Abstract
Objective
To evaluate longitudinal changes in prostate-specific antigen (PSA) levels in men with and without prostate disease.
Design
Case-control study of men with and without prostate disease who were participants in a prospective aging study.
Setting
Gerontology Research Center of the National Institute on Aging; the Baltimore (Md) Longitudinal Study of Aging.
Patients
Sixteen men with no prostate disease (control group), 20 men with a histologic diagnosis of benign prostatic hyperplasia (BPH), and 18 men with a histologic diagnosis of prostate cancer.
Outcome Measures
Multiple PSA and androgen determinations on serum samples obtained from 7 to 25 years prior to histologic diagnosis or exclusion of prostate disease.
Results
Changesin androgen levels with age did not differ between groups. Control subjects did not show a significant change in PSA levels with age. There was a significant difference in the age-adjusted rate of change in PSA levels between groups (prostate cancer>BPH>control; P<.01). At 5 years before diagnosis when PSA levels did not differ between subjects with BPH and prostate cancer, rate of change in PSA levels (0.75 μg/L per year) was significantly greater in subjects with prostate cancer compared with control subjects and subjects with BPH. Also, rate of change in PSA levels distinguished subjects with prostate cancer from subjects with BPH and control subjects with a specificity of 90% and 100%, respectively.
Conclusions
The most significant factor affecting serum PSA levels with age is the development of prostate disease. Rate of change in PSA levels may be a sensitive and specific early clinical marker for the development of prostate cancer.
PROSTATE-SPECIFIC ANTIGEN (PSA) is a serine protease produced by both benign and malignant prostatic epithelium that can be measured in serum samples by immunoassay.1 Cross-sectional analysis of serum PSA levels in men with and without prostate discent studies suggest that PSA may be useful in the early detection of prostate cancer,3,4 it is known that PSA elevations occur in men with BPH2,5,6 and that men with prostate cancer can have normal PSA levels.4-6 Therefore, based on cross-sectional studies, PSA elevations are not specific for prostate cancer, and a normal PSA level does not exclude the presence of cancer.
The longitudinal changes in PSA that occur with age in men with and without prostate disease have not been reported previously. In addition, although it is well known that PSA is under the influence of androgen,7,8 the influence of age-related decreases in androgen levels on PSA has not been studied. To better understand the factors that affect PSA levels and potentially improve the use of this valuable clinical marker in men with prostate disease, we evaluated PSA levels in a longitudinal, casecontrol study.
METHODS
Study Groups
Three groups of men were identified from subjects participating in the Baltimore Longitudinal Study of Aging (BLSA). The BLSA is an ongoing, long-term,prospective aging study of the National Institute of Aging, Bethesda, Md, which has as its goal the study of the processes of aging in humans.9 Since the BLSA was established in 1958, there have been approximately 1500 male participants in the study. Participants are community-dwelling volunteers who return approximately every 2 years for 2.5 days of physical examinations and a battery of physiological, psychological, and medical tests. During these visits, fasting and nonfasting serum samples are stored for current and future studies. Participants are predominantly white and well educated. Previous analysis of this population of men10 revealed the age-specific prevalence of prostate disease to be similar to that in the white male population.11
Control Group
All active male participants between the ages of 55 and 90 years who participated in the BLSA for at least 7 years were screened by one urologist (H.B.C.) during their routine BLSA visit for the presence of symptoms or physical findings of BPH, as described by Guess and colleagues.12 Between January 1990 and October 1990, approximately 200 men returned for a routine BLSA visit. From these men, 16 subjects who did not have a prior urologie history of disease and were thought to have no prostate disease were identified and constituted the control group of subjects.
BPH Group
One hundred thirty-four men who had reported having a simple prostatectomy for BPH were identified from 1459 male participants in the BLSA. Of the 134 men, there were 20 men older than 60 years whose age was similar to the control subjects that met the following criteria: (1) participation in the BLSA for at least 7 years prior to simple prostatectomy, (2) confirmation of the pathologic diagnosis from simple prostatectomy, and (3) no previous prostate surgery prior to simple prostatectomy. Pathologic diagnosis of BPH was confirmed to decrease the possibility of unsuspected prostate cancer in this group of subjects.
