04### | 11.3 ± 1.0 | 0.690 (p=0.058) | 9.0 ± 0.7### |
| D-V4dipicc | 7 | 0.60 ± 0.08 | 13.2 ± 0.9 | 0.821 (p=0.023) | 23.0 ± 1.4 |
| D-V5dipicc | 4 | 0.51 ± 0.09 | 14.3 ± 3.4 | 0.800 (p=0.200) | 26.4 ± 4.0 |
| D-V5dipic-OH | 8 | 1.03 ± 0.03## | 24.2 ± 1.5***,### | 0.262 (p=0.531) | 23.6 ± 1.4 |
| D-V5dipic-OH(HA) | 7 | 0.64 ± 0.08 | 18.4 ± 1.8** | 0.714 (p=0.071) | 31.0 ± 3.9*** |
| D-V5dipic(MeHA) | 7 | 0.54 ± 0.04 | 10.1 ± 0.4 | -0.286 (p=0.535) | 19.4 ± 1.8# |
| N-VS | 7 | 0.48 ± 0.05* | 14.2 ± 1.0 | -0.577 (p=0.175) | 32.9 ± 5.3*** |
Symbols
p ≤ 0.05,
p ≤ 0.01, and
p ≤ 0.001 compared to D-VS
p ≤ 0.05,
p ≤ 0.01, and
p ≤ 0.001 compared to N-VS
Average dose was calculated for end of the experiment from days 16 to 28.
Data previously published [19].
2.2 Effects of mode of administration on the anti-diabetic effect of V3dipic, V4dipic, and V5dipic
When complexes are chronically administered orally they experience changing environments and pH before they enter the cells of the gastrointestinal tract and are distributed into serum and tissues. The effects of passage through the gastrointestinal system can be avoided if the therapeutic agent is administered either into the peritoneal cavity or intravenously. The effects of intraperitoneal administration of a single dose of V3dipic, V4dipic, or V5dipic was examined in the McNeill laboratory (Fig 4) [32]. To differentiate this type of treatment from chronically administration in the drinking water; we are calling the one time ip injection mode of administration acute treatment. The oxidation state of the Vdipic complexes showed differential effects in this system with respect to lowering of diabetic hyperglycemia. In these acute treatment experiments, treatment with V3dipic and V5dipic maintained lowered diabetic hyperglycemia for 48 hours, while treatment with V4dipic showed only a transient 12 hour ability to slightly lower blood glucose (Fig. 4a).
Fig 4.
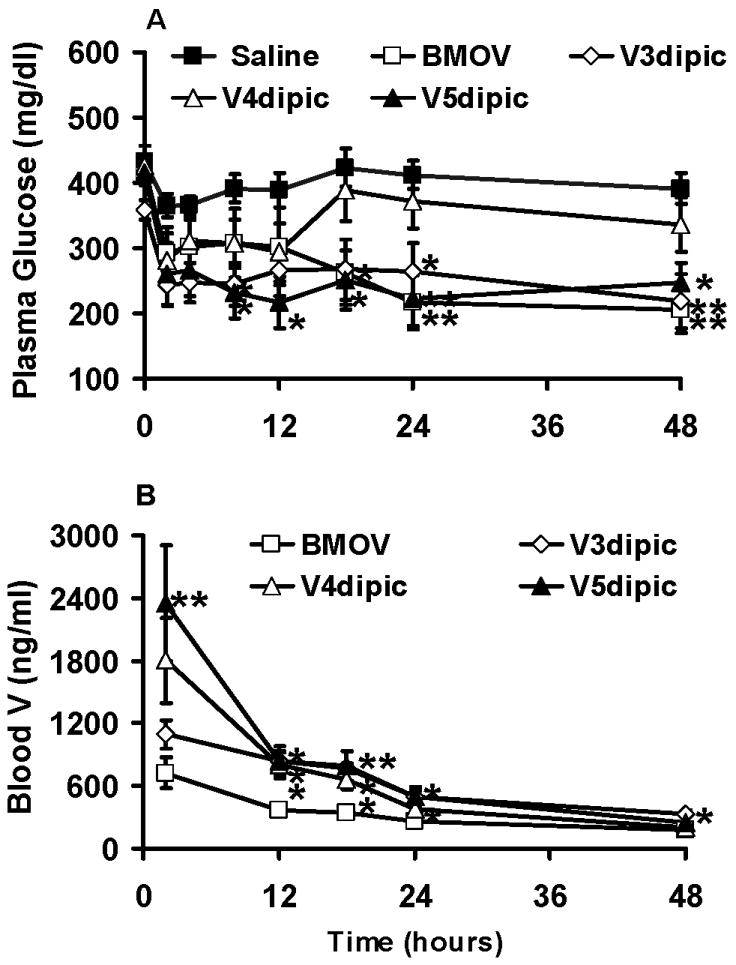
Effect of acute interperitoneal administration of V3dipic, V4dipic, or V5dipic on plasma glucose in rats with STZ-induced diabetes. A) Plasma glucose levels. Diabetic rats treated with saline (■), BMOV (□), V3dipic (◊), V4dipic (△), V5dipic (▲). B) Blood Total V levels as determined by atomic absorption spectrometry. BMOV (□), V3dipic (◊), V4dipic (△), V5dipic (▲). Data redrawn from previous publication [32]. Data analyzed by one way ANOVA with multiple means testing. ** represents p < .01 vs diabetic rats.
The amount of V in the blood was determined as a function of time in an acute administration experiment (Fig 4b). The concentration of V in blood over time was modeled using a one- or two-compartment model using the data shown in Fig 4b. Data gathered for V4dipic, V5dipic and the control, BMOV, fit a two-compartment model. Data obtained after treatment with V3dipic fit to a one-compartment model. Correlations between blood V and plasma glucose levels were also obtained in this experiment [32]. A significant correlation (0.777 R2) was only seen for animals given V5dipic. However, both V3dipic and V5dipic were equally effective at lowering BG. The results reported here demonstrate that although different, there are oxidation state differences in the ability to lower diabetic hyperglycemia when the V3dipic, V4dipic or V5dipic complexes are administered either orally or intraperitoneally. These results would imply that there are significant changes to the V complex while it passes through the gastro-intestinal tract affecting anti-diabetic efficacy.
2.3 Effects of oxidation state modification of VdipicCl complexes on diabetic hyperglycemia, hyperlipidemia, and serum metabolites
The effect of oxidation state in the Vdipic series was additionally examined using the dipicCl ligand. In this form of the ligand, the chlorine changes the electron density in the dipic ring. The dipicCl ligand alone showed a non-statistically significant trend to further raise diabetic hyperglycemia in rats[21, 22] is shown in Figure 5. Treatment with DipicCl did statistically significantly lower serum TG, but not serum cholesterol (Table 3), and did significantly lower the pathologically raised diabetic serum AST and ALP.
Table 3. Effects of treatment with V3dipicCl, V4dipicCl, V5dipicCl, dipicCl ligand and V5dipicNH2 on serum biochemical parameters in rats with STZ-induced diabetesa.
| Group | Chol (mg/dl) | TG (mg/dl) | AST (U/I) | ALP (U/I) |
|---|---|---|---|---|
| Normal Control | 1.44 + 0.29* | 0.67 + 0.21 | 216 + 33 | 63 + 7 *** ## |
| Diabetic | 2.04 + 0.42 | 0.94 + 0.09 | 257 + 133## | 747 + 326 ### |
| DipicCl | 1.54 + 0.12 | 0.90 + 0.19 | 147 + 14** | 356 + 146*** |
| V3dipicCl | 1.38 + 0.36 | 0.75 + 0.32 | 200 + 46 | 358 + 130** |
| V4dipicCl | 1.25 + 0.38* | 0.54 + 0.30 * # | 146 + 17** | 270 + 84 *** |
| V5dipicCl | 0.96 + 0.36* | 0.36 + 0.3 * # | 170 + 44* | 212 + 53 *** |
| V5dipicNH2 b | 1.10 + 0.22* | 0.47 + 0.28 | 166 + 27 | 223 + 113 |
Values are expressed as the mean + SD. N= 5-6,
p< 0.05,
p< 0.01 and
p< 0.001 vs Diabetic.
vs Dipic Cl group
Parameters measured were total serum cholesterol (Chol), Triglycerides (TG), Aspartate Aminotransferase (AST), and alkaline phosphatase (ASP).
