Abstract
Enkephalinergic neurons in the rostral ventrolateral medulla (rVLM), an important presympathetic region in the brainstem, are activated by 30 min of low frequency (2 Hz) electroacupuncture (EA) at acupoints P5–P6, which overlie the median nerves. To more closely model clinical application of acupuncture, we administered EA for 30 min twice over a 72 hr period to unsedated conscious rats to examine its prolonged action. We hypothesized that repetitive EA would increase preproenkephalin mRNA and met-enkephalin in the rVLM of unsedated conscious rats. Rats received either EA (1 – 4 mA, 0.5 ms, 2 Hz) or sham stimulation (needle placement without electrical stimulation) twice at P5–P6 acupoints bilaterally. Preproenkephalin mRNA and its peptide met-enkephalin in the rVLM were measured 24 or 48 hrs after the final EA or sham procedure. Relative ratios of preproenkephalin mRNA levels (normalized with the 18s housekeeping gene) were almost doubled at 24 hrs compared to sham (6.1 ± 0.79 vs. 3.1 ± 0.47). Met-enkephalin measured in rVLM tissue pooled from several rats exposed to the same treatment was increased by repeated EA by 68% after 24 hrs and 51% after 48 hrs, relative to sham. These findings suggest that repeated application of EA in the conscious rats enhances transcription and translation of enkephalin in rVLM for days. Since opioids in the rVLM contribute importantly to the action of EA on sympathetic outflow, this mechanism may contribute to the prolonged action of acupuncture on elevated blood pressure in patients.
Keywords: low frequency acupuncture, biotelemetry, opioids, preproenkephalin, blood pressure
Introduction
Acupuncture has been gaining acceptance among medical professionals as a therapeutic option with few or minimal side effects for a range of clinical conditions, including hypertension (Ho, et al., 1999; Li, et al., 1998; Lin, et al., 2001; Andersson, 1993; Chiu, et al., 1997; Flachskampf, et al., 2007; Li and Longhurst, 2007). A distinguishing aspect of acupuncture is its long-lasting action. The cardiovascular inhibitory response to a single application of acupuncture has been documented to last for at least 60 min in anesthetized reflex-induced hypertensive rats (Tjen-A-Looi, et al., 2003; Tjen-A-Looi, et al., 2007) and for 5–12 hr in unanesthetized spontaneous hypertensive rats (Yao, et al., 1982b; Yao, et al., 1982a). Repetitive EA applied weekly over a two month period to awake hypertensive human subjects may last for several weeks (Li and Longhurst, 2007).
The rVLM serves as an important site of regulation for respiration, circulation and pain, among other functions (Lovick and Li, 1989) and is an essential region controlling sympathetic outflow (Guyenet, 1990). We have shown previously that, in a point specific manner, stimulation of the P5–P6 acupoints overlying the median nerves on the forelimbs, activates enkephalinergic neurons in the rVLM and provides more input to the rVLM and for longer durations than any other set of acupoints located along meridians on the surface of the body (Tjen-A-Looi, et al., 2004). In this regard, anatomical and electrophysiological studies demonstrate that 30 min of EA at P5–P6 activates enkephalinergic rVLM neurons (Tjen-A-Looi, et al., 2003; Guo, et al., 2004). Stimulation of EA at P5–P6 attenuates excitatory responses of rVLM neurons, in part, through an opioid mechanism (Li, et al., 2006). The order of potency of opioid receptors during EA inhibition in the rVLM is δ=µ>>κ, suggesting that enkephalins, endorphin and endomorphin but not dynorphin are the important neuromodulators in this region of the brain stem during EA (Li, et al., 2001). We recently have shown that the mRNA of preproenkephalin, the precursor of enkephalin, in the rVLM of anesthetized rats is increased 1.5 hour after a single 30 min application of EA (Li, et al., 2010a). However, the influence of repetitive EA on enkephalin gene expression in rVLM neurons in conscious unsedated rats is not known. Generally acupuncture is applied once every two to seven days for several sessions (Li and Longhurst, 2007; Macklin, et al., 2006; Flachskampf, et al., 2007). As such, to more closely model the clinical application of acupuncture, we repetitively applied 30 min of EA (2 Hz, 1–2 mA, 0.5 ms duration) twice over a 72 hr period to unsedated conscious rats and evaluated the levels of preproenkephalin mRNA and met-enkephalin peptide 24 and 48 hr after the second EA treatment. We hypothesized that repetitive EA would increase preproenkephalin mRNA and metenkephalin peptide in the rVLM of conscious rats. Part of this study has been presented as a preliminary report (Li, et al., 2010b).