Prostate Cancer Group
Thirty-seven men with the diagnosis of prostate cancer were identified from 1459 male BLSA participants. Of the 37 men, there were 18 subjects older than 60 years whose age was similar to the control and BPH groups that met the following criteria: (1) participation in the BLSA for at least 7 years prior to diagnosis of prostate cancer, (2) confirmation of the pathologic diagnosis of prostate cancer, and (3) no previous prostate surgery prior to prostate cancer diagnosis. Clinical stage of the cancer was assigned as local or regional or metastatic disease based on clinical examination, prostatic acid phosphatase determinations, bone scan results, and pathology reports from the treating urologist’s records. Elevations of enzymatic acid phosphatase were considered as evidence of metastatic disease.
After identification of the study population, available serum samples from the BLSA serum bank were analyzed for visits prior to the time of diagnosis. Serum samples were not available for all subjects at each visit. Serum samples that had been stored at −70°C and not previously thawed were identified by date of collection so that results could be related to the date of diagnosis. The date of diagnosis for the control group was the date of exclusion of prostatic disease. The date of diagnosis for the BPH and prostate cancer group was the date of simple prostatectomy or histologie diagnosis of prostate cancer, respectively.
PSA Assay
All PSA determinations were made on routine serum samples using the Tandem-R monoclonal immunoradiometric assay (Hybritech Inc, San Diego, Calif). According to the manufacturer’s recommendations, the expected range for PSA in unaffected men using this assay is zero to 3.99 μg/L. The interassay coefficient of variation was calculated from three quality control samples (mean ± SD, percent of coefficient of variation): 0.84 μg/L± 0.063 μg/L, 7.5%; 2.9 μg/L±0.12 μg/L, 4%; and 40 μg/L± 1.6 μg/L, 4%. A mixed-effects regression model (see “Statistical Analysis” section) using length of storage of serum samples as an independent variable suggested that date of serum sample had no significant affect on PSA levels
Serum Testosterone and Luteinizing Hormone Determinations
Total testosterone, free testosterone, and luteinizing hormone determinations were performed in one laboratory (Hazleton Laboratories, Vienna, Va) by radioimmunoassay on fasting serum samples taken in the morning. Hormone measurements were evenly spaced over the time of follow-up prior to diagnosis.
Study Group Demographics
Table 1 shows the ages at diagnosis, years of follow-up, and number of serum determinations for the three groups. For men in the BPH group, the median age at diagnosis (75.9 years) was significantly greater than the median age of men in the control group (66.0 years). There were no significant differences in the years of follow-up and median number of hormone measurements between the three groups. Subjects with local or regional prostate cancer had a significantly greater number of PSA measurements than subjects in the control and BPH groups.
Statistical Analysis
A mixed-effects regression model13 was used to test the hypothesis that, after controlling for the effect of age at diagnosis, PSA values increase faster in subjects with prostate cancer compared with subjects with BPH or those without prostate disease.14 The model includes random effects to account for the natural heterogeneity in PSA values and the rate of change in PSA within the population. This heterogeneity would be expected due to uncontrolled factors affecting when the diagnosis was made and affecting the rate of progression of prostate cancers. Since the a priori hypothesis was that PSA levels would increase faster in the subjects with cancer than in the subjects with BPH or control subjects, one-tailed tests were used.
Several potential rules for detection of prostate cancer were developed based on the distributions of the observed rates of change in PSA levels in the diagnostic groups. One criterion was the rate of change in PSA between two consecutive visits (PSAR = [PSAt–PSAt–1]/[Yearst–Yearst–1]). Since the rate of change between two consecutive visits is subject to random measurement error and fluctuations in the rate of change, a second criterion was the running average of the rate of change over three consecutive visits (PSAR2 = [PSARt + PSARt–1]/2).
The sensitivity (true positives/[true positives+false negatives]) and specificity (true negatives/[true negatives+false positives]) of the detection criteria were compared by calculating the binomial probability of obtaining distributions equal to, or more deviant than, the observed distribution assuming the null hypothesis that the two rules had equal sensitivities or specificities.15
RESULTS
Hormone Levels
The mean serum hormone levels (free testosterone, total testosterone, and luteinizing hormone) were within the normal range for these tests in all groups (data not shown). A mixed-effects regression model using as independent variables age at entrance into the study, length of follow-up, and diagnostic group, revealed no differences in hormonal rates of change among the groups with age (results not shown). As expected, testosterone levels decreased with age and luteinizing hormone levels increased with age.
