Abstract
The aging kidney exhibits slowly developing injury and females are usually protected compared to males, in association with maintained renal nitric oxide (NO). Here, we compared renal injury in Fischer 344 (F344) rats in intact, ovariectomized, and ovariectomized + estrogen replaced young (6 month) and old (24 month) female rats with young and old intact male rats and measured renal protein abundance of NO synthase isoforms and oxidative stress. There was no difference in age-dependent glomerular damage between young or old intact male and female F344, and neither ovariectomy nor estrogen replacement affected renal injury; however, tubulointerstitial injury was greater in old males than old females. These data suggest that ovarian hormones do not influence these aspects of kidney aging in F344 rats and that the greater tubulointerstitial injury is caused by male sex. Old males had greater kidney cortex NOS3 abundance than females, and NOS1 abundance (alpha and beta isoforms) was increased in old males compared to both young males and old females. NOS abundance was preserved with age in intact females, ovariectomy did not reduce NOS1 or 3 protein abundance, and estrogen replacement did not uniformly elevate NOS proteins, suggesting that estrogens are not primary regulators of renal NOS abundance in this strain. NADPH oxidase-dependent superoxide production and nitrotyrosine immunoreactivity were increased in the aging male rat kidney compared to the female which could compromise renal NO production and/or bioavailability. In conclusion, the kidney damage expressed in the aging F344 is fairly mild and is not related to loss of renal cortex NOS3 or NOS1 alpha. This is in contrast to the aging male Sprague Dawley rat [1] where kidney damage is exacerbated and NOS3 and NOS1 alpha are lost compared to the old female.
Keywords: Nitric oxide, oxidative stress, tubulointerstitial injury, estrogen, ovariectomy
Introduction
The aging kidney exhibits a fall in glomerular filtration rate (GFR) due to renal vasoconstriction and structural damage [2]. In general, females enjoy marked protection against the cardiovascular and renal effects of aging [1,2,3] and premenopausal women and cycling rats with various forms of chronic kidney disease (CKD) exhibit a slower rate of progression compared to males [2,4,5]. However, much of this protection is lost after menopause, suggesting that ovarian hormones contribute to cardio-renal health. Protective actions of estrogens include acute (nongenomic) stimulation of NO release and long term stimulation of mRNA and/or protein expression of NO synthase (NOS) isoforms, leading to increased NOS activity in a variety of tissues [2,3]. Therefore, one goal of this study was to determine the impact of the loss of ovarian hormones and/or estrogen replacement on renal structural injury and NOS abundance.
We have previously reported that total NO production falls in the aging male Sprague Dawley rat, and there is a fall in renal cortical NOS1alpha and NOS3 with advancing age. However, NOS protein abundance and activity are maintained in the aging female Sprague Dawley rat, and kidney structure is also preserved [1], suggesting that preservation of renal NO and protection against age-dependent kidney injury are linked. Systemic and renal NO deficiency also contributes to the progression of CKD of different pathologies in both humans and experimental animals [6,7]. In particular, loss of renal cortical NOS1 alpha abundance plays a predictive, and possibly causal role in the progression of many forms of experimentally induced-CKD [6,7] with a fall in NOS1 alpha abundance occurring when >20% of glomeruli exhibit some degree of damage [8].
The Fischer 344 (F344) rat is a widely used animal model for aging research [9–12]; therefore, it is important to understand sex differences and age-related kidney injury in this strain. In contrast to the Sprague Dawley, aging F344 males develop slowly evolving glomerular structural damage and are resistant to hypertension [1,13,14]. We recently reported that in male F344 at 24–26 months of age, glomerular injury was mild (<15% damage) and there was no loss of renal NOS1 alpha [15]. The purpose of the present study was to test two hypotheses: (1) that aging intact F344 females exhibit less glomerular damage than similarly aged males, and (2) that loss of female ovarian hormones would lead to greater structural injury and dysregulation of the NOS system in the aging F344 kidney. We studied intact young (6 months) and old (24–26 months) male and female F344 rats with additional groups of young and old females subjected to ovariectomy with, or without, estrogen replacement, 8 weeks prior to sacrifice. We previously reported that estrogen replacement restores coronary flow-mediated dilation in these ovariectomized, aging females [16]. Since the level of oxidative stress plays a profound role in determining NO bioavailability [17,18], we also measured renal cortical NADPH oxidase-dependent superoxide production and nitrotyrosine levels in the kidney cortex of all groups.
Methods
All animal handling was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the West Virginia University and University of Florida Institutional Animal Care and Use Committees.
Animal procedures
Young (3–4 months) and old (22–24 months) male and female F344 rats supplied by Harlan (Indianapolis, IN) were purchased from the National Institute of Aging (NIA Bethesda, MD). All rats were housed in a temperature/light-controlled environment and given access to phytoestrogen-free rat chow and water ad libitum. At the time of arrival, female rats were maintained with ovaries intact (control), ovariectomized, or ovariectomized + estrogen-replaced and housed for 8 weeks post-operatively. Ages at time of death were 6 months (young) and 24–26 months (old), and in intact females, at least two complete estrous cycles were monitored by daily vaginal smears prior to death. The old females were in diestrus most of the time, and there was no impact of the stage of the estrus cycle on vascular responsiveness in young and old females [16], thus all data within each female group were pooled. The rats used in the current study were a subset of a group of rats from a previous study [16]. As previously reported by us, in the full set of rats (n=13–23), circulating estrogen levels fell from 62±9 pg/ml to 19±3 pg/ml in the young group, and levels were partially restored to 41±4 pg/ml after estrogen replacement. In the old females, ovariectomy did not reduce estradiol levels (29±3 pg/ml in intact vs 35±7pg/ml in ovariectomized old females), but estrogen replacement significantly increased estradiol to 54±9 pg/ml in aged rats.