Materials and Methods
Measurement of arterial pressure by biotelemetry and tail cuff
Experimental preparations and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. The study conformed to the American Physiological Society’s Guiding Principles for Research Involving Animals and Human Beings. Studies were performed on Sprague-Dawley rats (350–400g). A biotelemetry transmitter with high fidelity solid-state pressure and temperature sensors (Endosomatic Systems Inc.) was used in selected rats to measure blood pressure and temperature. Rats were fasted overnight and anesthetized with an intramuscular injection of ketamine (75–100 mg/kg) and xylazine (5–10 mg/kg). Supplemental doses (1/3–1/2 of the original dose) were given as needed, as assessed by the presence or absence of the corneal reflex. The abdominal aorta was isolated through a ventral midline abdominal incision. The pressure sensitive catheter was inserted into the abdominal aorta and guided upstream. Tissue adhesive (Vetbond, 3M Animal Care Products, St Paul, MN) and tissue mesh (PETKM2002, Textile Development Associates, Inc) was used to secure the position of the catheter. The telemetric pack was inserted subcutaneously. Incisions of the abdominal muscle and skin were closed with sutures. Breathing rate and pattern, heart rate, temperature, blood pressure and response to corneal probing were monitored throughout the recovery period for up to three hours. Buprenorphine (0.05 mg/kg, SQ) was administered every 8–12 hours during the first 48 hours. Penicillin G procaine (40,000 units/kg, SQ) was used every 12–24 hrs for seven days. The rats recovered under close supervision for one to two weeks. The blood pressure of the rats which did not receive an implanted biotelemetry device was measured non-invasively with a volume pressure recording sensor and an occlusion tail-cuff (CODA System, Kent Scientific, Torrington, CT). Briefly, the conscious animal was placed in a restrainer and permitted to rest for 10 to 15 min. The cuff was then placed on the tail and was inflated and released several times to condition the animal to the procedure. After stabilization, blood pressure was measured five times, and the average of the recorded values was used.
Conscious animal: acclimatization before EA
Prior to application of electroacupuncture, rats were trained for two weeks to become accustomed to handling. Rats were handled gently prior to restraint using a sling that safely and effectively immobilized the rats. The rats were restrained 30 min once daily, twice each week for two weeks before starting the course of electroacupuncture to prevent stress associated with this procedure. Rats were placed in the slings and acupuncture needles (Lekon disposal 40 gauge needles, 0.16 mm diameter) were inserted briefly into the P5–P6 acupoints for very short time periods (~10 s) to allow them to become accustomed to handling and the acupuncture procedure. The P5–P6 acupoints on the forelimbs of rats are analogous to those in humans (Hua, 1994).
Experimental procedures
Experimental protocol 1: EA in conscious rats. After familiarization, the freely moving rats were placed in the slings for 30 min of EA. Acupuncture needles were inserted bilaterally at the P5–P6 acupoints (located over the median nerves) and connected to a constant current stimulator with a stimulus isolation unit and stimulator (Grass, model S88, W. Warwick, RI, USA) to provide EA (2 Hz, 1 – 4 mA, 0.5-ms duration) for 30 min. Correct positioning of the needles at the acupoints was confirmed by observing slight repetitive paw twitches during stimulation, indicating stimulation of motor fibers in the mixed nerve bundle comprising the median nerve (Chao, et al., 1999).
Experimental protocol 2: Sham acupuncture in conscious rats. The same treatment protocol detailed above was used with the exception that needles were inserted but not stimulated electrically.
Experimental protocol 3: Restraint group. Rats were restrained as detailed above, but needles were not inserted.
High-fidelity blood pressure and heart rate were monitored before, during and after EA using biotelemetry. Brain stems were harvested 24 and 48 hrs following the second application of EA.
Tissue collection in rVLM and arcuate nucleus
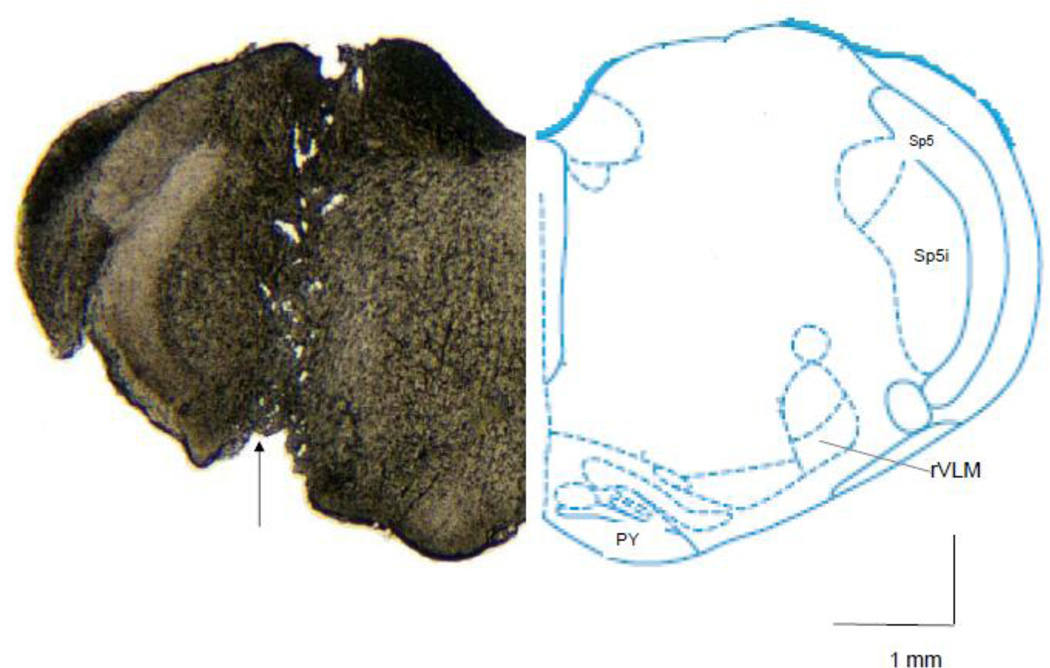