Changes in PSA
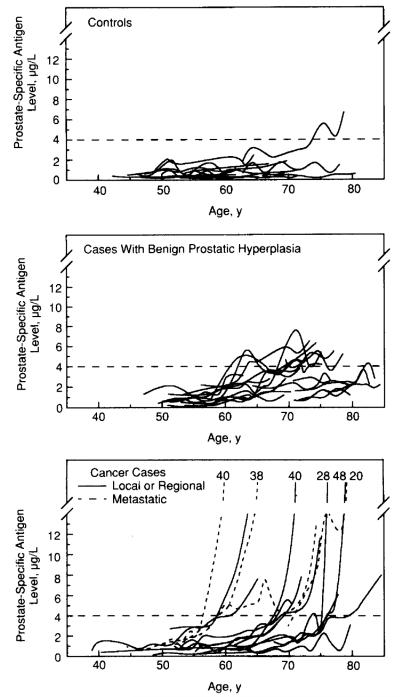
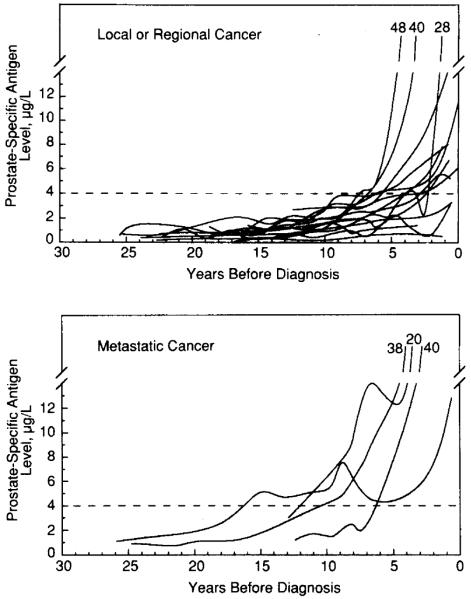
Observed PSA levels are shown for each participant in all groups as a function of age (Fig 1) and as a function of years prior to diagnosis for subjects with prostate cancer (Fig 2). The observed rate of change in PSA levels μg/L per year) and rate of change estimated from the mixed-effects model are given in Table 2. Subjects with prostate cancer had significantly greater rates of change in PSA levels than those without prostate cancer up to 10 years before diagnosis.
Fig 1.
Observed data on prostate-specific antigen (PSA) levels μg/L) plotted as a function of age for each individual in the three diagnostic groups (solid lines). The broken horizontal line marks the single PSA criterion of 4.0 μg/L The PSA values at visit closest to diagnosis are indicated for individuals who progress beyond a PSA value of 14 μg/L.
Fig 2.
Observed data on prostate-specific antigen (PSA) levels μg/L) plotted as a function of years before diagnosis for each individual subject with cancer (local or regional and metastatic). The PSA values at visit closest to diagnosis are indicated for individuals who progress beyond a PSA value of 14 μg/L
A mixed-effects regression analysis (Fig 3) revealed significant differences in the rate of change in PSA levels between all groups (metastatic cancer>local or regional cancer>BPH>control; P<.01). The PSA levels increased slowly over time in the control group but did not reach statistical significance. Compared with the gradual increase in PSA levels seen in subjects with BPH, subjects with prostate cancer had an exponential increase in PSA levels approximately 10 years before diagnosis.
Fig 3.