Surgical Procedures
Bilateral ovariectomy surgeries were performed as described previously [16]. In some rats, estrogen replacement was performed simultaneously with the ovariectomy procedure. Two 0.05 mg 17β-estradiol 60-day slow-release pellets (Innovative Research, Novi, MI) were implanted subcutaneously near the scapulas.
At the end of the study, rats were weighed and anesthetized with inhaled isoflurane (Abbott, North Chicago, IL) followed by thoracotomy and removal of the heart. The kidneys were isolated, and a transverse slice of the left kidney was saved in 10% phosphate buffered formalin for histology (see below), and the remaining tissue was separated into cortex and medulla. All tissues were flash-frozen in liquid nitrogen, then stored at −80°C until further analyses.
Renal pathology
Fixed kidney tissue was paraffin embedded, and 5μm sections were cut and stained with periodic acid Schiff (PAS, Sigma, St Louis, MO) followed by hematoxylin as the secondary stain. Glomerular and tubulointerstitial injury were scored, blinded, as previously described [15,19] using a 0–4 scale for glomerular injury (~100 glomeruli per slide) and a 0–5 scale for tubulointerstitial injury.
Western Blot
The proteins measured by Western blot, with their specific primary antibody and concentration given in parenthesis, were: 1) endothelial NOS (NOS3; BD Transduction; 1:250), 2) neuronal NOS alpha (NOS1α, Santa Cruz; 1:50), 3) neuronal NOS beta (NOS1β, Thermo Scientific; 1:500), and 4) nitrotyrosine (Millipore; 1:500). Homogenized samples of kidney cortex and kidney medulla, standardized by protein concentrations (200 μg), were separated by electrophoresis (7.5% or 12% acrylamide gel, 200 V, 65 min) and transferred onto nitrocellulose membranes as previously described [20]. Membranes were stained with Ponceau Red (Sigma) to check for transfer efficiency/uniformity and equal loading, blocked and washed then incubated overnight with primary antibody at 4°C. Membranes were then incubated with the appropriate secondary antibody for one hour at room temperature, and developed with enhanced chemiluminescent reagents (Thermo Scientific). Bands were quantified by densitometry using the VersaDoc Imaging System and One Analysis Software (BioRad). Protein abundance was calculated as integrated optical density (IOD) of the protein of interest (after subtraction of background), factored for Ponceau Red stain (a marker of total protein loading) and an internal positive control value (to allow for quantitative comparisons between different membranes).
NADPH Oxidase Assay
NADPH oxidase-dependent superoxide production was measured by lucigenin (5 μmol/L, Sigma) chemiluminescence in the presence of NADPH (100 μmol/L, Sigma) as previously described [21]. Kidney cortex samples were homogenized, and protein concentrations were measured by the Bradford assay (BioRad). 35 μg of kidney cortex homogenate diluted in physiological saline solution (PSS) was added to wells. Then PSS or Tempol (10 mmol/L final concentration, Sigma) was added, and the plate was incubated for 30 minutes at 37°C. Lucigenin and NADPH were added to the wells using an autoinjector, and after a 30-minute dark-adapt period, plates were counted on an Orion microplate luminometer. NADPH-stimulated superoxide detection was diminished by Tempol. Enzyme activity was expressed as RLU/μg protein –RLU/μg protein in the presence of Tempol.
Statistical analyses
Data are presented as means ± SEM and were analyzed using SigmaPlot software (San Jose, CA). The data for the two hypotheses (1:effect of age and sex, 2:effect of age and hormone status in females) were analyzed separately using two-way ANOVA followed by the Holm-Sidak Multiple Comparison test. Significance was defined as p<0.05. The sample size for Western blot analysis and NADPH oxidase activity was 6 in all groups, and the sample size ranged from 6–9 rats in the histological analysis.