Twenty four or 48 hrs after the second EA application, rats were decapitated followed with quick removal of the brain. The tissue was stored in RNA Later solution (Ambion) for preproenkephalin mRNA analysis or in dry ice and then frozen at −80°C for later met-enkephalin peptide detection. After overnight storage, the brain stem or hypothalamus was placed on dry ice for isolation and subsequent removal of the rVLM or arcuate nucleus, since both nuclei participate in EA modulation of sympathetic outflow through an opioid mechanism(Tjen-A-Looi, et al., 2003)(Guo, et al., 2004)(Tjen-A-Looi, et al., 2004)(Li, et al., 2001)(Tjen-A-Looi, et al., 2007)(Li and Longhurst, 2007)(Lantos, et al., 1995)(Bloom, et al., 1978)(Guo and Longhurst, 2007)(Li, et al., 2009)(Tjen-A-Looi, et al., 2006)(Li, et al., 2006). To obtain tissue from the rVLM, a punch biopsy was taken from the ventral side using a 20-gauge needle stub (the outer and inner diameter are 0.9 mm and 0.6 mm respectively). The biopsy was conducted by locating the outer wall of the needle 0.5 mm lateral to the lateral edge of the pyramid tract and just next to the caudal edge of the trapezoid body. This region overlaps with the rVLM area where we have recorded premotor cardiovascular sympathoexcitatory neurons that are influenced by EA (Crisostomo, et al., 2005; Tjen-A-Looi, et al., 2003). Bilateral rVLM tissue samples 0.5 mm from the ventral surface, excluding the first 0.2 mm, were collected (Fig. 1). Alternatively, arcuate nucleus samples were dissected by punch biopsy using a 20 gauge needle stub as described previously (Obici, et al., 2002). Preliminary studies indicated that there was no alteration in preproenkephalin mRNA in the arcuate 24 hrs after repetitive EA compared to sham (3.0 ± 0.2 vs 3.0 ± 0.1). As such, in the present study we focused on the rVLM.
Fig. 1.
Site of punch biopsy taken from rVLM region for measurement of preproenkephalin mRNA and met-enkephalin. Left: a tissue section shows the site of punch biopsy (arrow) taken from rVLM region (12.24 mm caudal to Bregma). Right: the same rostral level of brain section indicated in the rats atlas (Paxinos and Watson, 2009). PY: Pyramid tract. Sp5: Spinal trigeminal tract. Sp5i: Spinal trigeminal nucleus, interpolar.
Histology
Brain stems were fixed in 10% paraformaldehyde (pH 7.4) for at least 2 days following punch biopsy. They were sliced with a microtome cryostat at a thickness of 40 µm. Sequential cross sections of each brain stem were examined for absence of the rVLM with a light microscope.
RNA isolation and real time PCR
For RNA isolation, tissue samples were homogenized in a glass tissue grinder (DUALL 20, Kontes Glass Co.) by using 800 µl Trizol reagent (Life Technologies). Total RNA was extracted using the manufacturer’s protocol. The RNA was dissolved in 10 µl nuclease-free water, and the concentration and purity of RNA was determined spectrophotometerically using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies). One hundred nanograms of total RNA were transcribed using SuperScript II RT (Invitrogen) and a mixture of oligo (dT) (100 ng/reaction) and random primers (200 ng/reaction). Real-time quantitative PCR was performed with an Opticon 4 (Bio-Rad) using the SYBR green real-time master mix (Bio-Rad). The following primer sequences were used: preproenkephalin, forward (5’-tgc ctt ctt tca aaa tct gg-3’), reverse (5’-ggg gta aag ctc atc cat ct-3’); 18s, forward (5’-cgg aca gga ttg aca gat tg-3’), reverse (5’-acg cca ctt gtc cct cta ag-3’). The primer sequences were designed to span intron-exon boundaries to avoid amplification of genomic DNA. The sizes of the PCR products were: preproenkephalin, 191 bp; 18s, 185 bp. The PCR program was 95°C 10 min, (95°C 10 s, 59°C 45 s) × 50 cycles followed by the melting curve analysis (55–90°C) to verify product specificity. To create a standard curve for each gene of interest, rat cDNA corresponding to the region analyzed was amplified with the same specific primers. A solution containing the corresponding amplification fragment was analyzed with a spectrophotometer and the molecular number was calculated. A standard curve then was generated by analysis of the serial dilutions of fragment solutions (102–107 copies/µl). For each sample, the copy number of both preproenkephalin and the 18s housekeeping gene were extrapolated from their respective standard curves. The value of preproenkephalin mRNA expression was normalized by the number of 18s copies and expressed in arbitrary units. Reproducibility of results was determined by performing triplicate measurements of each cDNA aliquot.
Competitive enzyme immunoassay
Tissue samples from the rVLM for detection of met-enkephalin peptide were homogenized in 0.01 M HCl on ice then placed in boiling water for 10 min to inactivate peptidases. Homogenates were centrifuged at −4 °C and 12000 g for 30 min. The supernatant was collected after centrifugation then lyophilized overnight and stored at −20°C. The lyophilized samples were reconstituted in 1×assay buffer (CEK0230-40, AB BioLabs) for the competitive enzyme immunoassay of met-enkephalin. An aliquot of each reconstituted sample was used to determine the total protein (Quick Start Bradford Protein Assay, Bio-rad). Since the met-enkephalin peptide in the rVLM of individual rats was lower than the minimum detection level (0.1 ng/ml), we pooled rVLM tissue from 4–6 rats in each protocol to assay the concentration of this peptide.