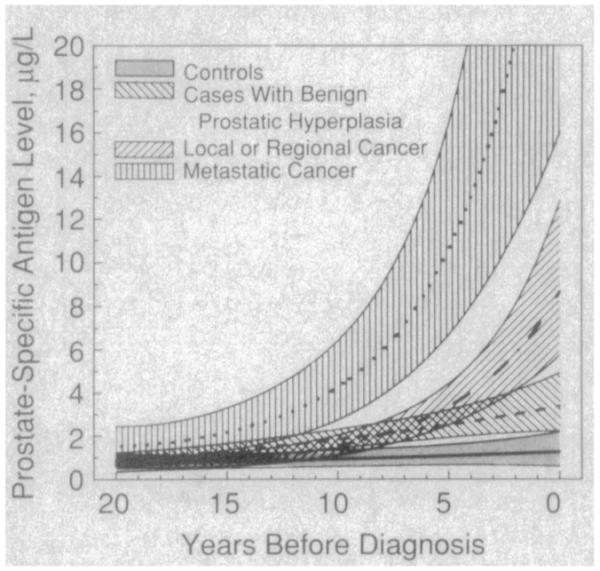
Average curves (±95% confidence intervals) of prostate-specific antigen levels μg/L) as a function of years before diagnosis for three diagnostic groups estimated from a mixed-effects model assuming an age of diagnosis of 75 years.
Differentiation Between Study Groups
One (6.3%) of 16 control subjects, 8 (40%) of 20 subjects with BPH, and 14 (78%) of 18 subjects with prostate cancer had at least one PSA measurement greater than 4.0 μg/L (Figs 1 and 2). In four men with local or regional prostate cancer, no PSA level was greater than 4.0 μg/L. No control subject or subject with BPH had a serum PSA level of greater than 10.0 μg/L. The PSA value closest to the time of diagnosis differed significantly between the study groups (prostate cancer>BPH>control) as shown in Table 3. The difference in PSA levels between subjects with local or regional prostate cancer and those with BPH disappeared at 5 years before diagnosis (Table 3). In contrast, the rate of change in PSA was significantly greater for subjects with local or regional prostate cancer compared with those without prostate cancer at 5 to 10 years before diagnosis (Table 2).
The sensitivity and specificity of single PSA levels and rate of PSA change in differentiating between subjects with and without prostate cancer are shown in Table 4 together with the time prior to diagnosis when cancers were detected using various detection criteria. When a rate of change in PSA levels between two consecutive visits (PSAR) was 1.0 μg/L or greater per year or an average rate of change between three consecutive visits (PSAR2) of 0.75 μg/L or greater per year was compared with single PSA measurements in the detection of prostate cancer, there was no significant difference in sensitivity. Using the detection criterion of a PSA level of 4.0 μg/L or greater resulted in detection of 78% of prostate cancers at a median of 4.9 years prior to diagnosis for subjects without metastatic disease. An average rate of change (PSAR2) of 0.75 μg/L or greater per year detected 72% of prostate cancers a median of 2.6 years prior to diagnosis for subjects without metastatic disease. The average rate of change in PSA levels of 0.75 μg/L or greater per year was more sensitive as a criterion of prostate cancer detection when PSA was 4.0 μg/L or greater than when PSA was less than 4.0 μg/L (Table 4).
When a single PSA level of 4.0 μg/L or greater was used as a criterion for prostate cancer detection, 40% of subjects with BPH were incorrectly classified as having cancer (specificity, 60%) (Table 4). However, when an average rate of change of 0.75 μg/L or greater per year was used as the criterion for prostate cancer detection, only 10% of subjects with BPH were misclassified (specificity, 90%). The average rate of change criterion yielded a significantly better specificity than a single PSA level of 4.0 μg/L or greater(P = .03). Inaddition, theaverage rate of change rule maintained a high level of specificity both when PSA levels were below and above 4.0 μg/L.
COMMENT
Previous cross-sectional studies have shown that prostate disease is an important factor affecting PSA levels.2 However, there are other important questions that remain unanswered: (1) the influence of age on changes in PSA in men without prostate disease, (2) the influence of age-related decreases in androgen levels on PSA, and (3) the magnitude of changes in PSA that occur with the development of prostatic disease over time. Knowledge of these factors that affect PSA is important to better use PSA measurements in men with prostate disease.
In this study we have demonstrated that the increasing prevalence of prostate disease that occurs with age is the most important factor affecting PSA levels in men. In men thought to have no evidence of prostate disease (control group), the rate of change in PSA was lower than in subjects with prostate disease and was not significantly different from 0.0 μg/L per year. This is consistent with the concept that PSA is produced by prostatic epithelium and that epithelial cell death and growth are balanced in men without prostate disease.16 This results in relatively little change in prostate size with age17 in these men after puberty. In the control group, one subject had a higher rate of change in PSA than the other control subjects (Fig 1). Since this group of men was screened by medical history and physical examination only, it is possible that underlying prostatic disease was undetected in this individual. Withdrawal of androgens in men with prostate disease results in a decrease in PSA levels presumably as a result of the androgen dependence of prostatic epithelium.7 In this study, androgen levels do not appear to account for the difference in PSA changes between men with and without prostate disease since the change in androgen levels with age did not differ among the groups.