Results
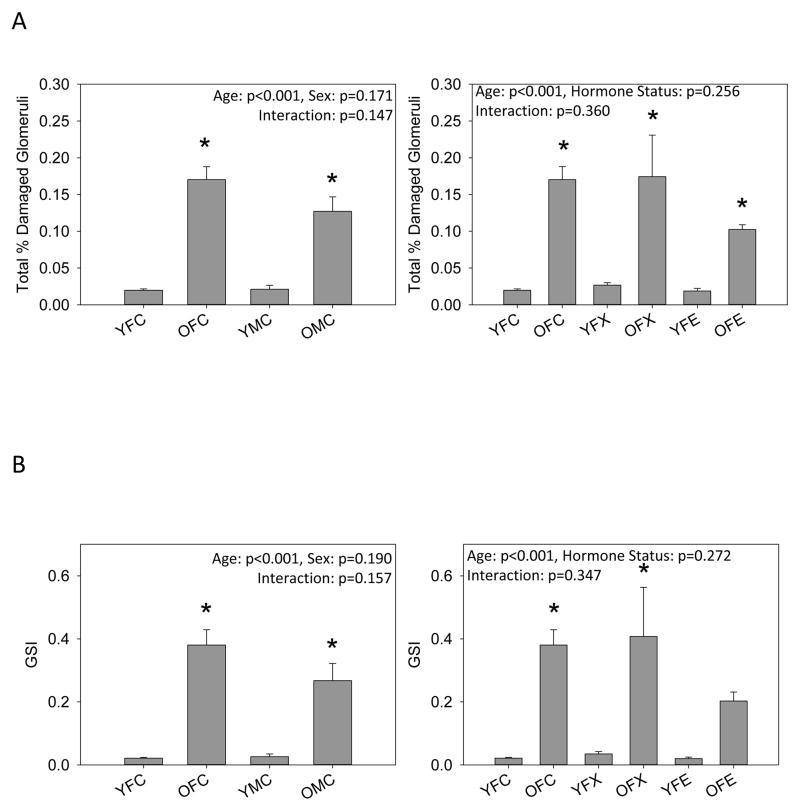
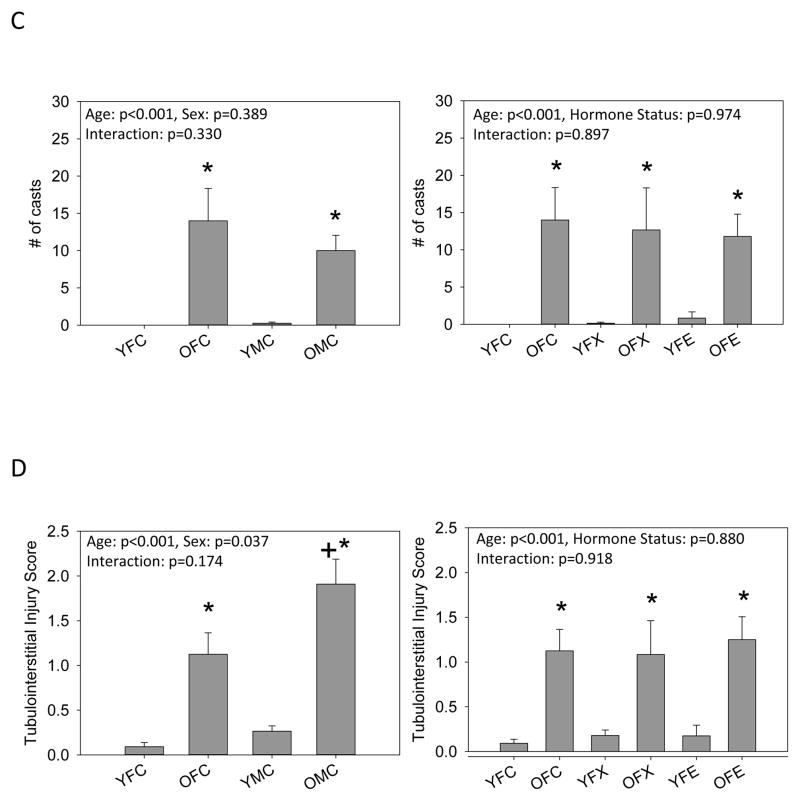
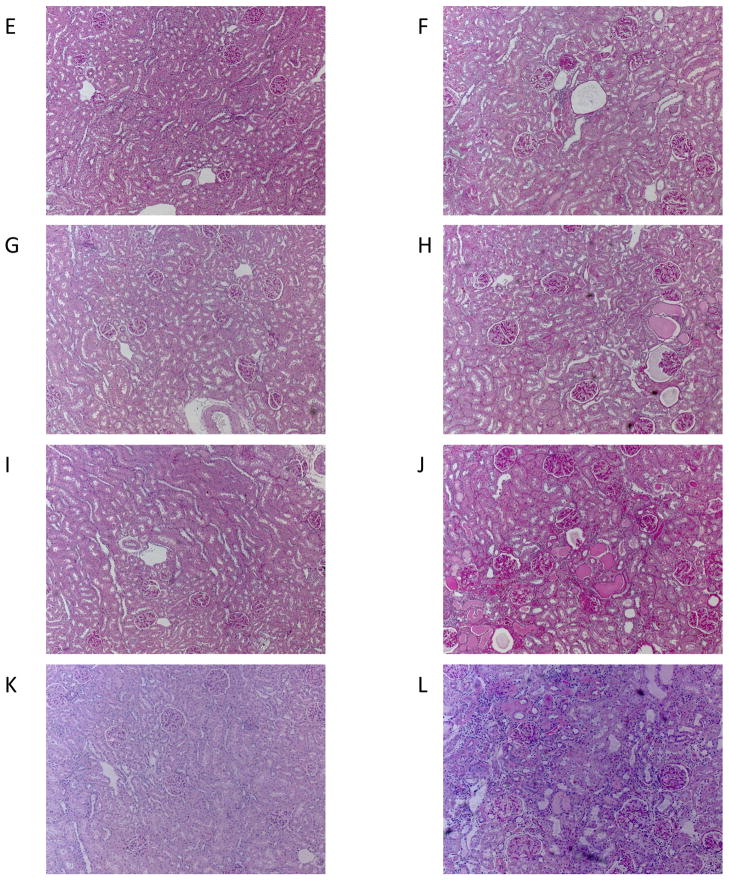
Histological analysis revealed minimal glomerular injury in young animals and that aging resulted in mild glomerular injury (~12–18%) and the appearance of protein casts in all groups (Figure 1). No differences in the number of damaged glomeruli or the severity of glomerular injury (GSI) were observed between intact old male and female rats or old females with ovariectomy with or without estrogen replacement. In contrast, tubulointerstitial injury was greater in old male rats compared to intact old females. Ovariectomy with and without estrogen replacement had no effect on tubulointerstitial injury in old females, but tubulointerstitial injury was greater in all old female groups compared to young female groups. Representative images of the renal histology for the 8 groups studied are given in Figure 1.