Statistical analysis
All values, except the concentration of met-enkephalin peptide, which was averaged by the number of animals in each group, are presented as means ± SEM. The Kolmogorov-Smirnoff test was used to determine if the data were normally distributed. Comparisons between two groups were analyzed statistically with the Student’s t-test, and values were considered to be significantly different when P<0.05. All statistical calculations were performed with a statistical software package (SigmaStat, Version 3.0).
Results
Cardiovascular responses
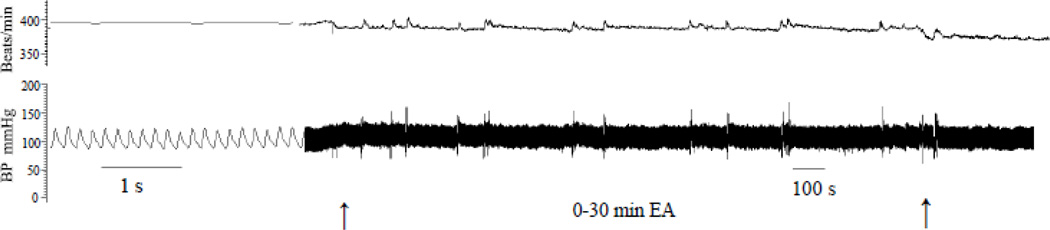
Blood pressure and heart rate did not change significantly before, during or after EA stimulation (Fig. 2). The systolic blood pressures and heart rates recorded 24 and 48 hours after EA were unchanged from the 24 hr values recorded before EA, which were 106 ± 4.2 mmHg and 384 ± 7 bpm, respectively. Additionally, heart rates and blood pressures remained constant in the sham and restraint groups.
Fig. 2.
Blood pressure and heart rate of a conscious rat before, during and after EA. The left and right arrows show the start and end times of EA.
Met-enkephalin expressions at 24 hours after EA
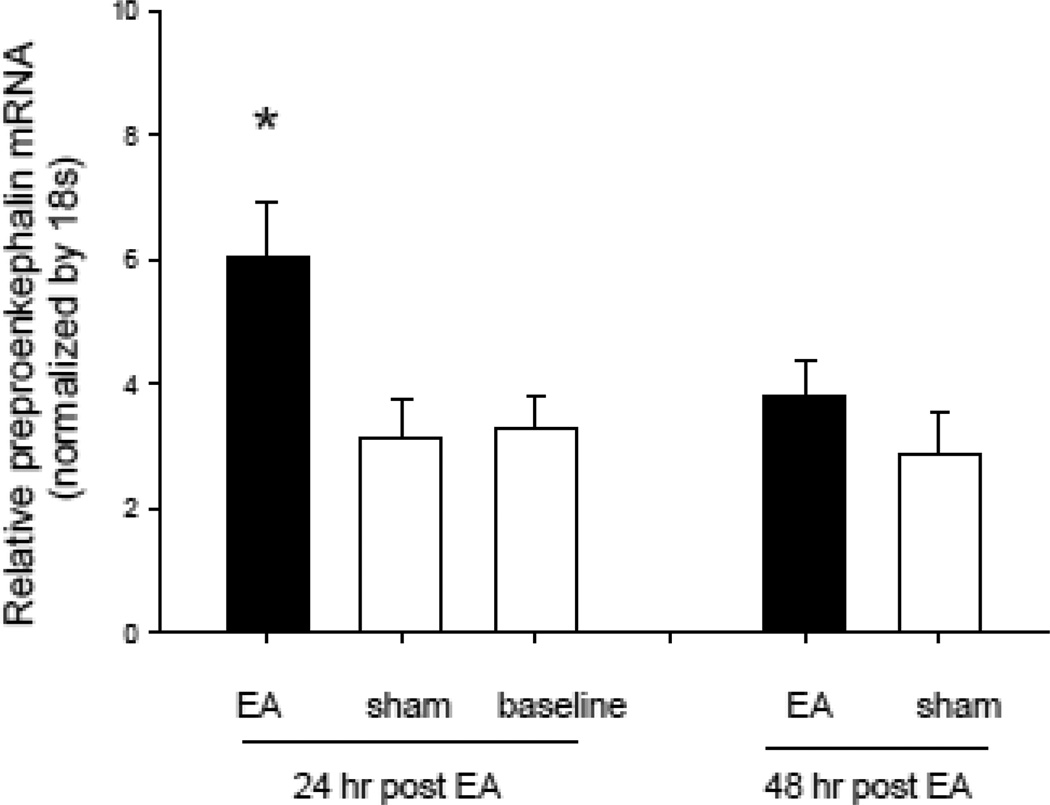
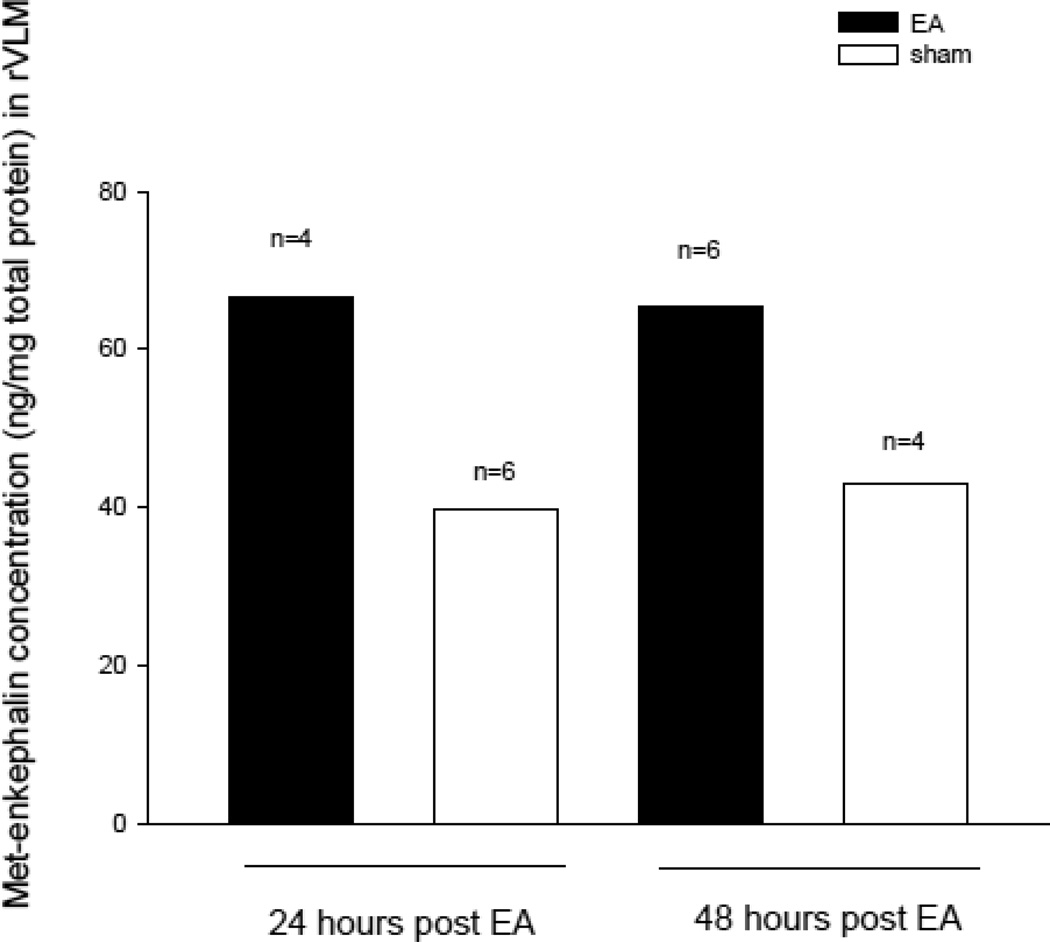
The expression of preproenkephalin mRNA and met-enkephalin in the rVLM was increased 24 hrs after the last application of EA (Fig. 3). Specifically, the relative ratio of preproenkephalin mRNA following EA (normalized with the 18s housekeeping gene) was increased significantly (P < 0.05) 24 hour after EA (6.1 ± 0.79, n=8) relative to the sham (3.1 ± 0.47, n=7) and restraint groups (3.3 ± 0.50, n=6). Figure 3 shows the EA-related increase in preproenkephalin mRNA expression determined with reverse transcription and quantitative real-time PCR. Met-enkephalin in the rVLM also was increased by 68%, relative to sham (67, n=4 vs 40 pg peptide/mg protein, n=6) (Fig. 4).
Fig. 3.
Relative preproenkephalin mRNA levels in the rVLM 24 and 48 hours after two applications of EA, separated by 72 hours. * P < 0.05 compared with the sham group.
Fig. 4.
Concentration of met-enkephalin in the rVLM. Heights of bars represent the concentration measured from pooled brains of rats, with animal numbers shown above each bar.