The magnitude of changes in PSA were significantly different in the three groups of men in this study. In men with BPH, the increase in PSA was gradual up to the time of diagnosis (Fig 3). This is consistent with the fact that PSA levels increase proportionately with increases in weight of BPH tissue.2 Autopsy studies also suggest a prostate doubling time of 10 years or more in men with BPH.17 The observed differences in rates of change in PSA levels among men with BPH (Fig 1) may reflect the known histologie heterogeneity of BPH. BPH is a heterogeneous disease that is characterized histologically by a variable degree of stromal and epithelial hyperplasia. Thus, men with a predominance of epithelial hyperplasia would be expected to have greater increases in PSA during periods of epithelial proliferation than those men with primarily stromal hyperplasia. It is also possible that some of the subjects with BPH that have PSA changes similar to subjects with cancer may represent individuals with undetected prostate cancer.
We hypothesized that men with prostate cancer would have a greater change in serial PSA levels compared with men who had BPH based on several clinical observations. It has been shown that serum PSA levels increase directly with the volume of prostate cancer.2 Furthermore, on a volume-for-volume basis, prostate cancer is thought to contribute 10 times more to serum PSA concentrations than benign tissue.18 Finally, the doubling time of prostate cancer19 is estimated to be 100 times faster than for benign tissue.17 We found an exponential increase in PSA concentration (Fig 3) in subjects with prostate cancer 5 to 10 years prior to diagnosis, which is consistent with the known increase in PSA that occurs with increasing tumor volume and the kinetics of tumor growth. The observed differences in the rate of PSA change between subjects with prostate cancer (Figs 1 and 2) is consistent with the known variability in the biologic behavior of prostate cancers. Four men with local or regional disease demonstrated minimal change in PSA concentrations with levels remaining below 4.0 μg/L up to the time of diagnosis. Whether men with prostate cancer and minimal PSA change have biologically different tumors than men with greater changes in PSA is currently unknown.
Recent data suggest that measurement of serum PSA concentrations may allow earlier detection of prostate cancer.3,4 However, men with BPH have PSA elevations in 21% to 86% of cases,2,5,6 and men with prostate cancer have normal PSA levels in 21% to 35% of cases.4-6 Therefore, PSA elevations are not specific for prostate cancer, and a normal PSA level does not exclude the presence of cancer. This is reflected in the 78.7% sensitivity and 59.2% specificity using a PSA of 4.0 μg/L or greater as a detection criterion in a recent prostate cancer screening study.4 Using the same criterion for prostate cancer detection we found a 78% sensitivity and a 60% specificity in this case-control study. When we used a prostate cancer detection criterion based on the average rate of change in PSA between three consecutive visits (PSAR2≥0.75 μg/L per year), we found no difference in the sensitivity of prostate cancer detection compared with the use of a PSA of 4.0 μg/L or greater. However, the use of this rate of change criterion in prostate cancer detection significantly increased the ability to differentiate between subjects with BPH and prostate cancer (specificity, 90%). More importantly, the rate of change rule maintained a high degree of specificity at PSA levels both below and above 4.0 μg/L. Thus, serial PSA measurement and assessment of the rate of change in PSA concentrations may allow the physician to differentiate between those men with BPH and prostate cancer. This may identify more accurately the men who will benefit from further diagnostic tests, such as prostatic ultrasound and/or biopsy. Since BPH is more prevalent than prostate cancer, differentiation between men with prostate cancer and BPH would avoid subjecting a large number of men with lower PSA levels to unnecessary workup and the psychological trauma associated with cancer suspicion.