Figure 1.
Histological analysis of kidneys from young (Y) and old (O), female (F) and male (M) F344 rats. Control (C) rats were gonad intact, and some females were ovariectomized (X) with or without estrogen replacement (E), n=6–9, * = p<0.05 vs respective young rat, + = p<0.05 vs intact female of same age. (A) the % of glomeruli showing any degree of injury, (B) Glomerulosclerosis index (GSI) gives a measure of the severity of individual glomerular damage as well as the % of damaged glomeruli, with 0=100% completely normal glomeruli and 4.0 = 100% damage to 100% of glomeruli [15,19], (C) the total number of casts observed in the kidney section, and (D) the tubulointersititial injury score as 0=none, 1=10%, 2=10–25%, 3=25–50%, 4=50–75%, 5=75–100% [19]. Representative images of sections from kidneys from (E) YFC, (F) OFC, (G) YFX, (H) OFX, (I) YFE, (J) OFE, (K) YMC, (L) OMC rats.
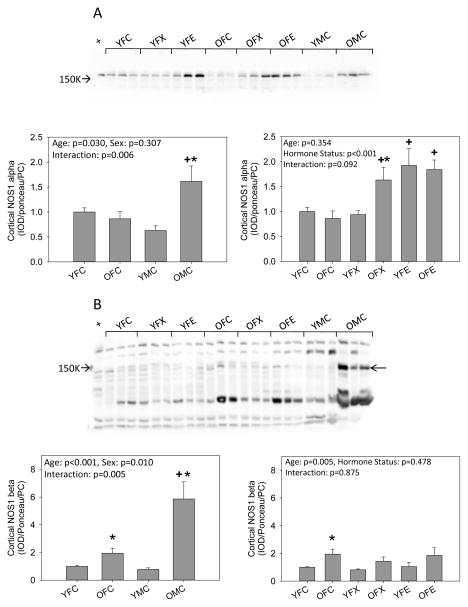
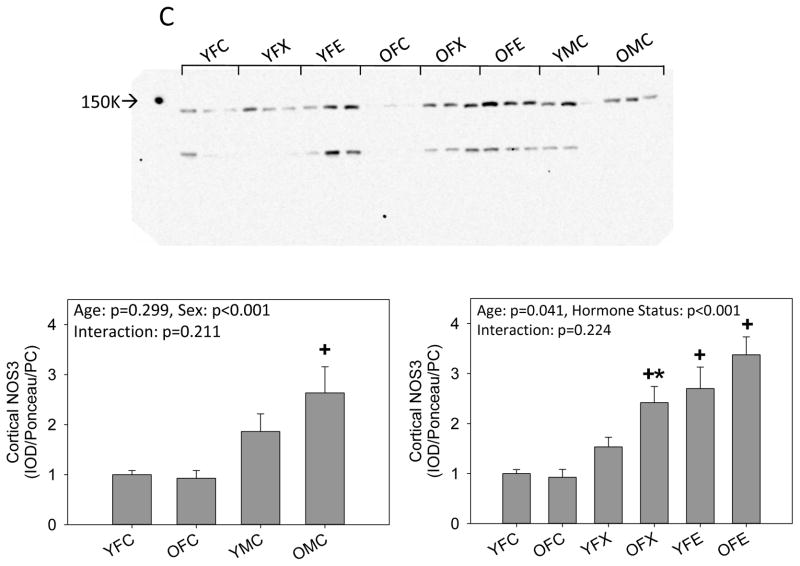
We next examined the abundance of the NOS isoforms. There was no difference in cortical NOS1 alpha abundance between young intact males and females. NOS1 alpha increased with age in males, but no age-related change in NOS1 alpha occurred in females. Ovariectomy alone had no impact in young females but when combined with estrogen replacement cortical NOS1 alpha increased. Compared to intact young and old females, there was an increase in NOS1 alpha in the old ovariectomized females which was not further influenced by estrogen replacement (Fig 2a). The abundance of NOS1 beta was also similar in young intact males and females and increased with age in both sexes, with a greater increment seen in the males. There was no effect of ovariectomy with or without estrogen replacement in either young or old females (Fig 2b). NOS3 abundance was higher in old but not young (p=0.08) intact males vs. females, and there was no effect of aging,. Ovariectomy had no impact in young females although estrogen supplementation increased cortical NOS3 abundance. In old females, ovariectomy increased cortical NOS3, and estrogen supplementation had no further impact (Fig 2c).
Figure 2.
Western blot analysis of (A, B) neuronal NOS (NOS1) isoforms and (C) endothelial NOS (NOS3) in the kidney cortex from young (Y) and old (O), female (F) and male (M) F344 rats. Control (C) rats were gonad intact, and some females were ovariectomized (X) with or without estrogen replacement (E). + = positive control on representative blot. Arrow on Panel B indicates NOS1 beta specific band. n=6, * = p<0.05 vs respective young rat, + = p<0.05 vs intact female of same age.