Treatment with the V3dipicCl, V4dipicCl or V5dipicCl complexes did show oxidation state differences. Administration of any of these three complexes caused lowered diabetic hyperglycemia by day 8. However, at day 5, significantly lowered BG was only seen after treatment with V5dipiCl (Fig 5). Treatment with all three complexes lowered the elevated chol levels in the diabetic rats,while treatment with only V4dipicCl or V5dipicCl lowered the elevated TG levels (Table 3). Additionally, treatment with V4dipicCl or V5dipicCl significantly lowered AST from elevated diabetic levels in the animals. Treatment with all VdipicCl complexes lowered the elevated serum ALP. The oxidation state differences observed in the treatment of diabetic rats with the dipicCl series was different from those observed in treatment with the dipic series.
3. Insulin-enhancing effects of V5dipic with OH modification of ligand and amine coordination at the metal
3.1 Effects on diabetic hyperglycemia, diabetic hyperlipidemia and absorption into serum when the V5dipic complex is modified with OH and hydroxylamine is coordinated to the V
The aromatic ring of the V5dipic complex was first substituted with hydroxyl group in the para position altering the electronic properties of the complex. The effect of changing the coordination geometry around the V atom was done using the V5dipicOH complex, which was the first V5dipic complex for which the details of the insulin-enhancing effects of the V5dipicOH complex were reported [18]. The coordination number of the V5dipic complex was increased to 7 by the addition of a dimethyl hydroxylamido group to the V complex [V5dipic(MeHA)]. The effects of coordinating the hydroxylamine to both parent complexes V5dipic and V5dipicOH has been described in detail [58].
The effect of administration of these hydroxylamine complexes on BG in Wistar rats with STZ-induced diabetes is shown in Fig. 6 (unpublished). The coordination of dimethyl hydroxylamine to the V5dipic interfered with the ability of the V5dipic complex to significantly lower BG when administered to diabetic rats. Previously it was shown that treatment with V5dipicOH was effective in lowering BG for the first two weeks of the experiment. However, BG values returned to those of the untreated diabetic control by the end of the experiment at 4 weeks. The effective time period for BG normalization in this animal model is very dependent upon the specific V compound used. In fact, treatment with BMOV showed that the BG lowering effect has been reported to continue for months after treatment was terminated [6]. In an attempt to lengthen the time of effectiveness of the dipic series, V5dipicOH was complexed to a hydroxylamine group, which improved the BG lowering effect at the end of the experimental time period. Treatment with V5dipicOH(HA) caused the lowered BG level to be maintained throughout the one month experiment. None of these modifications produced a complex that was more effective at lowering of BG than the parent, V5dipic. The effect of treatment with the complex with an addition of an amino group at the para position was also studied. In Fig. 5 it can be seen that the NH2 substitution on the dipic ligand resulted in a V5dipicNH2 complex that when administered also significantly lowered diabetic hyperglycemia to similar levels as VS administration [22].
Fig 6.
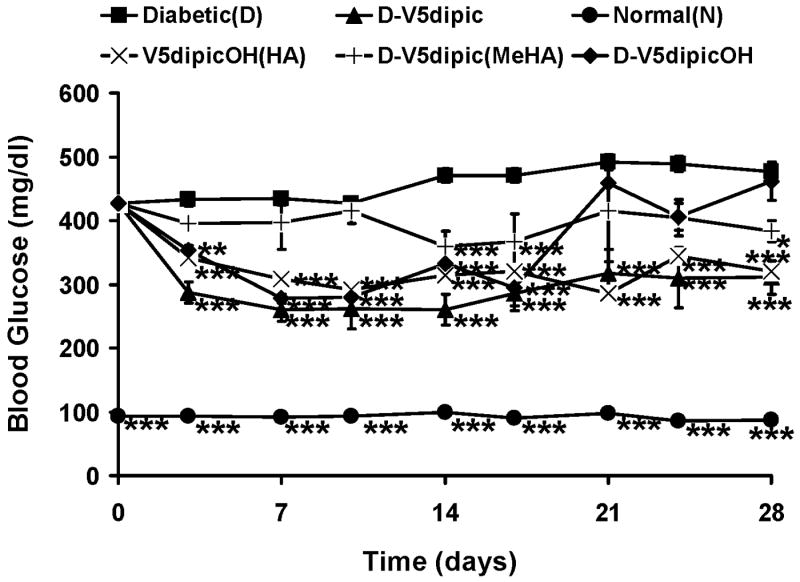
Effect of oral chronic administration of hydroxylamine coordinated to V5dipic and V5dipicOH on BG levels in rats with STZ-induced diabetes. Data obtained as described in previous publications [18, 19, 32]. N (●), D (■), V5dipic (▲), V5dipic(MeHA) (|), V5dipicOH (◆), V5dipicOH(HA)(X). Data analyzed by one way ANOVA with multiple means testing. *** represents p < 0.001 vs diabetic rats.
The effects of treatment with hydroxylamine modified V5dipic and V5dipicOH complexes on diabetic hyperlipidemia were monitored by measuring serum TG, chol and FFA (Table 1). All of the measured lipids were significantly lowered by administration of all of theses V5dipic complexes. While V5dipicOH was the most effective complex at lowering the elevated TG levels associated with diabetes, administration of V5dipicNH2 also significantly lowered serum chol and serum TG (Table 3) [22]. The V5dipic(MeHA) lowered serum TGs to normal levels, while the ternary complexes of V5dipic and V5dipicOH formed by addition of hydroxylamine were more effective at lowering FFAs than the five-coordinate complexes. The coordination of HA to the metal appears to have increased the ability of treatment with the V5dipic or V5dipicOH complexes to lower diabetic hyperlipidemia. Overall, the V5dipicOH(HA) was the best complex with respect to lowering serum lipids after treatment since it was the only complex that lowered both serum TG and FFA to normal levels.
The absorption into serum was measured and compared to the dose of V ingested by the animals treated with hydroxylamine modified Vdipic complexes. These results were compared to those seen in diabetic animals treated with VS or Vdipic complexes with differing oxidation states as described in Section 2.1 (Table 2). As discussed before the ratio of the serum V to the average ingested dose of V is the best measure of absorption into serum when the animals are dosed with vanadium complexes in the drinking water. Of the new complexes studied (V5dipicOH, V5dipicOH(HA) and V5dipic(MeHA), animals treated with V5dipicOH accumulated the most V in serum of any in this study and significantly more than both the Normal animals and Diabetic animals treated with VS. However, these animals also ingested much more V, so the ratio of serum V to dose ingested was not significantly different from that seen with these two other groups. Animals dosed with V5dipicOH(HA) accumulated significantly more V into serum than the D animals treated with VS and the statistical significance of this was also observed when the ratio of serum V to dose is used as the metric. In the diabetic animals treated with V5dipic(MeHA) the ratio of serum V to average dose was significantly lower than that seen with normal animals treated with VS but not significantly higher than the ratio seen with diabetic animals treated with VS. Limiting the comparisons to the diabetic animals only the V5dipicOH(HA) significantly increased the absorption of V into serum with a 2.3 fold increase being observed in the ratio.