Met-enkephalin expression at 48 hours after EA
The relative ratios of preproenkephalin mRNA 48 hrs after EA were insignificantly increased at 48 hours after EA (3.8 ± 0.48, n=4), relative to sham (2.9 ± 0.46, n=5) (Fig. 3). However, we observed that its peptide product, met-enkephalin, was increased by 51% relative to sham 48 hrs after the final application of EA (65, n=6, vs 43 pg peptide/mg total protein, n=4) (Fig 4).
Histological confirmation
As indicated by an example shown in Figure 1, each biopsy was found to be located within the rVLM as defined by the atlas of Paxinos and Watson (Paxinos and Watson, 2009). Specifically, it was located 12 to 12.48 mm caudal to Bregma, 2.0 to 2.6 mm lateral to the midline and 0.2 mm to 0.5 mm dorsal to the ventral surface.
Discussion
The current study is the first to demonstrate that repeated 30 min stimulations of EA at P5–P6 acupoints overlying the median nerves over a 72 hr period increases preproenkephalin mRNA in the rVLM 24 hrs after termination of EA. Furthermore, the peptide product, met-enkephalin, was increased 24 and 48 hrs following EA. Previous work has shown that a single 30 min application of EA at P5–P6 in anesthetized animals increases preproenkephalin mRNA 90 min after EA, but not for longer time periods (Li, et al., 2010a). The present study suggests that repeated acupuncture is capable of inducing more prolonged increases in the expression of this opioid precursor (preproenkephalin) mRNA as well as its peptide product (met-enkephalin).
An important aspect of this study was the repeated application of EA to conscious rats. These rats were preconditioned by repeated handling and brief acupuncture needle insertion to minimize stress-related preproenkephalin expression. Mansi and co-workers (Mansi, et al., 2000) have shown that acute psychogenic stress activates enkephalin containing perikarya within the paragigantocellularis and lateral reticular nuclei in rats. Therefore, the habituation to both handling and restraint is critical to prevent stress related enkephalin gene expression in the brain. To limit stress as much as possible, we allowed ample time (2 weeks) for the rats to become accustomed to handling and restraint before EA application. We observed that all rats were relaxed during restraint and EA stimulation.