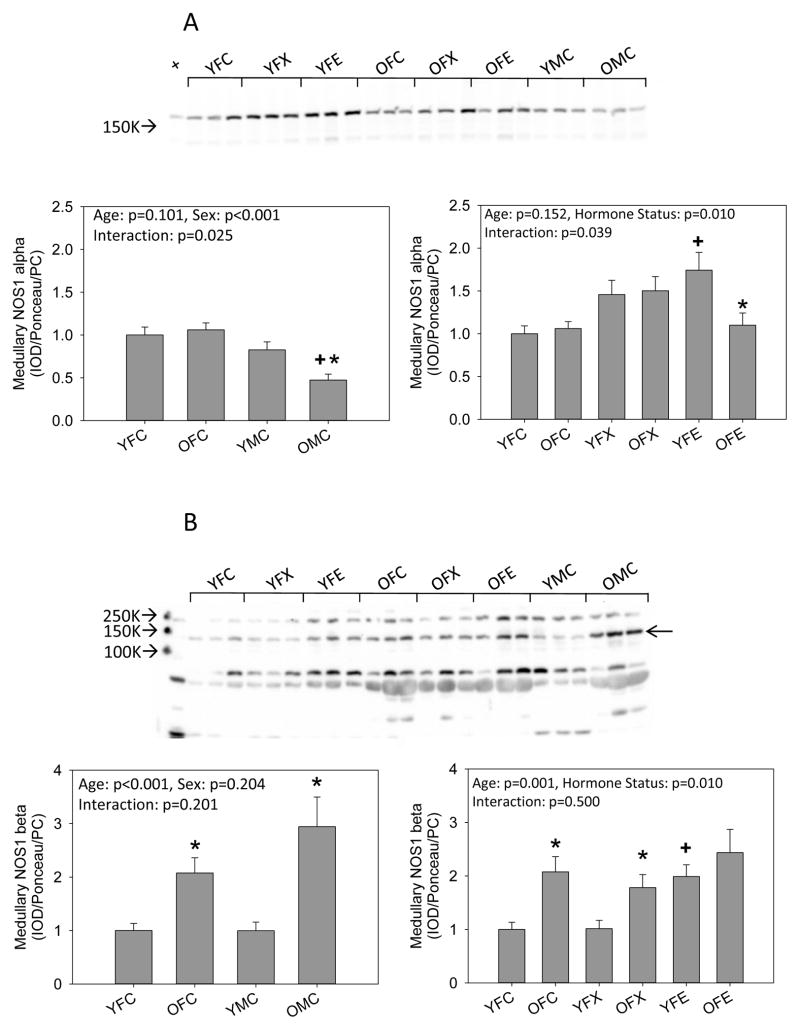
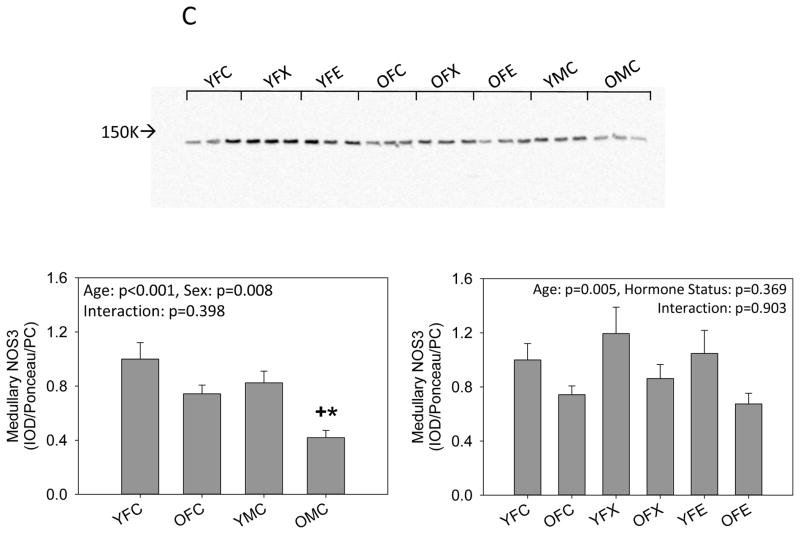
In medulla, NOS1 alpha was similar in young males and females and unaffected by age in females but fell in males. Ovariectomy had no effect on medullary NOS1 alpha in either young or old females. Estrogen replacement increased medullary NOS1 alpha abundance, but this increase was not seen in old females (Fig 3a). Medullary NOS1 beta was similar in young intact males and females and increased with age in both sexes. Ovariectomy was without effect in young females but estrogen replacement increased medullary NOS1 beta abundance while ovariectomy with or without estrogen replacement had no further effect in the old females (Fig 3b). There was no sex difference in medullary NOS3 in young rats, and there was no effect of aging or ovariectomy with or without estrogen in females. Medullary NOS3 was lower in old males than in either young males or old females (Figure 3c).
Figure 3.
Western blot analysis of (A, B) neuronal NOS (NOS1) isoforms and (C) endothelial NOS (NOS3) in the kidney medulla from young (Y) and old (O), female (F) and male (M) F344 rats. Control (C) rats were gonad intact, and some females were ovariectomized (X) with or without estrogen replacement (E). + = positive control on representative blot. Arrow on Panel B indicates NOS1 beta specific band. n=6, * = p<0.05 vs respective young rat, + = p<0.05 vs intact female of same age.