3.2 The effects on global gene expression of treatment with V5dipicOH and VS
Treating outbred Wistar rats with STZ-induced diabetes with the simple salt VS results in approximately 60% of the animals responding with both lowered diabetic hyperglycemia and hyperlipidemia. VS treated diabetic rats that responded to treatment with both lowered hyperlipidemia and hyperglycemia were originally selected for gene expression profiling studies. An Affymetrix rat chip with approximately 8000 probe sets was used to study gene expression in normal and diabetic animals in the presence and absence of VS treatment [71]. The Affymetrix probe sets used were sets of 16 short oligonucleotides specifically designed to monitor the expression of a single gene as monitored by various algorithms. An important conclusion of that earlier study was that in a two way ANOVA analysis (using diabetes and treatment as variables) of the 5100 probe sets that were expressed in any of our experimental conditions showed a statistically significant interaction. This result confirms what has been observed in individual assays that V treatment affects normal and diabetic animals differently. The response of the diabetic rats to VS when only hyperlipidemia and not hyperglycemia was controlled was similar to that observed for the diabetic rats treated with V5dipicOH (Fig. 6 and Table1). In order to see the effect of the dipicOH ligand on global gene expression we extended our previous gene expression study to include data from these two new treatment groups which had not previously been published.
In our previous work 66 probe sets altered in diabetes and restored to normal levels in diabetic animals treated with VS in which both hyperlipidemia and hyperglycemia were controlled were identified. We now examined the expression of these same probe sets in the animals treated with VS with elevated BG levels and normalized hyperlipidemia and those treated with V5dipicOH as illustrated in Fig 8. In this experiment each column represents data from one rat and there are five rats in each group. The expression data in each row are normalized to the median value seen in the normal control group. The pattern of gene expression obtained in Figure 8 for the diabetic rats treated with VS who responded with lowered BG and lowered lipid levels resembles the expression pattern seen for the normal rats more than that of the diabetic rats. Interestingly the pattern of gene expression seen in the normal animals treated with VS shows a resemblance to that seen in diabetic animals, especially for the down regulated genes. The expression pattern seen with the rats treated with VS or V5dipicOH in which BG remained high and only the lipids were lowered resembled the diabetic gene expression pattern for this set of both up regulated and down regulated genes.
Fig 8.
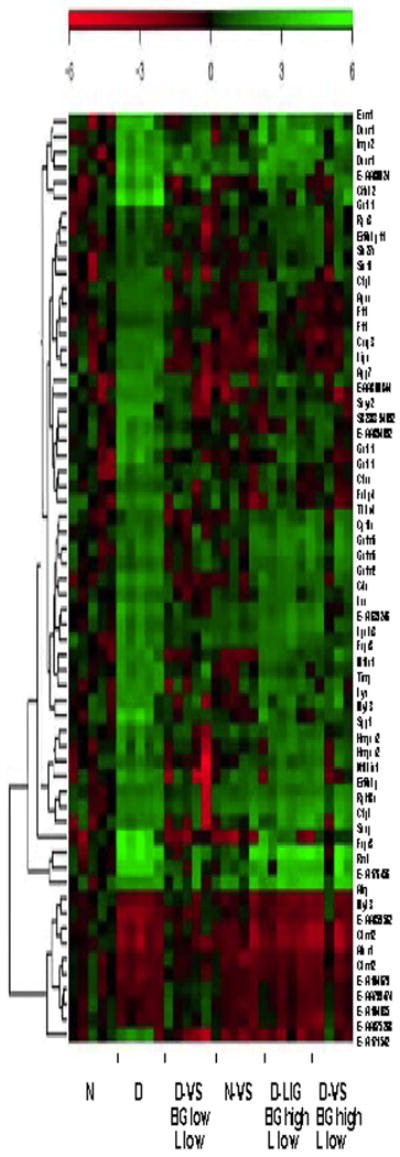
Heatmap for the 62 probe sets selected as being both altered by diabetes and corrected by oral administration of VS. The expression of each individual gene is compared with that of the median of the N group, represented by black in the group of arrays for the N rats. The intensity of the color indicates the variability of the expression of that probe set in the group. Data from N and D untreated and treated with VS (where the treated rats showed lowered diabetic hyperlipidemia and hyperglycemia (D-VS BG low L low) have previously been published [71]. New data for D animals treated with VS in which only lipids were lowered (D-VS BG high, L low) and D animals treated with the liganded V5dipicOH (D-Lig BG high, L low).
The number of probe sets differentially expressed when comparing the expression in three treated groups to that observed in the normal and diabetic animals was then examined (Table 4) for all of the 5100 genes showing some expression changes in the overall study. In comparisons of gene expression in diabetic animals with gene expression in VS treated diabetic groups and the V5dipicOH treated diabetic animals, hundreds of differentially expressed probe sets were identified (First Column, Table 4). Hundreds of differentially expressed genes were identified when gene expression in both the VS treated diabetics with high BG and low lipids and the V5dipicOH treated diabetic animals were compared to gene expression in normal animals (Second Column, Table 4). In contract, when gene expression in the VS treated diabetics in which BG and serum lipids were lowered was compared to normal gene expression, only 33 differentially expressed probe sets were identified. Identification of 33 genes in this type of experiment is similar to a baseline measurement. These results imply that the changes in gene expression seen when diabetic hyperlipidemia and hyperglycemia are alleviated by V treatment returns gene expression to normal. However, the changes in gene expression when only diabetic hyperlipidemia is returned to normal are mediated via changes that do not involve a return to normal gene expression.
Table 4. Comparisons of Gene Expression Diabetic Rats Treated with VS and V5dipicOHa.
| Group | Number of Probe Sets Expressed compared to Untreated Diabetic b | Number of Probe Sets Expressed compared to Untreated Normal b |
|---|---|---|
| VS - BG low, Lipid Low b | 523 | 33 |
| VS - BG high, Lipid Low | 476 | 516 |
| V5dipic – BG high, lipid Low | 274 | 460 |
Experimental methods for animal treatment, RNA extraction, and analysis with Affymetrix Rat Chip U34 described in Willsky et al (2006) Physiological Genomics [71]. Probe sets identified by standard t test comparisons with False Discovery Rate of 0.05 using affymetrix expression data.
Data for this group previously published [71].
Gene expression data from Wistar rast classified as normal, diabetic, diabetic treated with vanadate, and diabetic treated with insulin have been obtained using the 96 genes on the GE Array for the insulin pathway [72]. Although the expression of most of the diabetes altered genes were returned to normal levels with VS and insulin treatment, expression patterns were not identical. This result is in agreement with data for mice with STZ-induced diabetes treated with VS or insulin using the Affymetrix array [73]. In addition, the identified probe sets with altered gene expression found in this mouse experiment using the Affymetrix chip[73] with VS treatment were very similar to those reported described above for the rat Affymetrix experiment using VS treatment[71].
4.0 Interpretations of the observed effects of the Vdipic complexes used as treatment in STZ-induced diabetic rats
4.1 Summary of the insulin-enhancing effects seen in administration of Vdipic complexes to diabetic rats and toxicity implications
Substitution to the 4-position of the dipic ligand in the Vdipic complexes screened had a limited effect on insulin-enhancement. When the OH or NH2 functionalities perturbed the aromatic ring of the V5dipic complex, chronic administration did not significantly improve insulin-enhancement, as a matter of fact the BG lowering effect on diabetic hyperglycemia was reduced when V5dipicOH was used. [18, 22]. Administration of the ternary complex formed upon hydroxylamine coordination to the V improved the normalization effect on hyperlipidemia when compared to both V5dipic and V5dipicOH (Fig 6. and Table 1). Complexation of the HA moiety to V5dipicOH extended the BG lowering effects on diabetic hyperglycemia to the end of the treatment period.