There is an approximate 10% day-to-day variation in PSA levels that may explain some of the variability in PSA measurements observed in subjects with BPH. This variation would mean that an increase in PSA concentrations of 0.75 μg/L would be more likely to represent a true change for men with lower baseline PSA levels compared with those with higher baseline PSA levels. However, despite this PSA variation, we observed a similar specificity for rate of change in cancer detection when PSA values were less than 4.0 μg/L or 4.0 μg/L to 10.0 μg/L (Table 4). This may be due to the use of three consecutive PSA measurements in the determination of rate of change (average rate of change) that “dampens out” some of the variability in repeated measurements.
The changes in PSA levels observed in this case-control study may not be representative of changes that occur in all men being monitored by PSA levels in clinical practice. In this study, PSA measurements were performed on stored serum samples and not on consecutively collected samples over many years as would be the case in clinical practice where the interassay variation might be greater. Greater interassay variation could decrease the specificity of serial PSA measurements and evaluation of the rate of change in PSA in clinical practice. Furthermore, the limited number of subjects in this study requires that a large prospective study verify our results to establish if rate of change in PSA might be of use in clinical practice. However, since subjects with BPH in our study all presumably had symptoms severe enough to warrant surgery, the rate of change in PSA in men with less severe voiding symptoms may be lower. If this proves to be true, the use of rate of change in PSA may be even more specific in an unselected population of patients with BPH.
In summary, the changes that occur in PSA with age and the magnitude of the change appear to be primarily dependent on the development of prostate disease with age. Because the rate of change in PSA appears to be greater in men with prostate cancer compared with men without the disease, and because this change may occur at a time when the disease is not clinically evident, serial measurement of PSA may be a useful clinical marker of prostate cancer. This information is important in designing prospective clinical trials to evaluate prostate cancer screening. The questions of who may benefit from serial measurements of PSA, at what age, and at what intervals will require further study.
Table 1.
Description of Diagnostic Groups*
| Cancer Cases |
|||||
|---|---|---|---|---|---|
| Controls | BPH Cases | Local or Regional |
Metastatic | Significance Tests† |
|
| No. of participants | 16 | 20 | 14 | 4 | |
|
| |||||
| Age at diagnosis, y Median (range) |
66.0 (56.7-80.5) | 75.9 (64.6-86.7) | 73.8 (63.6-85.4) | 72.1 (62.7-82.8) | BPH>C |
|
| |||||
| Follow-up, y Median (range) |
15.1 (9.4-16.8) | 14.3 (6.9-24.1) | 17.2 (10.6-24.9) | 17.4 (10.0-25.3) | NS |
|
| |||||
| No. of repeated prostate-specific antigen measurements per individual Median (range) |
8.0 (4-10) | 8.0 (5-11) | 11.0 (7-15) | 9.5 (7-12) | LR>C,BPH |
|
| |||||
| No. of repeated hormone measurements per individual Median (range) |
4.0 (3-5) | 4.0 (3-6) | 4.0 (3-5) | 4.0 (3-6) | NS |
BPH indicates benign prostatic hyperplasia; C, controls; NS, no comparisons significant at P= .05; and L/R, local or regional cancer cases.
Results of pairwise comparisons using Mann-Whitney U tests.15
Table 2.
Observed and Predicted Rates of Change in Prostate-Specific Antigen Levels (μg/L per year)*
| Cancer Cases |
|||||
|---|---|---|---|---|---|
| Time Interval Before Diagnosis, y | Controls | BPH Cases | Local or Regional | Metastatic | Significance Tests† |
| Zero-5.0 | |||||
| Observed median (observed range) | 0.03 (−0.30-0.56) | 0.12 (−0.32-0.53) | 0.88 (−0.04-13.83) | 5.35 (1.74-7.69) | LR, M>C, BPH |
|
| |||||
| Predicted | 0.04 | 0.18 | 0.75 | 4.40 | |
|
| |||||
| 5.0-10.0 | |||||
| Observed median (observed range) | 0.01 (−0.28-0.37) | 0.09 (−0.28-1.1) | 0.27 (−0.04-1.78) | 1.33 (−0.07-1.83) | LR>C, BPH |
|
| |||||
| Predicted | 0.03 | 0.14 | 0.37 | 1.26 | |
|
| |||||
| 10.0-15.0 | |||||
| Observed median (observed range) | 0.02 (−0.07-0.28) | 0.09 (−0.14-0.62) | 0.14 (−0.01-0.37) | 0.30 (0.12-1.18) | LR>C; M>C, BPH |
|
| |||||
| Predicted | 0.02 | 0.10 | 0.13 | 0.42 | |
Predicted from the mixed-effects regression model assuming an age at diagnosis of 75.0 years. BPH indicates benign prostatic hyperplasia; LR, local or regional cancer; M, metastatic cancer cases; and C, controls.