We examined enkephalin expression in the rVLM following EA stimulation to determine the long term expression of this opioid. The rVLM is highly relevant since it serves as an important center that regulates sympathetic discharge (Guyenet, 1990) and is modulated by the opioid system during application of EA at acupoints P5–P6 (Tjen-A-Looi, et al., 2003; Guo, et al., 2004).
The opioid system including endorphin and enkephalins, which act through µ- and δ-opioid receptors, respectively, in the rVLM, contributes to the sympathoinhibitory effects of EA (Li, et al., 2001). Anatomical studies employing confocal microscopy to identify c-Fos acupuncture responsive neurons have documented that enkephalin but not endorphin is synthesized in EA-activated neurons in the rVLM (Guo, et al., 2004; Guo and Longhurst, 2007). Repetitive EA in the present study caused similar increases in preproenkaphalin mRNA as we previously observed 1.5 hours after termination of a single application of EA for 30 min (3.1 to 7.8 vs 2.8 to 6.1, respectively). Thus, the observations that single and repeated EA application increases preproenkephalin mRNA expression in the rVLM support the importance of EA-activated enkephalin gene expression in this sympathoexcitatory regulatory nucleus and suggests that this transcriptional response participates in lowering of blood pressure in anesthetized animals following a single application of EA (Tjen-A-Looi, et al., 2007) and possibly in patients receiving a course of acupuncture therapy (Li and Longhurst, 2007).
While the mRNA of preproenkephalin was increased significantly only 24 hrs after EA, the met-enkephalin peptide was elevated at both 24 and 48 hrs. Eiden and associates (Eiden, et al., 1984) have demonstrated that the increased synthesis of met-enkephalin in the bovine chromaffin cells is linked to increased preproenkephalin mRNA and possibly post-transcriptional events such as translation or preproenkephalin processing. In addition to synthesis, the concentration of met-enkephalin in the rVLM is influenced by storage and release of endogenous peptide. Consistent with our results, Almeida's group (Almeida, et al., 1992) has shown that met-enkephalin is increased in the hypothalamus for as long as seven days after adrenalectomy compared to sham surgery, suggesting that elevations in this peptide can be detected days after its synthesis. Thus, we were not surprised to find increases in met-enkephalin that outlasted the increases in mRNA of its precursor. This finding also provides an explanation about why clinical acupuncture repeated applied only once or twice weekly can achieve clinically meaningful responses like prolonged decreases in blood pressure in patients with mild to moderate hypertension (Li and Longhurst, 2007).
The long-lasting blood pressure response to a single application of EA (for 30 min) often is maintained for at least 60 minutes (Tjen-A-Looi, et al., 2007). This prolonged response, which typically outlasts brief somatosympathetic neural occlusive responses in the rVLM (Tjen-A-Looi, et al., 2003), is the result of activation of a long-loop pathway involving the arcuate nucleus in hypothalamus, ventrolateral periaqueductal gray (vlPAG) in midbrain, nucleus raphé pallidus (NRP) and rVLM in the medulla (Li, et al., 2009). A number of excitatory and inhibitory neurotransmitters including glutamate (arcuate nucleus and vlPAG), acetylcholine (arcuate nucleus), endocannabinoids (vlPAG), γ-aminobutyric acid (vlPAG and rVLM), serotonin (rVLM) and nociceptin (rVLM) underlie acupuncture’s action and hence indirectly or directly contribute to the prolonged actions of EA in anesthetized animals (Crisostomo, et al., 2005; Tjen-A-Looi, et al., 2007; Tjen-A-Looi, et al., 2009; Moazzami, et al., 2010; Li, et al., 2010c; Li, et al., 2010d; Zhou, et al., 2007). Although future studies will need to examine the roles of the other neurotransmitters in the various pathway nuclei that might participate in the very prolonged acupuncture-cardiovascular response lasting days to weeks with repetitive EA in conscious subjects, in the rVLM only opioids and γ-aminobutyric acid have been shown to contribute to the prolonged action of EA on elevated blood pressure in anesthetized animals (Tjen-A-Looi, et al., 2007).
We found it interesting that repeated EA increased preproenkephalin mRNA and met-enkephalin without apparent changes to blood pressure in normotensive conscious rats studied in these experiments. We were not surprised to observe that the increase in preproenkephalin gene expression could occur without an accompanying change in blood pressure since we have found that acupuncture typically does not influence baseline blood pressure in normotensive awake humans (Li, et al., 2004) or anesthetized animal subjects (Crisostomo, et al., 2005; Tjen-A-Looi, et al., 2007; Tjen-A-Looi, et al., 2009; Moazzami, et al., 2010; Li, et al., 2010c; Li, et al., 2010d). Thus, the increase in enkephalin gene expression in rVLM is not dependent on changes in blood pressure.
In summary, the present study for the first time demonstrates that repetitive EA separated by a three day period, as commonly employed in clinical acupuncture, increases the expression of preproenkephalin mRNA and the concentration of it peptide product, met-enkephalin, in the rVLM of conscious rats. This study thus provides quantitative data suggesting that the prolonged action of repetitive EA may be related to the increased gene expression of met-enkephalin in the rVLM. As such, repetitive EA, in contrast to a single application, appears to provide a unique stimulus to the rVLM to increase the production of preproenkephalin mRNA and ultimately met-enkephalin, which then is available to reduce the action of excitatory neurotransmitters such as glutamate that mediates the sympathoexcitation resulting from visceral reflex input to rVLM premotor sympathetic neurons (Zhou, et al., 2007). The extended action of EA suggests that it may be a suitable therapeutic application for chronic diseases such as hypertension (Li and Longhurst, 2007).