Differing results were also seen in the oxidation state series for both Vdipic [19] and VdipicCl [21]. Although there are significantly different changes after treatment of diabetic rats with Vdipic or VdipicCl complexes in oxidation states III, IV, or V; there is no statistically significant linear relationship of oxidation state and diabetic hyperglycemic (Figs 3 and 5) or hyperlipidemic (Tables 1 and 3) effects. In the VdipicCl oxidation state series, treatment with V5dipicCl showed greater effectiveness than administration of V3dipicCl or V4dipicCl at normalizing diabetic hyperglycemia in the earlier timepoints, while all were equally effective after 12 days. A linear trend with oxidation state in the effectiveness at lowering diabetic hyperlipidemia was observed with the VdipicCl complexes. Treatment with V5dipicCl also was the most effective at normalizing elevated Chol and TG levels in the diabetic animals.
In the chronic administration model [47] the Vdipic oxidation state series showed a trend in effectiveness (V5>V4>V3) for the treatment of diabetic hyperglycemia (Fig. 3). However, in the acute model [32]V3dipic and V5dipic effectively lowered BG while V4dipic did not (Fig. 4) Since the administration routes of the complexes were different it is possible that the modification of efficacy is due to increased interactions with metabolites when the complex passes through the gastrointestinal tract. Interactions with other ligands, and possibly also with membranes within the animal, during the treatment process could also contribute to the results observed.
Of all the Vdipic complexes studied, treatment with V5dipic, V5dipicCl, V5dipicOH(HA) and V5dipicNH2 appeared to alleviate most of the diabetic symptoms in our studies. V5dipic appears to normalize diabetic hyperglycemia to the greatest extent of the complexes examined. The hydroxylamine V complexes appeared to be most effective at normalizing diabetic hyperlipidemia. Currently we have only performed a two-week experiment with the V5dipicNH2 and no data on absorption into serum is available for this complex. The V5dipicOH(HA) was the only Vdipic complex that accumulated more V into serum compared to VS after doses were normalized (Table 2). One could speculate that addition of HA to V5dipicNH2 would further improve alleviation of diabetic symptoms.
It is encouraging to see a correlation between V in blood and dose for the complexes VS, V5dipic and V5dipcOH(HA) in chronic studies. However, corresponding studies are lacking for the other V complexes explored here. An inverse Spearman correlation of -0.488 (p<0.05, n=19) was seen for BG and serum V when Vdipic in all oxidation states was administered using the data presented here in Fig 3 and Table 2. An inverse Spearmen correlation of -0.503 (p<0.05 n=22) for serum V and cholesterol in animals treated with the V5dipic complexes described in Tables 1 and 2. Additional correlations are expected for V and metabolite markers in serum however such analyses would require additional animal samples. Treatment with V5dipic complex resulted in a correlation between V levels in blood, and BG levels in plasma using the acute administration protocol [32]. These results are consistent with the concept that the blood/serum compartment or another biological compartment in equilibrium with these is important in the anti-diabetic properties of the Vdipic complexes in this animal model.
The toxic effects of administration of the Vdipic complexes and their derivatives varied from minimal to significant morbidity and mortality(section 1.3). Short term human clinical trials with vanadium salts have been completed with minimal toxicity observed. Phase 1 and phase 2 clinical trials with BEOV, the first V coordination complex to be used in human studies, have concluded; and kidney toxicity has been reported in the phase 2 trial [74]. Therefore a brief discussion of how the results reported here relate to other rodent and human studies with V is warranted.
Worker exposure to excess vanadium has largely resulted from inhalation of vanadium pentoxide in workplace dust[75]. Respiratory effects predominate, but many organ systems can demonstrate adverse effects[76]. Non-occupational vanadium exposure is predominantly from the food supply and typical daily doses consumed by humans have been estimated at 0.01 to 0.03 mg V per day. Normal serum levels range from 0.02 to 0.9 ng V/ml[77]. Chronic one year oral exposure studies in rats indicate that doses as high as 19 mg V /kg /day as vanadyl sulfate in the drinking water caused no hematological or pathological effects, and a dose of 28 mg V/kg/day led to a small decrease in body weight gain [78-81]. Similarly, lifetime studies in rats and mice showed no adverse effects at doses of up to 4.1 mg V/kg/day[82, 83].
One reason for the ability of animals to tolerate chronic exposures to high dose rates of vanadium is the poor absorption of vanadium salts in the GI tract. Less than one percent of the ingested vanadium is typically absorbed by this route of exposure. Although there may be higher absorption rates for various complexes of vanadium as indicated by the serum to dose rate ratios in Table 2, the magnitude of the differences in absorption are within a factor of 2 to 3 when compared to VS. Dissociation of vanadium from the complex is likely necessary for both its toxicological and insulin-like effects. In addition, there may be compound specific differential distributions of vanadium to tissues, which may depend on the physico-chemical properties of each compound, and on the rate of release of V from each compound in body fluids and tissues. Additional studies are necessary to assess tissue specific pharmacokinetic parameters for individual vanadium complexes, and their role in producing adverse effects.
4.2 Chemical stability and electrochemistry/catalytic studies of the V5dipic complexes with anti-diabetic effects
The chemistry and properties of a range of dipic-complexes have been studied in detail by our group [16-18, 20-23, 32, 53, 55, 58-60, 65, 70, 84-90]. Small changes in the properties of Vdipic complexes manifest themselves as changes in stability, redox properties and solubility. Our objective here was to associate these differences, with the observed effects of modified Vdipic complexes on insulin-enhancement. Formation of the V5dipic complexes can be determined using NMR spectroscopy, however, formation of V4 and V3dipic complexes must involve alternative methods, such as EPR and UV-vis spectroscopy. Although many excellent studies have been carried out on a range of V complexes other ligands,[16,25,26, 68,69] less has been done with V4 and V3dipic complexes [17,19, 54-56]. For V5dipic and the substituted complexes, the stability maximum is around pH 3.4. Because the pKa for H2dipic is 4.49[16, 52, 53] this observation is not due to the chemistry of the ligand, but due to the chemistry of V(5) in the aqueous solution. Below pH 3.4, complex formation is less pronounced because monomeric vanadate concentration is low. Above pH 3.4, less V5dipic complex is observed because of a combination of V(5) chemistry and dipic deprotonating, forming the dianion ligand species. Pertubation of the dipic ring with both electron donating and withdrawing substituents lowers complex stability. These changes trace with the trend of lowered effectiveness as insulin-enhancing agents. It is interesting that in the Vdipic complexes, as in the case of the BMOV-BEOV series, the parent and unsubstituted system is the most effective [7].
The stability of dipicolinate complexes are pH dependent as is illustrated in Fig. 9 for the [VO(dipic)(Me2HNO)(H2O)]. A large and a small signal is shown from pH 2.2 to 5.2 reflect the two different isomers of this asymmetric hydroxylamine. Above pH 6.1 the complex is beginning to hydrolyze to form vanadates and the oxovanadium hydroxylamine complex. The effect of substituent perturbation is shown in Fig. 10 where the amount of intact V5dipicX is plotted as a function of pH [20, 23, 53, 55, 58]. As shown in Fig. 10 V5dipic, V5dipicOH, V5dipicNH2, V5dipicCl, and V5dipicNO2 have stability maxima near pH 3.4. The V5dipic complex is the most stable over the tested pH range. Of particular interest to biology, V5dipic is also the most stable complex at physiological pH ranges. Interestingly, when X = OH, NH2, NO2 or Cl, the resulting V complexes are less stable than V5dipic [20, 23, 55, 58]. The higher relative stability of V5dipic presumably arises due to counteracting electronic effects created by the substituents reducing the chelating abilities of the modified ligands. Binding affinity reduction would result in the stability of monomeric vanadate becoming more favorable over complex formation. Since the parent version of the ligand creates the Vdipic complex possessing the greatest insulin-enhancement effect in animals, these properties correlate well with the chelating effect of the dipic ligand.