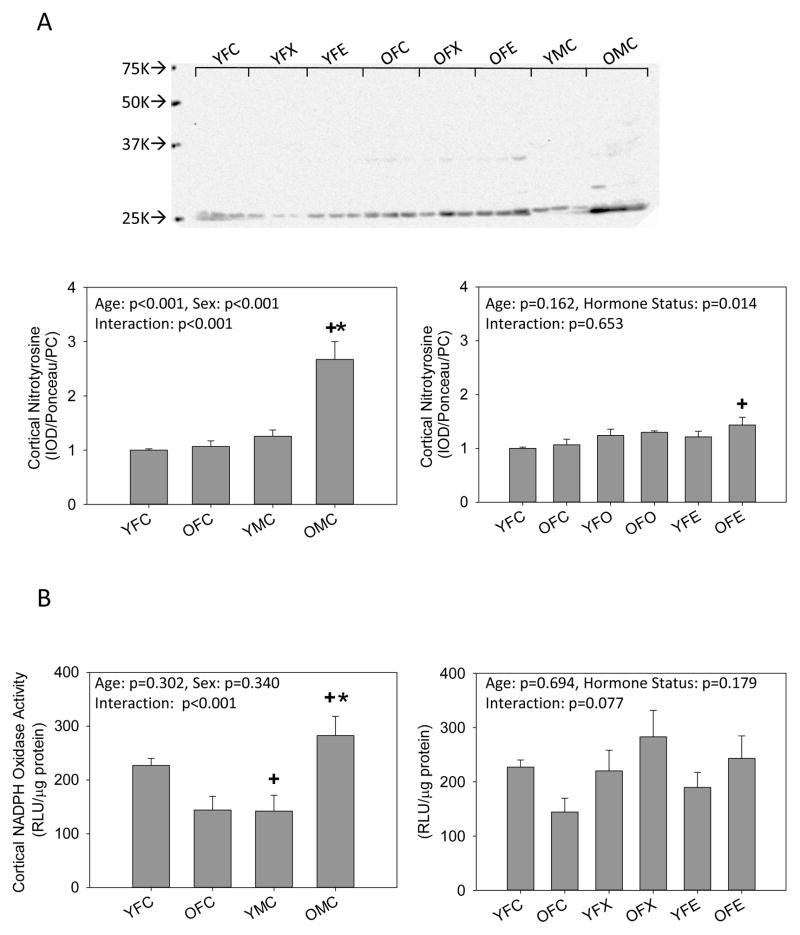
Because NO bioavailability is decreased during oxidative stress, we measured nitrotyrosine immunoreactivity and NADPH oxidase-dependent superoxide production in the kidney cortex. No changes in nitrotyrosine or NADPH oxidase activity were observed with age or manipulation of ovarian hormones in female rats, with the exception of an increase in nitrotyrosine immunoreactivity in the old ovariectomized females with estrogen replacement, but both nitrotyrosine and superoxide production increased with age in intact male rats. Both nitrotyrosine and superoxide production were also greater in old males as compared to old females (Fig 4).
Figure 4.
Western blot analysis of (A) nitrotyrosine immunoreactivity and (B) NADPH oxidase activity levels in the kidney cortex of young (Y) and old (O), female (F) and male (M) F344 rats. Control (C) rats were gonad intact, and some females were ovariectomized (X) with or without estrogen replacement (E), n=6, * = p<0.05 vs respective young rat, + = p<0.05 vs intact female of same age.
Discussion
A surprising finding from this study is that the mild glomerular damage seen in the aging F344 develops similarly in males and females, which differs from most other rat strains where the male exhibits a more rapid rate of age-dependent glomerular injury [1,22,23]. In contrast, tubulointerstitial injury was greater in aged intact males vs. intact females. The lack of effect of ovariectomy, with or without estrogen replacement, on glomerular and tubulointerstitial injury in aging females suggests that ovarian hormones do not influence these aspects of kidney aging in F344 rats and that the greater tubulointerstitial injury in old males is caused by male sex. Of course, rats in the present study were only ovariectomized 8 weeks prior to study, but earlier work by us, in the Munich Wistar rat showed no effect on renal structure or function ~18 months post ovariectomy and this study also suggested that male gonadal hormones were associated with worse age-dependent kidney injury [23].
In the young adult F344 male, the abundance of both NOS1 isoforms and NOS3 was similar to females in cortex and medulla. This contrasts with the Sprague Dawley rat where whole kidney NOS3 was higher in the young intact female vs. male [24]. Neugarten and colleagues localized this increase to the medulla and reported that the elevated medullary NOS3 was estrogen – dependent, being lost with ovariectomy and restored with estrogen replacement [25]. In contrast, we report here that ovariectomy had no impact on renal cortical or medullary NOS1 or NOS3 in young ovariectomized females. In the present study, estrogen replacement in ovariectomized young females raised cortical NOS1 alpha and NOS3 to supernormal levels and had no impact on medullary NOS1 alpha or NOS3. Exactly why these changes occurred with estrogen replacement are not clear since we previously reported that the estrogen levels achieved with estrogen replacement in young ovariectomized rats were lower than in the intact females [16]. We also observed higher cortical NOS3 in old male F344 vs. old intact females, that neither cortical or medullary NOS3 fell with age in intact females, despite ~50% reduction in circulating estrogen levels and that estrogen replacement in old ovariectomized females had no effect on renal NOS3 abundance despite restoring estrogen levels to the young intact female value [16]. These variable findings emphasize the importance of strain/genetic background when considering sex differences in cardiovascular/renal variables. Our recent report that reduction of estrogen levels by aromatase inhibition leads to unchanged NOS1 and 3 protein abundance but increased NOS activity in cortex of young Sprague-Dawley females [26] also cautions against assumptions regarding NOS proteins based on sex and ovarian hormone status.