Results of pairwise comparisons using Mann-Whitney U tests.
Table 3.
Prostate-Specific Antigen Levels (μg/L) of Diagnostic Groups*
| Cancer Cases |
|||||
|---|---|---|---|---|---|
| Controls | BPH Cases | Local or Regional | Metastatic | Significance Tests† |
|
| Diagnosis (±3.1 y) Median (range) |
0.9 (0.2-6.7) | 2.8 (1.1-6.5) | 7.7 (0.5-47.7) | 28.8 (12.8-40.2) | LR, M>BPH>C |
|
| |||||
| 5 y before diagnosis (±1.9 y) Median (range) |
0.7 (0.1-3.6) | 2.6 (0.2-7.6) | 2.8 (0.6-8.7) | 7.8 (3.2-12.3) | BPH, LR, M>C; M>BPH |
|
| |||||
| 10 y before diagnosis (±2.2 y) Median (range) |
0.5 (0.2-2.4) | 1.8 (0.5-4.7) | 1.9 (0.2-3.1) | 4.9 (1.5-7.7) | BPH, LR, M>C |
|
| |||||
| 15 y before diagnosis (±2.1 y) Median (range) |
0.5 (0.1-3.2) | 0.9 (0.2-2.6) | 0.8 (0.3-1.9) | 3.0 (2.4-5.0) | M>C, BPH, LR |
BPH indicates benign prostatic hyperplasia; LR, local or regional cancer; M, metastatic cancer cases; and C, controls.
Results of pairwise comparisons using Mann-Whitney U tests.
Table 4.
Sensitivity, Specificity, and Time of Detection With Various Screening Criteria
| Specificity at Diagnosis |
Median (Range) No. of Years Before Diagnosis When True-Positive Patients Met Screening Criterion |
||||
|---|---|---|---|---|---|
| Screening Criteria | Sensitivity at Diagnosis | BPH Cases† | Controls | Local or Regional Cancers | Metastatic Cancers |
| Single measurement (μg/L) PSA≥4.0 |
.78 | .60 | .94 | 4.9 (2.9-7.4) | 11.2 (6.0-16.4) |
|
| |||||
| PSA≥10.0 | .50 | 1.0 | 1.0 | 2.2 (0.5-5.1) | 5.4 (1.5-7.9) |
|
| |||||
| Single rate of change (μg/L per year)† PSAR≥1.0 |
.78 | .60 | .94 | 3.3 (0.0-9.0) | 7.9 (2.3-8.9) |
|
| |||||
| Average rate of change (μg/L per year)‡ PSAR2≥0.75 in total sample |
.72 | .90§ | 1.0 | 2.6 (0.0-9.0) | 7.5 (2.3-8.7) |
|
| |||||
| PSAR2≥0.75 when PSA<4 μg/L | .11 | 1.0 | 1.0 | 4.8 (0.6-9.0) | NA∥ |
|
| |||||
| PSAR2s≥0.75 when PSAs<4 and PSA<10 μg/L | .79 | .88 | 1.0 | 2.6 (0.7-7.2) | 7.9 (4.5-8.9) |
|
| |||||
| PSAR2≥0.75 When PSA≥10 μg/L | 1.0 | NA∥ | NA∥ | 0.6 (0.0-4.3) | 3.5 (0.6-7.9) |
BPH indicates benign prostatic hyperplasia.
Rate of change in prostate-specific antigen levels over two consecutive visits.
Rate of change in prostate-specific antigen levels over three consecutive visits.
Significantly different from specificity for single PSA≥4.0 μg/L (P= .03).
No subjects in this category.
Acknowledgments
Research for this study was conducted by the National Institute on Aging Intramural Research Program, Bethesda, Md.
The PSA kits were contributed by Hybritech Ine, San Diego, Calif.
We are indebted to Christopher H. Morrell, PhD, for his comments on the statistical analyses, Patricia Kaminski for her assistance in the statistical analyses and graphics, Howard Baldwin, MA, for his help with the BLSA serum bank, and Cynthia Kelley for technical assistance.