Fig 9.
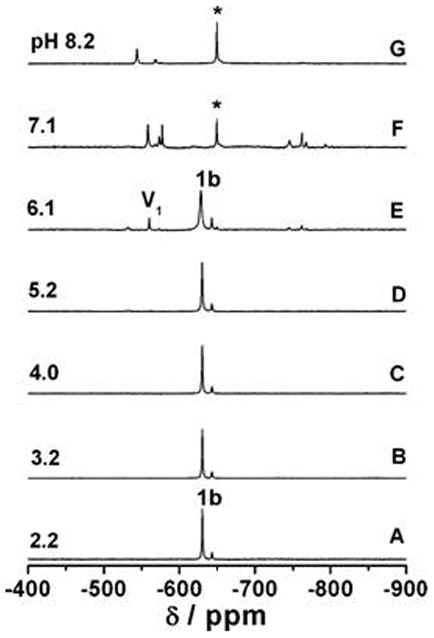
The 51V NMR spectra of [VO(dipic)(MeHNO)(H2O) recorded as the pH was varied from 2-8 (A-G). Each spectrum was recorded from a separate sample with an initial concentration of 2 mm complex. Monomeric vanadate and the predominant hydroxylamido:V species are labeled as V1 and *, respectively. Figure reproduced from Smee et al [20] with permission.
Fig 10.
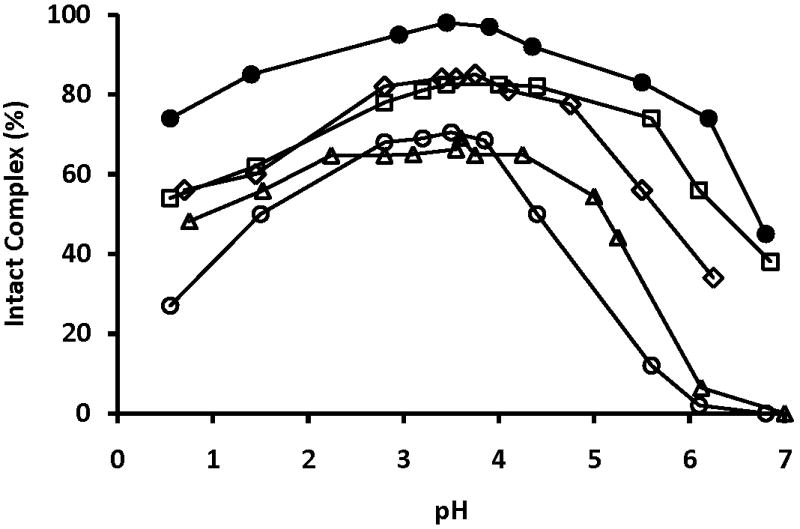
Percentage of intact V5dipicX as a function of pH. 51V-NMR data obtained from previously published work by Smee et al. [20, 23]. X= H (○); OH (◆); NH2 (□); NO2 (●); Cl (*)
Coordination of the hydroxylamine (HA) ligand to the V atom forms a ternary complex which significantly changes the properties of the V complexes [20, 60, 91, 92]. Although this ligand can rapidly dissociate in hydrophobic environments, the presence of HA significantly stabilizes the complex. Reductions have been reported during Vdipic chelation to HA [20, 23]. Subtle variations in the HA structure have a profound effect on the electronic properties exhibited by V and is readily observed by 51V NMR chemical shifts. For the V5dipicOH complex a chemical shift of -534 ppm is observed, while -605 ppm is found for V5dipicOH(MeHA) and -671 ppm for V5dipicNO2(HA) [20]. For V5dipicCl(HA) a chemical shift of -679 ppm was observed, while -630 ppm is found for V5dipicCl(MeHA), -604 ppm for V5dipicCl(Me2HA) and -594 ppm for V5dipicCl(Et2HA), all of which are significantly further upfield compared to the parent complex V5dipicCl at -533 ppm [23]. The electronic changes in these systems have been characterized in detail [60].
Hydrolysis of these HA ternary complexes is more involved than the simple V5dipic system. Not only do the oxovanadates form, but the parent complexes (V5dipic and V5dipicCl) [23, 53] as well as the Vdipic(HA) complexes form [91, 93, 94]. Indeed, the Vdipic(HA) complexes have been characterized in detail, showing an optimum pH near neutral pH with the existence of several species. These species include the oxovanadates, simple Vdipic(HA) complexes, as well as the ternary complexes [91, 93, 94]. Interestingly, the ternary HA ligands extend the stability of the ternary complexes by one to two pH units to the neutral pH range. As the Vdipic(HA) complex approaches neutral pH it begins to hydrolyze. However, the hydrolysis point is several pH units higher than the hydrolysis point of the parent complex, which occurs around pH 5 [23]. Combined, these studies show that the stabilization provided by the additional ligand on the V results in complexes that exist into the neutral pH range. These results suggest a mechanism for how the parent complexes are able to exert activities in biological systems. Interestingly, fine-tuning the properties of V5dipic could be important to rationalize the insulin-enhancing effects of these compounds [95].
V compounds undergo redox chemistry under physiological conditions [26, 96]. Generally the redox cycling of V compounds under physiological conditions involves the transfer of one-electron. Recently evidence of two-electron transfer reactions with Vdipic complexes have been reported [97, 98]. Redox cycling, involving a range of metabolites, forms reactive oxygen species (ROS) [99]. ROS are no longer considered toxic in all environments, and low concentrations have some beneficial effects [100]. In fact, insulin-induced ROS are believed to be involved in the insulin-signaling pathway [95]. Cell signaling processes involve receptor ligand interactions which are based on electrostatic potentials associated with the ions and dipoles in the receptor ligand complex[66]. These types of interactions take place with all types of ions including the many cations and phosphates in biological systems and shift the energetics associated with electron transfer processes[101]. Redox properties of V complexes can be tuned from favoring one-electron transfer reactions to two-electron transfer reactions, potentially decreasing the toxicity of these complexes[66].
Electronic properties of V complexes are altered upon substitution with electron withdrawing and donating groups [60]. It is well known that metal chelation will alter redox potential and was recently demonstrated for vanadate under physiological conditions [96]. Formation of ternary complexes under cellular conditions is likely, considering the increased stability of such complexes. Ternary Vdipic complexes are reported to form with peroxide, a series of hydroxylamines, pyridine, pinacol, and alcohols. These occur in hydrophobic environments and thus support the suggestion of membrane interaction being involved in the biological actions of V[66]. V5dipic readily penetrates lipid interfaces [65] residing in hydrophobic environments under near physiological conditions. Therefore, the potential exists that alteration of the insulin-enhancing and/or toxic actions of V complexes may be triggered by the movement of V complexes into membrane environments.[66].
5.0 Conclusions
Structure-activity relationship studies were carried out using a range of Vdipic complexes. It was demonstrated that changes in coordination geometry caused the greatest improvement in the insulin-enhancing properties of these complexes. Changes in V oxidation state also impact the insulin-enhancing properties, however these effects were less pronounced. We conclude that with regard to improving the insulin-enhancing properties, dipic ligand substitution is less important when compared to the V oxidation state and ternary complex formation. We propose that the compound stability and the ability to interact with cellular redox reactions are key components for the insulin-enhancing activity exerted by V compounds. Recently, the possibility that membrane interactions are influenced by the ligand was suggested, and such membrane effects may affect uptake and action of the V complexes [66]. Interestingly, the classes of compounds discussed here with the greatest insulin-enhancing effects are found for compounds that are most compatible with the lipid environment.