The primary goal of this study was to investigate renal NOS abundance in the context of sexual dimorphism in kidney aging. This was based on our earlier findings in the Sprague Dawley rat where aging resulted in a decline in renal NOS only in males in association with marked kidney damage while females exhibited stable renal NOS and little renal pathology [1]. Furthermore, the middle aged male Sprague Dawley showed a fall in the renal cortex NOS1 alpha which preceded the development of significant structural damage and also preceded loss of renal cortical NOS3. This suggested that the early fall in the renal cortex NOS1 alpha might be part of the pathogenesis of rapid progression of age-dependent CKD seen in the male. In fact, we have investigated a number of models of CKD in the male rat and we invariably observe that severe glomerular damage is associated with a loss of renal cortex NOS1 alpha, which is present in the macula densa, proximal tubules, and collecting duct of the normal kidney [2,6,7]. Our earlier work in the young adult male Sprague Dawley with severe renal mass reduction showed that renal cortical NOS1 alpha was maintained until >20% glomeruli were damaged, after which the abundance fell markedly [8] and was lost from macula densa and tubules [28]. In the present study we report that the aging male and female F344 display <20% glomerular damage without sexual dimorphism and in agreement with our earlier work [8] the cortical NOS1 alpha abundance was not reduced with age, being unchanged in females and increased in males. We have previously reported that as NOS1 alpha abundance falls during progression of CKD in the rat, the abundance of the NOS1 beta isoform increases [27–29]. The distribution of the NOS1 beta in the damaged renal cortex is in proximal tubules and also in interstitium, but not macula densa. While we initially concluded that the increase in NOS1 beta following kidney injury was in compensation for the loss of NOS1 alpha, we recently observed an increased renal cortical NOS1 beta in 30 month old male F344/BN rats which show minimal age-dependent glomerular damage (<5% injury) [15]. Similarly, in the present study we found that there were age-dependent increases in NOS 1 beta in both male and female F344 rats despite no fall in NOS1 alpha and <20% glomerular injury. Together, our findings suggest that renal cortical NOS1 beta induction occurs early during kidney injury and may even play a pathogenic role in the later glomerular injury. The NOS 1 beta is produced by alternative splicing and little is known about its regulation although it is a functional enzyme. One key difference is that the NOS 1 beta lacks the PDZ binding domain at the amino terminal and is thus likely to be exclusively cytosolic, which resembles the inducible NOS often associated with inflammatory injury [28].
In addition to regulation of the NOS enzymes, nitric oxide bioavailability is also diminished by the presence of superoxide and other reactive oxygen species. In this study, we observed evidence of increased oxidative stress in the aged male kidney (increased nitrotyrosine immunoreactivity and NADPH oxidase activity) that is not observed in kidneys from female rats with aging. Neither ovariectomy nor estrogen replacement uniformly altered these measures of oxidative stress suggesting that male sex or androgens are responsible for the elevated oxidative stress observed in the aged F344 males. The level of oxidative stress in the kidney has important implications for renal NOS. For example, high levels of superoxide will scavenge NO and produce peroxynitrite leading to increased tyrosine nitration, as seen in old males. The increased superoxide will also decrease tetrahydrobiopterin availability which “switches” the NOS to become superoxide generators [18,30], thus maintained or elevated levels of renal cortical NOS (as seen in the aging male F344 kidney cortex) can actually become part of the pathology by exacerbating the oxidative stress.
In conclusion, although there is no sex difference in the age-dependent mild glomerular injury that develops in the F344, there is greater tubulointerstitial injury in males that is probably not related to the absence of estrogens. The greater tubulointerstitial injury may be due to the greater renal oxidative stress that also occurs in aging male F344s. Future studies should examine the effects of androgens in the progression of age-related kidney injury in the F344 strain.