References
- 1.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate-specific antigen. Invest Urol. 1979;17:159–163. [PubMed] [Google Scholar]
- 2.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 3.Cooner WH, Mosley BR, Rutherford CL, et al. Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination, and prostate-specific antigen. J Urol. 1990;143:1146–1154. doi: 10.1016/s0022-5347(17)40211-4. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 5.Hudson MA, Bahnson RR, Catalona WJ. Clinical use of prostate-specific antigen in patients with prostate cancer. J Urol. 1989;142:1011–1017. doi: 10.1016/s0022-5347(17)38972-3. [DOI] [PubMed] [Google Scholar]
- 6.Oesterling JE, Chan DW, Epstein JI, et al. Prostate-specific antigen in the preoperative and postoperative evaluation of localized prostatic cancer treated with radical prostatectomy. J Urol. 1988;139:766–772. doi: 10.1016/s0022-5347(17)42630-9. [DOI] [PubMed] [Google Scholar]
- 7.Weber JP, Oesterling JE, Peters CA, Partin Aw, Chan DW, Walsh PC. The influence of reversible androgen deprivation on serum prostate-specific antigen levels in men with benign prostatic hyperplasia. J Urol. 1989;141:987–992. doi: 10.1016/s0022-5347(17)41083-4. [DOI] [PubMed] [Google Scholar]
- 8.Stamey TA, Kabalin JN, Ferrari M, Yang N. Prostate-specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate, IV: anti-androgen treated patients. J Urol. 1989;141:1088–1090. doi: 10.1016/s0022-5347(17)41177-3. [DOI] [PubMed] [Google Scholar]
- 9.Shock NW, Greulich RC, Andres R, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. National Institutes of Health; Washington, DC: 1984. pp. 84–2450. National Institutes of Health publication. [Google Scholar]
- 10.Arrighi HM, Guess HA, Metter EJ, Fozard JL. Symptoms and signs of prostatism as risk factors for prostatectomy. Prostate. 1990;16:253–261. doi: 10.1002/pros.2990160309. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health . 1987 AnnualAnnual Cancer Statistics Review. National Institutes of Health; Washington, DC: 1988. pp. 88–2789. National Institutes of Health publication. [Google Scholar]
- 12.Guess HA, Arrighi HM, Metter EJ, Fozard JL. Cumulative prevalence of prostatism matches the autopsy prevalence of benign prostatic hyperplasia. Prostate. 1990;17:241–246. doi: 10.1002/pros.2990170308. [DOI] [PubMed] [Google Scholar]
- 13.Lindstrom MJ, Bates DM. Newton-Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J Am Stat Assoc. 1988;83:1014–1022. [Google Scholar]
- 14.Pearson JD, Kaminski P, Morrell CH, et al. Modeling longitudinal rates of change in prostate-specific antigen during aging; Proceedings of the American Statistical Association Meetings: Social Statistics Section; Alexandria, Va: American Statistical Association. 1991.pp. 580–585. [Google Scholar]
- 15.Rosner B. Fundamentals of Biostatistics. 2nd ed Vol. 79. Duxbury Press; Boston, Mass: 1986. [Google Scholar]
- 16.Kyprianou N, Martikainen P, Isaacs JT. Programmed cell death of normal and malignant prostatic cells. In: Voigt KD, Knabbe C, editors. Endocrine Dependent Tumors. Raven Press; New York, NY: 1991. pp. 69–81. [Google Scholar]
- 17.Berry SJ, Coffey DS, Walsh PC. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 18.Stamey TA, Kabalin JN, McNeal JE, et al. Prostate-specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate, II: radical prostatectomy–treated patients. J Urol. 1989;141:1076–1083. doi: 10.1016/s0022-5347(17)41175-x. [DOI] [PubMed] [Google Scholar]
- 19.Meyer JS, Sufrin G, Martin SA. Proliferative activity of benign human prostate, prostatic adenocarcinoma, and seminal vesicle evaluated by thymidine labeling. J Urol. 1982;128:1353–1356. doi: 10.1016/s0022-5347(17)53506-5. [DOI] [PubMed] [Google Scholar]