Acknowledgments
We thank Jesse Ho for his technical assistance. We also thank Doug Hoi Cheung, Emma Choi and Brian Lee for their technical assistance. This study was supported by AHA postdoctoral fellowship 10POST4190125, NIH HL-63313 and HL-072125.
Abbreviations
- EA
Electroacupuncture
- P5–P6
Jianshi–Neiguan acupoints
- rVLM
Rostral ventrolateral medulla
- vlPAG
Ventrolateral periaqueductal gray
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Almeida OF, Hassan AH, Harbuz MS, Linton EA, Lightman SL. Hypothalamic corticotropin-releasing hormone and opioid peptide neurons: functional changes after adrenalectomy and/or castration. Brain Res. 1992;571:189–198. doi: 10.1016/0006-8993(92)90654-r. [DOI] [PubMed] [Google Scholar]
- Andersson The functional background in acupuncture effects. Scand.J.Rehabil.Med.Suppl. 1993;29:31–60. [PubMed] [Google Scholar]
- Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R. Neurons containing beta-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc.Natl.Acad.Sci.U.S.A. 1978;75:1591–1595. doi: 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DM, Shen LL, Tjen-A-Looi SC, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am.J.Physiol. 1999;276:H2127–H2134. doi: 10.1152/ajpheart.1999.276.6.H2127. [DOI] [PubMed] [Google Scholar]
- Chiu YJ, Chi A, Reid IA. Cardiovascular and endocrine effects of acupuncture in hypertensive patients. Clin.Exp.Hyperten. 1997;19:1047–1063. doi: 10.3109/10641969709083204. [DOI] [PubMed] [Google Scholar]
- Crisostomo M, Li P, Tjen-A-Looi SC, Longhurst JC. Nociceptin in rVLM mediates electroacupuncture inhibition of cardiovascular reflex excitatory response in rats. J.Appl.Physiol. 2005;98:2056–2063. doi: 10.1152/japplphysiol.01282.2004. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Giraud P, Affolter HU, Herbert E, Hotchkiss AJ. Alternative modes of enkephalin biosynthesis regulation by reserpine and cyclic AMP in cultured chromaffin cells. Proc.Natl.Acad.Sci.U.S.A. 1984;81:3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachskampf FA, Gallasch J, Gefeller O, Gan J, Mao J, Pfahlberg AB, Wortmann A, Klinghammer L, Pflederer W, Daniel WG. Randomized trial of acupuncture to lower blood pressure. Circulation. 2007;115:3121–3129. doi: 10.1161/CIRCULATIONAHA.106.661140. [DOI] [PubMed] [Google Scholar]
- Guo Z-L, Longhurst J. Expression of c-Fos in arcuate nucleus induced by electroacupuncture: relations to neurons containing opioids and glutamate. Brain Res. 2007;1166:65–76. doi: 10.1016/j.brainres.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z-L, Moazzami AR, Longhurst JC. Electroacupuncture induces c-Fos expression in the rostral ventrolateral medulla and periaqueductal gray in cats: relation to opioid containing neurons. Brain Res. 2004;1030:103–115. doi: 10.1016/j.brainres.2004.09.059. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Role of ventral medulla oblongata in blood pressure regulation. In: Loewy and AD, Spyer KM, editors. Central regulation of autonomic functions. New York: Oxford University Press; 1990. pp. 145–167. [Google Scholar]
- Ho F-M, Huang P-J, Lo H-M, Lee F-K, Chern T-H, Chiu T-W, Liau C-S. Effect of acupuncture at Nei-Kuan on left ventricular function in patients with coronary artery disease. Am.J.Chin.Med. 1999;27:149–156. doi: 10.1142/S0192415X99000197. [DOI] [PubMed] [Google Scholar]
- Hua XB. Acupuncture manual for small animals experimental acupuncture. Shanghai: Shanghai Science and Technology Publisher; 1994. pp. 269–290. [Google Scholar]
- Lantos TA, Gorcs TJ, Palkovits M. Immunohistochemical mapping of neuropeptides in the premamillary region of the hypothalamus in rats. Brain Res.Brain Res.Rev. 1995;20:209–249. doi: 10.1016/0165-0173(94)00013-f. [DOI] [PubMed] [Google Scholar]
- Li M, Tjen ALS, Longhurst JC. Electroacupuncture enhances preproenkephalin mRNA expression in rostral ventrolateral medulla of rats. Neurosci Lett. 2010a;477:61–65. doi: 10.1016/j.neulet.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Tjen-A-Looi SC, Longhurst JC. Repetitive electroacupuncture causes prolonged enhancement of preproenkephalin mRNA expression in rostral ventrolateral medulla of unanesthetized rats. FASEB J. 2010b;24:809.5. doi: 10.1016/j.neulet.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Ayannusi O, Reed C, Longhurst JC. Inhibitory effect of electroacupuncture (EA) on the pressor response induced by exercise stress. Clinical Autonomic Research. 2004;14:182–188. doi: 10.1007/s10286-004-0175-1. [DOI] [PubMed] [Google Scholar]
- Li P, Longhurst JC. Long-lasting inhibitory effect of EA on blood pressure in patients with mild to moderate hypertension. Soc Neurosci. 2007;37 [Google Scholar]