Fig 7.
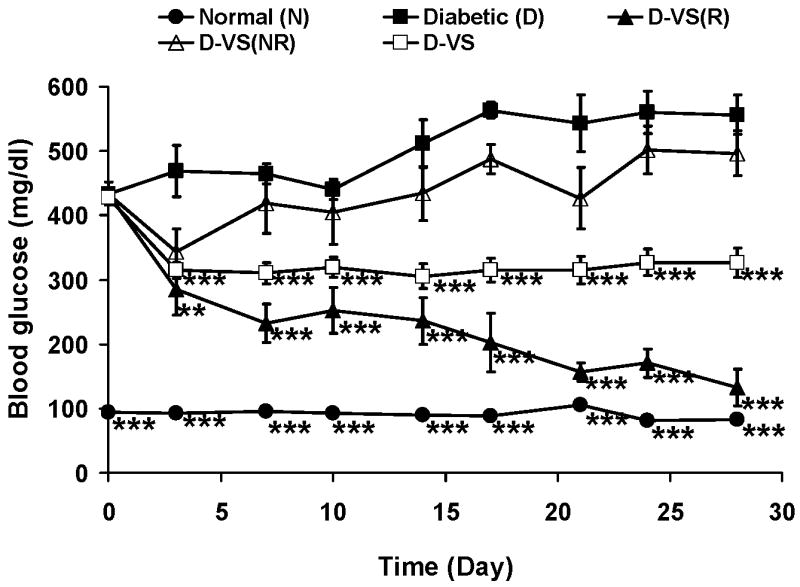
Effect of oral chronic administration of VS on rats with STZ-induced diabetes. Parts of this figure has been previously published [71]. N (●), D (■), D-VS(R) responding with lowered BG and lowered Lipids (▲), D-VS not responding (NR) with lowered BG and but showing lowered Lipids (△), D-VS all responses of animals treated with VS (□).Data analyzed by one way ANOVA with multiple means testing. *** represents p < 0 .001 vs diabetic rats.
Acknowledgments
DCC and GRW thank NIGMS Grant No. 40525 and the American Diabetes Association for funding this work. DCC also thanks NSF CHE-0628260 for funding. AMT was supported by NSF Bridge to the Doctorate fellowship, grant NSF HRD 0832932 and NSF CHE-0628260.
Abbreviations
- ANOVA
Analysis of Variance is the standard way to statistically analyze biological results when 2 or more groups are being compared
- AST
Aspartate aminotransferase
- ALP
Alkaline phosphatase
- BB
Biobreeding
- BG
Blood glucose
- BEOV
Bis(ethylmaltolato)oxovanadium(IV)
- BMOV
Bis(maltolato)oxovanadium(IV)
- Chol
Cholesterol
- Dipic
Dipicolinate or 2,6-pyridinedicarboxylate
- DipicCl
4-chlorodipicolinate or 4-chloro-2,6-pyridinedicarboxylate
- DipicOH
4-hydroxydipicolinate or 4-hydroxy-2,6-pyridinedicarboxylate
- FFA
Free fatty acids
- HA
Hydroxylamine
- MeHA
Methylhydroxylamine
- N
Normal
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- STZ
Streptozotocin
- TG
Triglyceride
- V
vanadium
- VS
vanadyl sulfate
- Vdipic
refers to all the vanadium-containing dipicolinate complexes of oxidation states III, IV and V
- VdipicCl
refers to all the vanadium-containing chlorodipicolinate complexes of oxidation states III, IV and V
- V3dipic
Bis(dipicolinato)oxovanadium(III)
- V4dipic
Dipicolinatooxovanadium(IV)
- V5dipic
Dipicolinatooxovanadium(V)
- V3dipicCl
Bis(4-chlorodipicolinato)oxovanadium(III)
- V4dipicCl
4-chlorodipicolinatooxovanadium(IV)
- V5dipicCl
4-chlorodipicolinatooxovanadium(V)
- V5dipic(MeHA)
Dipicolinatooxovanadium(V)methylhydroxylamine
- V5dipicNH2
4-aminodipicolinatooxovanadium(V)
- V5dipicOH
4-hydroxydipicolinatooxovanadium(V)
- V5dipicOH(HA)
4-hydroxydipicolinatooxovanadium(V) hydroxylamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heyliger CE, Tahiliani AG, Mcneill JH. Science. 1985;227:1474. doi: 10.1126/science.3156405. [DOI] [PubMed] [Google Scholar]
- 2.Degani H, Gochin M, Karlish SJD, Shechter Y. Biochemistry. 1981;20:5795. doi: 10.1021/bi00523a023. [DOI] [PubMed] [Google Scholar]
- 3.J.H. McNeill, (Ed.), CRC Press Boca Raton FL USA,1999.
- 4.Cros GH, Cam MC, Serrano JJ, Ribes G, McNeill JH. Mol Cell Bioche. 1995;153:191. doi: 10.1007/BF01075937. [DOI] [PubMed] [Google Scholar]
- 5.Battell ML, Yuen VG, McNeill JH. Pharmacol Commun. 1992;1:291. [Google Scholar]
- 6.Cam MC, Brownsey RW, McNeill JH. Can J of Phys Pharmacol. 2000;78:829. [PubMed] [Google Scholar]
- 7.Thompson KH, Orvig C. J Inorg Biochem. 2006;100:1925. doi: 10.1016/j.jinorgbio.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Thompson KH, Lichter J, Lebel C, Scaife MC, McNeill JH, Orvig C. J Inorg Biochem. 2009;103:554. doi: 10.1016/j.jinorgbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Thompson KH, Orvig C. Metal Ions in Biological Systems, Vol 41: Metal Ions and Their Complexes in Medication. 2004;41:221. [PubMed] [Google Scholar]
- 10.Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L. J Clin Invest. 1995;95:2501. doi: 10.1172/JCI117951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden G, Chen X, Ruiz J, Van Rossum GDV, Turco S. Metabolism. 1996;45:1130. doi: 10.1016/s0026-0495(96)90013-x. [DOI] [PubMed] [Google Scholar]
- 12.Goldfine AB, Patti ME, Zuberi L, Goldstein BJ, Leblanc R, Landaker EJ, Jiang ZY, Willsky GR, Kahn CR. Metabolism. 2000;49:400. doi: 10.1016/s0026-0495(00)90418-9. [DOI] [PubMed] [Google Scholar]
- 13.Cusi K, Cukier S, Defronzo RA, Torres M, Puchulu FM, Redondo JCP. J Clin Endocrin Metabol. 2001;86:1410. doi: 10.1210/jcem.86.3.7337. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai H, Yasui H. J Tr Elem Exp Med. 2003;16:269. [Google Scholar]
- 15.Hiromura M, Adachi Y, Machida M, Hattori M, Sakurai H. Metallomics. 2009;1:92. [Google Scholar]
- 16.Crans DC. J Inorg Biochem. 2000;80:123. doi: 10.1016/s0162-0134(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 17.Crans DC, Mahroof-Tahir M, Johnson MD, Wilkins PC, Yang LQ, Robbins K, Johnson A, Alfano JA, Godzala ME, Austin LT, Willsky GR. Inorg Chim Acta. 2003;356:365. [Google Scholar]
- 18.Crans DC, Yang LQ, Alfano JA, Chi LAH, Jin WZ, Mahroof-Tahir M, Robbins K, Toloue MM, Chan LK, Plante AJ, Grayson RZ, Willsky GR. Coord Chem Rev. 2003;237:13. [Google Scholar]
- 19.Buglyo P, Crans DC, Nagy EM, Lindo RL, Yang LQ, Smee JJ, Jin WZ, Chi LH, Godzala ME, Willsky GR. Inorg Chem. 2005;44:5416. doi: 10.1021/ic048331q. [DOI] [PubMed] [Google Scholar]
- 20.Smee JJ, Epps JA, Teissedre G, Maes M, Harding N, Yang L, Baruah B, Miller SM, Anderson OP, Willsky GR, Crans DC. Inorg Chem. 2007;46:9827. doi: 10.1021/ic701233y. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Ding W, Smee JJ, Baruah B, Willsky GR, Crans DC. Biometals. 2009;103:585. doi: 10.1007/s10534-009-9241-4. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Smee JJ, Ding WJ, Crans DC. J Inorg Biochem. 2009;103:585. doi: 10.1016/j.jinorgbio.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Smee JJ, Epps JA, Ooms K, Bolte SE, Polenova T, Baruah B, Yang LQ, Ding WJ, Li M, Willsky GR, La Cour A, Anderson OP, Crans DC. J Inorg Biochem. 2009;103:575. doi: 10.1016/j.jinorgbio.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Rehder D, Pessoa JC, Geraldes C, Castro M, Kabanos T, Kiss T, Meier B, Micera G, Pettersson L, Rangel M, Salifoglou A, Turel I, Wang DR. J Biol Inorg Chem. 2002;7:384. doi: 10.1007/s00775-001-0311-5. [DOI] [PubMed] [Google Scholar]