Acknowledgments
This work was supported by the NIH (R01 DK56843 (C.B.), T32DK076541 (N.M./Charles Wood), R25HL103181 (O.A./Charles Wood), and R01 HL077224 (J.M.D.)) and the American Heart Association (11SDG06910000 to J.M.S). We thank Kevin Engels for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erdely A, Greenfeld Z, Wagner L, Baylis C. Sexual dimorphism in the aging kidney; inverse relationship between injury and nitric oxide system. Kidney International. 2003;63:1021–1026. doi: 10.1046/j.1523-1755.2003.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nature Clinical Practice Nephrology. 2009;5:384–96. doi: 10.1038/nrneph.2009.90. [DOI] [PubMed] [Google Scholar]
- 3.Dubey RK, Jackson EK. Protective effects of estrogen on the cardiovascular and renal systems: Potential cellular, biochemical and molecular mechansims. American Journal Physiology; Renal Physiology. 2001;280:F365–88. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- 4.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. Journal of the American Society of Nephrology. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 5.Mulroney SE, Woda C, Johnson M, Pesce C. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney International. 1999;56:944–953. doi: 10.1046/j.1523-1755.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 6.Baylis C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease (CKD) Nature Clinical Practice Nephrology. 2006;2:209–220. doi: 10.1038/ncpneph0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baylis C. Nitric oxide deficiency in chronic kidney disease. Gottschalk Lecture 2007. American Journal Physiology; Renal Physiology. 2008;294:F1–F9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- 8.Szabo A, Wagner L, Erdely A, Lau K, Baylis C. Renal neuronal nitric oxide synthase protein expression as a marker of renal function. Kidney Int. 2003;64:1765–1771. doi: 10.1046/j.1523-1755.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 9.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of Brown Norway, Brown Norway x Fischer 344, and Fischer 344 x Brown Norway rats with relation to age. Journal of Gerontology A Biological Sciences and Medical Sciences. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razzaque MS, Shimokawa I, Nazneen A, Higami Y, Taguchi T. Age-related nephropathy in the Fischer 344 rat is associated with overexpression of collagens and collagen-binding heat shock protein 47. Cell Tissue Research. 1998;293:471–8. doi: 10.1007/s004410051139. [DOI] [PubMed] [Google Scholar]
- 11.Abrass CK, Adcox MJ, Raugi GJ. Aging-associated changes in renal extracellular matrix. American Journal of Pathology. 1995;146:742–52. [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman GL, Barthold SW, Osbaldiston GW, Foster SJ, Jonas AM. Pathological changes during aging in barrier-reared Fischer 344 male rats. Journal of Gerentology. 1977;32:258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein RS, Tarloff JB, Hook JB. Age-related nephropathy in laboratory rats. FASEB Journal. 1988;2:2241–5I. doi: 10.1096/fasebj.2.7.3280378. [DOI] [PubMed] [Google Scholar]
- 14.Hall CE, Ayachi S, Hall O. Immunity of Fischer 344 rats to salt hypertension. Life Sciences. 1976;18:1001–1008. doi: 10.1016/0024-3205(76)90422-7. [DOI] [PubMed] [Google Scholar]
- 15.Moningka NC, Sindler AL, Muller-Delp JM, Baylis C. Twelve weeks of treadmill exercise provides does not ameliorate the development of age-dependent kidney structural injury in the Fisher 344 male rat. Journal of Physiology. 2011;589:6129–38. doi: 10.1113/jphysiol.2011.214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. American Journal Physiology; Regulatory Integrative and Comparative Physiology. 2009;297:R1713–23. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolin MS. Interactions of oxidants with vascular signaling systems. Arteriosclerosis Thrombosis and Vascular Biology. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 18.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. Journal of Clinical Investigation. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moningka NC, Sasser JM, Croker B, Carter C, Baylis C. Protection against age-dependent renal injury in the F344xBrown Norway male rat is associated with maintained nitric oxide synthase. Mechanisms of Ageing and Development. 2011;132:1–7. doi: 10.1016/j.mad.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasser JM, Moningka NC, Cunningham MW, Jr, Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. American Journal Physiology; Regulatory Integrative and Comparative Physiology. 2010;298:R740–6. doi: 10.1152/ajpregu.90875.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasser JM, Sullivan JC, Elmarakby AA, Kemp BE, Pollock DM, Pollock JS. Reduced NOS3 phosphorylation mediates reduced NO/cGMP signaling in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2004;43:1080–5. doi: 10.1161/01.HYP.0000122804.32680.c9. [DOI] [PubMed] [Google Scholar]
- 22.Baylis C, Corman B. The aging kidney: insights from experimental studies. Journal of the American Society of Nephrology. 1998;9:699–709. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- 23.Baylis C. Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. Journal of Clinical Investigation. 1994;94:1823–9. doi: 10.1172/JCI117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reckelhoff JF, Hennington BS, Moore AG, Blanchard EJ, Cameron J. Gender differences in the renal nitric oxide (NO) system: dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. American Journal of Hypertension. 1998;11:97–104. doi: 10.1016/s0895-7061(97)00360-9. [DOI] [PubMed] [Google Scholar]
- 25.Neugarten J, Ding Q, Friedman A, Lei J, Silbiger S. Sex hormones and renal nitric oxide synthases. Journal of the American Society of Nephrology. 1997;8:1240–1246. doi: 10.1681/ASN.V881240. [DOI] [PubMed] [Google Scholar]
- 26.Santmyire BR, Venkat V, Beinder E, Baylis C. Impact of the estrus and of reduction in estrogen levels with aromatase inhibition, on renal function and nitric oxide activity in female rats. Steroids. 2010;75:1011–5. doi: 10.1016/j.steroids.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tain YL, Muller V, Szabo AJ, Erdely A, Smith C, Baylis C. Sex differences in response to rapamycin in kidney transplantation: Impact on constitutive nitric oxide synthase. Nitric Oxide Biology and Chemistry. 2008;18:80–86. doi: 10.1016/j.niox.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith C, Merchant M, Fekete A, Nyugen H-L, Oh P, Tain Y-L, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrology, Dialysis and Transplantation. 2009;24:1422–1428. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tain YL, Ghosh S, Krieg RJ, Baylis C. Reciprocal changes of renal neuronal nitric oxide synthase-α and –β associated with renal progression in a neonatal 5/6 nephrectomized rat model. Pediatric Neonatology. 2011;52:66–72. doi: 10.1016/j.pedneo.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]