- Li P, Pitsillides KF, Rendig SV, Pan H-L, Longhurst JC. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation. 1998;97:1186–1194. doi: 10.1161/01.cir.97.12.1186. [DOI] [PubMed] [Google Scholar]
- Li P, Tjen-A-Looi SC, Guo ZL, Fu L-W, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J.Appl.Physiol. 2009;106:620–630. doi: 10.1152/japplphysiol.91277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Tjen-A-Looi SC, Guo ZL, Longhurst JC. An arcuate-ventrolateral periaqueductal gray reciprocal circuit participates in electroacupuncture cardiovascular inhibition. Autonomic Neuroscience: Basic & Clinical. 2010c;158:13–23. doi: 10.1016/j.autneu.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Tjen-A-Looi SC, Longhurst JC. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Autonomic Neuroscience. 2001;89:38–47. doi: 10.1016/S1566-0702(01)00247-8. [DOI] [PubMed] [Google Scholar]
- Li P, Tjen-A-Looi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am.J.Physiol. 2006;209:H2535–H2542. doi: 10.1152/ajpheart.00972.2005. [DOI] [PubMed] [Google Scholar]
- Li P, Tjen-A-Looi SC, Longhurst JC. Nucleus raphé pallidus participates in midbrainmedullary cardiovascular sympathoinhibition during electroacupuncture. Am J Physiol Regul Integr Comp Physiol. 2010d;299:R1369–R1376. doi: 10.1152/ajpregu.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MC, Nahin R, Gershwin ME, Longhurst JC, Wu KK. State of complementary & alternative medicine in cardiovascular, lung and blood. Circulation. 2001;103:2038–2041. doi: 10.1161/01.cir.103.16.2038. [DOI] [PubMed] [Google Scholar]
- Lovick T, Li P. Integrated function of neurones in the rostral ventrolateral medulla. Prog Brain Res. 1989;81:223–231. [PubMed] [Google Scholar]
- Macklin EA, Wayne PM, Kalish LA, Valaskatgis P, Thompson J, Pian-Smith MC, Zhang Q, Stevens S, Goertz C, Prineas RJ, Buczynski B, Zusman RM. Stop Hypertension with the Acupuncture Research Program (SHARP): results of a randomized, controlled clinical trial. Hypertension. 2006;48:838–845. doi: 10.1161/01.HYP.0000241090.28070.4c. [DOI] [PubMed] [Google Scholar]
- Mansi JA, Laforest S, Drolet G. Effect of stress exposure on the activation pattern of enkephalin-containing perikarya in the rat ventral medulla. J Neurochem. 2000;74:2568–2575. doi: 10.1046/j.1471-4159.2000.0742568.x. [DOI] [PubMed] [Google Scholar]
- Moazzami A, Tjen-A-Looi SC, Guo Z-L, Longhurst JC. Serotonergic projection from nucleus raphe pallidus to rostral ventrolateral medulla modulates cardiovascular reflex responses during acupuncture. J.Appl.Physiol. 2010;108:1336–1346. doi: 10.1152/japplphysiol.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat.Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 2009. [DOI] [PubMed] [Google Scholar]
- Tjen-A-Looi SC, Li P, Longhurst JC. Midbrain vIPAG inhibits rVLM cardiovascular sympathoexcitatory responses during acupuncture. Am J Physiol. 2006;290:H2543–H2553. doi: 10.1152/ajpheart.01329.2005. [DOI] [PubMed] [Google Scholar]
- Tjen-A-Looi SC, Li P, Longhurst JC. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: Roles of endocannabinoids and GABA. J Appl Physiol. 2009;106:1793–1799. doi: 10.1152/japplphysiol.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjen-A-Looi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton Neurosci. 2003;106:119–131. doi: 10.1016/S1566-0702(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Tjen-A-Looi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular response during stimulation of specific acupoints. Am J Physiol. 2004;287:R852–R862. doi: 10.1152/ajpregu.00262.2004. [DOI] [PubMed] [Google Scholar]
- Tjen-A-Looi SC, Li P, Longhurst JC. Role of medullary GABA, opioids, and nociceptin in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am J Physiol. 2007;293:H3627–H3635. doi: 10.1152/ajpheart.00842.2007. [DOI] [PubMed] [Google Scholar]
- Yao T, Andersson S, Thoren P. Long-lasting cardiovascular depression induced by acupuncture-like stimulation of the sciatic nerve in unanaesthetized spontaneously hypertensive rats. Brain Res. 1982a;240:77–85. doi: 10.1016/0006-8993(82)90645-x. [DOI] [PubMed] [Google Scholar]
- Yao T, Andersson S, Thoren P. Long-lasting cardiovascular depressor response following sciatic stimulation in spontaneously hypertensive rats. Evidence for the involvement of central endorphin and serotonin systems. Brain Res. 1982b;244:295–303. doi: 10.1016/0006-8993(82)90088-9. [DOI] [PubMed] [Google Scholar]
- Zhou W, Fu L-W, Guo ZL, Longhurst JC. Role of glutamate in rostral ventrolateral medulla in acupuncture-related modulation of visceral reflex sympathoexcitation. Am J Physiol. 2007;292:H1868–H1875. doi: 10.1152/ajpheart.00875.2006. [DOI] [PubMed] [Google Scholar]