- 25.Rehder D. Inorg Chem Comm. 2003;6:604. [Google Scholar]
- 26.Crans DC, Smee JJ, Gaidamauskas E, Yang LQ. Chem Rev. 2004;104:849. doi: 10.1021/cr020607t. [DOI] [PubMed] [Google Scholar]
- 27.Scior T, Guevara-Garcia A, Bernard P, Do QT, Domeyer D, Laufer S. Mini-Rev Med Chem. 2005;5:995. doi: 10.2174/138955705774575264. [DOI] [PubMed] [Google Scholar]
- 28.Hanson GR, Sun Y, Orvig C. Inorg Chem. 1996;35:6507. doi: 10.1021/ic960490p. [DOI] [PubMed] [Google Scholar]
- 29.Fukui K, Fujisawa Y, Ohya-Nishiguchi H, Kamada H, Sakurai H. J Inorg Biochem. 1999;77:215. doi: 10.1016/s0162-0134(99)00204-4. [DOI] [PubMed] [Google Scholar]
- 30.Dikanov SA, Liboiron BD, Orvig C. J Am Chem Soc. 2002;124:2969. doi: 10.1021/ja011104s. [DOI] [PubMed] [Google Scholar]
- 31.Liboiron BD, Thompson KH, Hanson GR, Lam E, Aebischer N, Orvig C. J Am Chem Soc. 2005;127:5104. doi: 10.1021/ja043944n. [DOI] [PubMed] [Google Scholar]
- 32.Willsky GR, Godzalla ME, Kostyniak PJ, Chi LH, Gupta R, Yuen VG, McNeill JH, Mahroof-Tahir M, Smee JJ, Yang LQ, Lobernick A, Watson S, Crans DC. In: ACS symposium Series 974. Kustin K, Pessoa JC, Crans DC, editors. 2007. p. 93. [Google Scholar]
- 33.Hiromura M, Nakayama A, Adachi Y, Doi M, Sakurai H. J Biol Inorg Chem. 2007;12:1275. doi: 10.1007/s00775-007-0295-x. [DOI] [PubMed] [Google Scholar]
- 34.Sakurai H. Vanadium: The Versatile Metal. In: Kustin K, Pessoa JC, Crans DC, editors. Amer Chem Soc. 2007. p. 110. [Google Scholar]
- 35.Kawabe K, Yoshikawa Y, Adachi Y, Sakurai H. Life Sciences. 2006;78:2860. doi: 10.1016/j.lfs.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Kiss T, Jakusch T, Hollender D, Dornyei A, Enyedy EA, Pessoa JC, Sakurai H, Sanz-Medel A. Coord Chem Rev. 2008;252:1153. [Google Scholar]
- 37.Harris WR. Biochemistry. 1985;24:7412. doi: 10.1021/bi00346a057. [DOI] [PubMed] [Google Scholar]
- 38.Bordbar AK, Creagh AL, Mohammadi F, Haynes CA, Orvig C. J Inorg Biochem. 2009;103:643. doi: 10.1016/j.jinorgbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Sanna D, Micera G, Garribba E. Inorg Chem Inorg Chem. 2009;48:5747. doi: 10.1021/ic802287s. [DOI] [PubMed] [Google Scholar]
- 40.Pessoa JC, Tomaz I. Curr Med Chem. 2010;17:3701. doi: 10.2174/092986710793213742. [DOI] [PubMed] [Google Scholar]
- 41.Corot C, Idee JM, Hentsch AM, Santus R, Mallet C, Goulas V, Bonnemain B, Meyer D. J Mag Res Imag. 1998;8:695. doi: 10.1002/jmri.1880080328. [DOI] [PubMed] [Google Scholar]
- 42.Melchior M, Thompson KH, Jong JM, Rettig SJ, Shuter E, Yuen VG, Zhou Y, McNeill JH, Orvig C. Inorg Chem. 1999;38:2288. [Google Scholar]
- 43.Mehdi MZ, Pandey SK, Theberge JF, Srivastava AK. Cell Biochem Biophys. 2006;44:73. doi: 10.1385/CBB:44:1:073. [DOI] [PubMed] [Google Scholar]
- 44.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. J Biolog Chem. 1997;272:843. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 45.Fantus IG, Tsiani E. Molec Cell Biochem. 1998;182:109. [PubMed] [Google Scholar]
- 46.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Nature. 2003;423:769. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Ding WJ, Baruah B, Crans DC, Wang J. J Inorg Biochem. 2008;102:1846. doi: 10.1016/j.jinorgbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Mclauchlan CC, Hooker JD, Jones MA, Dymon Z, Backhus EA, Youkhana MA, Manus LM. J Inorg Biochem. 2010;104:274. doi: 10.1016/j.jinorgbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Minasi LA, Willsky GR. J Bacteriol. 1991;173:834. doi: 10.1128/jb.173.2.834-841.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cortizo AM, Caporossi M, Lettieri G, Etcheverry SB. Eur J Pharmacol. 2000;400:279. doi: 10.1016/s0014-2999(00)00356-3. [DOI] [PubMed] [Google Scholar]
- 51.Crans DC, Keramidas AD, Drouza C. Phosphorus Sulfur Rel Elem. 1996;109:245. [Google Scholar]
- 52.Wieghardt K. Inorg Chem. 1978;17:57. [Google Scholar]
- 53.Crans DC, Yang LQ, Jakusch T, Kiss T. Inorg Chem. 2000;39:4409. [Google Scholar]
- 54.Bersted BH, Belford RL, Paul IC. Inorg Chem. 1968;7:1557. [Google Scholar]
- 55.Jakusch T, Jin WZ, Yang LQ, Kiss T, Crans DC. J Inorg Biochem. 2003;95:1. doi: 10.1016/s0162-0134(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 56.Chatterjee M, Maji M, Ghosh S, Mak TCW. Dalton Trans. 1998:3641. [Google Scholar]
- 57.Norkus E, Stalnioniene I, Crans DC. Heteroatom Chemistry. 2003;14:625. [Google Scholar]
- 58.Yang LQ, La Cour A, Anderson OP, Crans DC. Inorg Chem. 2002;41:6322. doi: 10.1021/ic0201598. [DOI] [PubMed] [Google Scholar]
- 59.Bolte SE, Ooms KJ, Polenova T, Baruah B, Crans DC, Smee JJ. J Phys Chem. 2008;128:052317/1. doi: 10.1063/1.2830239. [DOI] [PubMed] [Google Scholar]
- 60.Ooms KJ, Bolte SE, Smee JJ, Baruah B, Crans DC, Polenova T. Inorg Chem. 2007;46:9285. doi: 10.1021/ic7012667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakurai H, Fujii K, Watanabe H, Tamura H. Biochemi Biophys Res Comm. 1995;214:1095. doi: 10.1006/bbrc.1995.2398. [DOI] [PubMed] [Google Scholar]
- 62.Sakurai H, Sano H, Takino T, Yasui H. J Inorg Biochem. 2000;80:99. doi: 10.1016/s0162-0134(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 63.Gatjens J, Meier B, Kiss T, Nagy EM, Buglyo P, Sakurai H, Kawabe K, Rehder D. Chemi Eur J. 2003;9:4924. doi: 10.1002/chem.200305019. [DOI] [PubMed] [Google Scholar]
- 64.Crans DC, Mahroof-Tahir M, Keramidas AD. Mol Cell Biochem. 1995;153:17. doi: 10.1007/BF01075914. [DOI] [PubMed] [Google Scholar]
- 65.Crans DC, Rithner CD, Baruah B, Gourley BL, Levinger NE. J Am Chem Soc. 2006;128:4437. doi: 10.1021/ja0583721. [DOI] [PubMed] [Google Scholar]
- 66.Crans DC, Trujillo A, Pharazyn P, Cohen M. Coord Chem Rev. 2011;(V7) in press. [Google Scholar]
- 67.Crans DC, Trujillo AM, Bonetti S, Rithner CD, Baruah B, Levinger NE. J Org Chem. 2008;73:9633. doi: 10.1021/jo801707y. [DOI] [PubMed] [Google Scholar]
- 68.A.S. Tracey, G.R. Willsky, E.S. Takeuchi, CRC Press, Boca Raton, FL (USA), 2007.
- 69.D. RehderJohn, Wiley & Sons Ltd., West Sussex, England, 2008.
- 70.Yang Y, Crans DC, Miller SM, Cour AL, Anderson AP, Kaszynski PM, Godzala ME, I, Austin LD, Willsky GR. Inorg Chem. 2002;41:4859. doi: 10.1021/ic020062l. [DOI] [PubMed] [Google Scholar]
- 71.Willsky GR, Chi LH, Liang YL, Gaile DP, Hu ZH, Crans DC. Physiolog Genomics. 2006;26:192. doi: 10.1152/physiolgenomics.00196.2005. [DOI] [PubMed] [Google Scholar]
- 72.Wei D, Li M, Ding W. J Biolog Inorg Chem. 2007;12:1265. doi: 10.1007/s00775-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 73.Yechoor VK, Patti ME, Saccone R, Kahn CR. Proc Natl Acad Sci USA. 2002;99:10587. doi: 10.1073/pnas.142301999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biospace. 2009 http://www.biospace.com/news_story.aspx?NewsEntityId=123583. Last checked May 25, 2011.
- 75.Irsigler GB, Visser PJ, Spangenberg PA. Am J Ind Med. 1999;35:366. doi: 10.1002/(sici)1097-0274(199904)35:4<366::aid-ajim7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 76.Rom WN, editor. LIttle,Brown and Co, Boston, MA. 1992. [Google Scholar]
- 77.Byerrum RU. In: Metals and their compounds in the Environment. Merian E, editor. VCH; Weinheim, Fed Rep Germany: 1991. p. 1289. [Google Scholar]
- 78.Dai S, McNeill JH. Pharmacol Toxicol. 1994;74:110. doi: 10.1111/j.1600-0773.1994.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 79.Dai S, Thompson KH, McNeill JH. Pharmacol Toxicol. 1994;74:101. doi: 10.1111/j.1600-0773.1994.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 80.Dai S, Thompson KH, Vera E, McNeill JH. Pharmacol Toxicol. 1994;75:265. doi: 10.1111/j.1600-0773.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 81.Dai S, Vera E, McNeill JH. Pharmacol Toxicol. 1995;76:263. doi: 10.1111/j.1600-0773.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 82.Schroeder HA, Balassa JJ. J Nutr. 1967;92:245. doi: 10.1093/jn/92.2.245. [DOI] [PubMed] [Google Scholar]
- 83.Schroeder HA, Mitchener M, Nason AP. J Nutr FIELD Full Journal Title:The Journal of nutrition. 1970;100:59. doi: 10.1093/jn/100.1.59. [DOI] [PubMed] [Google Scholar]
- 84.Crans DC, Yang LQ, Gaidamausks E, Khan AR, Jin W, Simonis U. ACS Symposium Series 858. 2009:304. [Google Scholar]
- 85.Stover J, Rithner CD, Inafuku RA, Crans DC, Levinger NE. Langmuir. 2005;21:6250. doi: 10.1021/la0508137. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Yang XD, Wang K, Crans DC. J Inorg Biochem. 2006;100:80. doi: 10.1016/j.jinorgbio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Aureliano M, Henao F, Tiago T, Duarte RO, Moura JJG, Baruah B, Crans DC. Inorg Chem. 2008;47:5677. doi: 10.1021/ic702405d. [DOI] [PubMed] [Google Scholar]
- 88.Cohen MD, Sisco M, Yoshida K, Chen LC, Zelikoff JT, Smee JJ, Holder AA, Stonehuerner JD, Crans DC, Ghio AJ. Inhal Toxicol. 2010;22:169. doi: 10.3109/08958370903161232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen MD, Sisco M, Prophete C, Chen LC, Zelikoff JT, Ghio AJ, Stonehuerner JD, Smee JJ, Holder AA, Crans DC. J of Immunotoxicol. 2007;4:49. doi: 10.1080/15476910601119350. [DOI] [PubMed] [Google Scholar]
- 90.Yang XG, Yang XD, Yuan L, Wang K, Crans DC. Pharm Res. 2004;21:1026. doi: 10.1023/b:pham.0000029293.89113.d5. [DOI] [PubMed] [Google Scholar]
- 91.Nxumalo F, Tracey AS. J Biolog Inorg Chem. 1998;3:527. [Google Scholar]
- 92.Wieghardt K. Adv in Inorg & Bioinorg Mech. 1984;3:213. [Google Scholar]
- 93.Angusdunne SJ, Paul PC, Tracey AS. Canadian Journal of Chemistry-Revue Canadienne De Chimie. 1997;75:1002. [Google Scholar]
- 94.Paul PC, Angus-Dunne SJ, Batchelor RJ, Einstein FWB, Tracey AS. Can J Chem. 1997;75:429. [Google Scholar]
- 95.Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Antiox & Redox Signal. 2005;7:1021. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crans DC, Zhang B, Gaidamauskas E, Keramidas AD, Willsky GR, Roberts CR. Inorg Chem. 2010;49:4245. doi: 10.1021/ic100080k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanson SK, Baker RT, Gordon JC, Scott BL, Sutton AD, Thorn DL. J Am Chem Soc. 2009;131:428. doi: 10.1021/ja807522n. [DOI] [PubMed] [Google Scholar]
- 98.Hanson SK, Baker RT, Gordon JC, Scott BL, Thorn DL. Inorg Chem. 2010;49:5611. doi: 10.1021/ic100528n. [DOI] [PubMed] [Google Scholar]
- 99.Ardanaz N, Pagano PJ. Exp Biol Med. 2006;231:237. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 100.Biswas S, Chida AS, Rahman I. Biochem Pharmacol. 2006;71:551. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 101.Kovacic P, Draskovich CD, Pozos RS. J Recept Sign Transduct. 2007;27:433. doi: 10.1080/10799890701699702. [DOI] [PubMed] [Google Scholar]


